Abstract
Although natural killer (NK) cells have been implicated in regulating immune responses, their ability to modulate disease development in autoimmune arthritis has not been analyzed. Here we investigate the contribution of NK cells to regulating collagen-induced arthritis, a well-characterized preclinical model of human rheumatoid arthritis. We find that the disease is induced by the combined action of two CD4+ T helper (TH) subsets: follicular TH cells and TH17 cells. Both CD4+ TH subsets are highly susceptible to lysis by NK cells after activation. Administration of antibody that activates NK cells through blockade of its inhibitory CD94/NKG2A receptor allows enhanced elimination of pathogenic follicular TH and TH17 cells and arrest of disease progression. These results suggest that antibody-dependent enhancement of NK activity may yield effective, previously undescribed therapeutic approaches to this autoimmune disorder.
Keywords: antibody therapy, Qa-1, T helper subsets
The prevalence of rheumatoid arthritis (RA) has increased markedly over the last decade, along with the increased size of the aging population (1). New therapeutic approaches have come from studies of animal models of RA. Induction of arthritis in mice after immunization with type II collagen (CII), termed collagen-induced arthritis (CIA), shares several similarities with human RA, including breach of self-tolerance, generation of autoantibodies, and the development of inflammatory changes in multiple joints (2). This animal model has been used to evaluate several FDA-approved therapies for RA, including anti-TNF Ab, IL-1 antagonists, and methotrextate (3, 4). Clinically relevant features of CIA include development of enlarged and hyperactive germinal centers (GCs), T cell-dependent production of pathogenic autoantibodies, and robust inflammatory responses in synovial tissues (3).
New approaches to treatment depend on definition of the subsets of T cells that induce this autoimmune disorder and regulatory pathways that inhibit their activity. Although pathogenic collagen-reactive CD4 cells belonging to the T helper 17 (TH17) subset may contribute to inflammatory responses (5), the T helper (TH) lineage that induces anti-collagen autoantibody responses has not been established. Because follicular TH cells (TFH) that provide cognate help to GC B cells can provoke autoantibody responses (6, 7), we compared the contribution of TFH cells to other TH subsets to induction of autoantibody. We found that TFH cells are primarily responsible for induction of the autoantibody response and that cooperation between TFH cells and TH17 cells induces development of clinical arthritis.
Although activated natural killer (NK) cells may inhibit expansion of autoreactive T cells in other settings (8, 9), the contribution of NK cells to the development of RA is not well understood. We noted that both TH17 and TFH cells are highly susceptible to NK lysis. We reasoned that enhanced NK-dependent elimination of these pathogenic subsets might inhibit development of arthritic disease. Here we found that Ab blockade of the inhibitory interaction between the CD94/NKG2A receptor and its Qa-1 ligand enhances NK cell-dependent elimination of pathogenic T cells, resulting in abrogation of disease development. These findings suggest a unique approach to the treatment of this disease.
Results
Cooperation Between TH17 and TFH Cells Induces CIA.
The development of CIA is associated with T- and B-lymphocyte responses that result in anti-CII autoantibodies and induction of inflammatory responses in joints. We initially defined the contribution of distinct TH subsets—TH1, TH17, and TFH—to these pathogenic autoimmune responses by cotransfer of collagen-reactive TH subsets and B cells into Rag2−/−Prf1−/− mice. TH subsets were (i) obtained from donors immunized to chicken CII and restimulated in vitro under culture conditions that generate >98% pure TH1, TH17, or TFH cells (ref. 10; Fig. S1) or (ii) cells recovered directly from CII-immune donors without an intermediate in vitro culture step before transfer into Rag2−/−Prf1−/− hosts (Fig. 1).
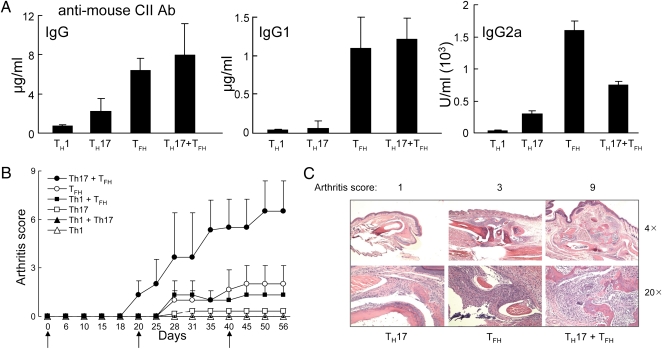
Fig. 1.
The contribution of TH17 and TFH cells to CIA. ICOS+CXCR5+BTLA+CD25−CD4+ TFH cells were FACS-sorted from arthritic mice (score = 12) and TH1 and TH17 cells were generated in vitro after stimulation with chicken CII. Each TH cell subset (2 × 106) or mixtures of the indicated TH subsets (106 each) were cotransferred with B cells (4 × 106) into Rag2−/−Prf1−/− mice followed by CII immunization and boosting at d 21 and 40. (A) Serum Ig and autoantibody titers (anti-mouse CII) were measured at d 45. (B) Arthritis scores of three mice per group are shown. Arrows indicate CII immunization and boosting. (C) Representative images of joint histology along with the arthritis score are shown for mice given the indicated TH subsets. Data represent one of three identical experiments.
Consistent with findings that TFH cells are primarily responsible for autoantibody production (7, 10), collagen-reactive TFH, but not TH1 or TH17, cells induced adoptive autoantibody responses to mouse collagen (Fig. 1A). Although cotransfer of TFH and TH17 subsets did not cooperate in the induction of autoantibody (Fig. 1A), their combined activity was essential for induction of robust arthritic disease (Fig. 1B). The development of arthritis in reconstituted Rag2−/−Prf1−/− hosts was similar to development in donor B6 mice—e.g., joint swelling and inflammation was apparent as early as day (d) 18 and prominent by d 35. Examination of joint histopathology revealed that, whereas TH17 or TFH cells each induced marginal levels of intraarticular inflammation, mixtures of TH17 and TFH cells induced a severe and destructive polyarthritis that included severe bone resorption and fibrovascular pannus formation (Fig. 1C). These findings suggest a cooperative mechanism that may reflect TFH-dependent autoantibody responses (Fig. 1A) and TH17-dependent inflammatory responses (5). The Ab-dependent pathway may result in immune complex formation in joint tissues and complement activation, resulting in enhancement of intraarticular inflammatory responses induced by TH17 cells (3). These findings also suggest that targeting of these autoreactive TH subsets may represent an effective therapeutic approach to CIA.
Effect of Perforin+ NK Cells on CIA.
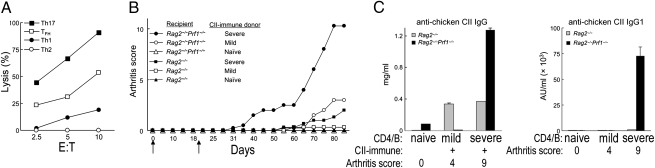
Both TH17 and TFH cells are highly sensitive to NK lysis compared with TH1 and TH2 cells (Fig. 2A), suggesting that NK cells might inhibit the response of both pathogenic TH subsets. The possibility that NK cells might inhibit CIA initially came from findings that adoptive transfer of CII-immune CD4+ T cells and B cells into Rag2−/−Prf1−/−, but not Rag2−/−, mice resulted in robust arthritis and autoantibody development (Fig. 2 B and C). The latter Rag2−/− recipients, but not the former, harbor perforin+ NK cells.
Fig. 2.
The contribution of NK cells to regulation of CIA. (A) IL-2–expanded NK cells were incubated for 4 h with CII-specific TH subsets (induced in vitro) at the indicated E:T ratios. Percent lysis is shown. (B) Purified CD4 cells (lacking CD25+CD4+ Treg) and B cells from arthritic mice with indicated disease severity were transferred into Rag2−/−Prf1−/− (n = 4 per transfer) or Rag2−/− hosts (n = 3 per transfer). Arthritis was induced as described and scores are shown. Naïve, score = 0; mild, score= 2–4; severe, score = 10–12. Arrows indicate CII immunization and boosting. (C) Serum anti-chicken CII IgG and IgG1 titers were measured at d 45 and represent one of three identical experiments for B and C.
Engagement of Inhibitory CD94/NKG2A Receptor on NK Cells Regulates CIA Development.
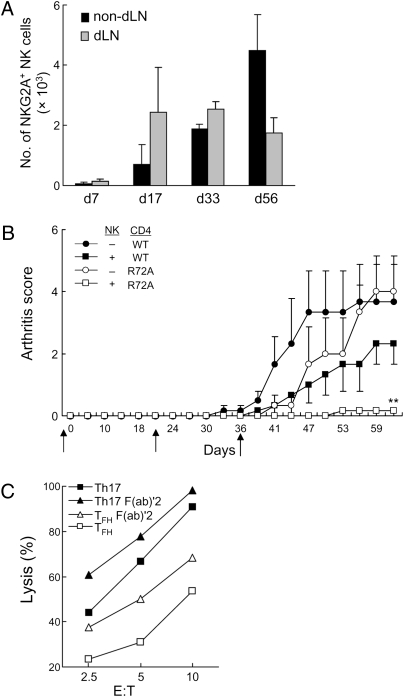
Elimination of pathogenic T cells by NK cells in vivo is normally held in check through engagement of the CD94/NKG2A inhibitory receptor on NK cells by the Qa-1/Qdm ligand expressed on activated T cells (8). Consistent with findings that NKG2A+ NK cells accumulate in human arthritic synovia (11, 12), the numbers of NKG2A+ NK cells increased systemically in all lymph nodes (LNs) with disease progression (Fig. 3A). We asked whether NK cells might inhibit the development of CIA in animals given CII-immune CD4+ T cells and determined whether the inhibitory CD94/NKG2A receptor might represent a suitable molecular target for therapeutic intervention. To this end, we evaluated arthritis development in adoptive hosts (Rag2−/−Prf1−/−) given CD4+ T cells from B6.WT or B6.Qa-1 R72A knock-in mice along with (B6) NK cells. CD4+ T cells from Qa-1 R72A mice express a mutant Qa-1 protein that prevents binding of Qa-1 to the inhibitory CD94/NKG2A receptor and increases their susceptibility to NK lysis (8). Infusion of either Qa-1 WT or Qa-1 mutant CD4 cells and B cells promoted arthritis development (Fig. 3B). Cotransfer of NK cells reduced disease in hosts given Qa-1 WT CD4 cells and abolished arthritis development in hosts coinjected with Qa-1 R72A CD4 cells (Fig. 3B). These findings suggest that genetic interruption of the inhibitory CD94/NKG2A–Qa-1 interaction resulted in increased levels of NK cell-dependent inhibition of CIA.
Fig. 3.
Regulation of NK cell activity through a CD94/NKG2A–Qa-1 interaction. (A) Numbers of NKG2A+ NK cells from draining LNs (dLNs) and nondraining LNs (nondLNs) during CIA development are shown for groups of three mice for each time point. (B) Purified CD4+ T cells (depleted of CD25+CD4+ Treg) from arthritic B6.WT or B6.Qa1 R72A mice were transferred into Rag2−/−Prf1−/− hosts along with B6.WT B cells. In some cases, sorted B6.WT NK cells were transferred into hosts before infusion of CD4 and B cells. Mice were immunized and boosted with CII at d 21 and 36 (black arrows). **P < 0.01. Arthritis scores are shown for adoptive hosts (three per transfer). (C) IL-2–expanded NK cells were preincubated with 10 μg⋅mL−1 20d5 F(ab)′2 for 1 h at 37 °C followed by incubation for 4 h with in vitro-differentiated CII-specific TH subsets at the indicated E:T ratios. Percent lysis is shown.
Anti-NKG2A F(ab)′2 Treatment Inhibits Arthritis.
In vitro cytotoxicity assays indicated that addition of anti-NKG2A F(ab)′2 Ab to block the inhibitory CD94/NKG2A receptor enhanced NK lysis of collagen-specific TFH and TH17 target cells (Fig. 3C). These findings suggested that Ab-dependent interruption of the inhibitory interaction between the Qa-1/Qdm ligand and the CD94/NKG2A receptor might enhance NK lysis of arthrogenic TFH and TH17 cells. We therefore evaluated the impact of administration of anti-NKG2A F(ab)′2 Ab on CIA development in vivo.
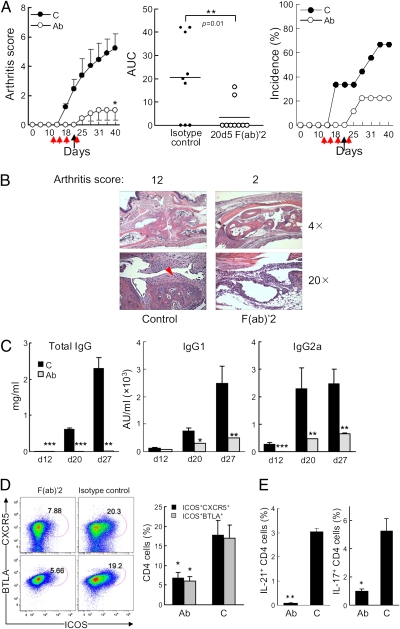
Injection of anti-NKG2A F(ab)′2 Ab after CII immunization resulted in prolonged suppression of CIA that persisted after cessation of treatment (Fig. 4A). Anti-NKG2A F(ab)′2 Ab treatment ameliorated joint inflammation/destruction, prevented pannus formation—a bona fide histological feature of severe arthritis (Fig. 4B)—and reduced collagen-specific recall responses of LN cells (Fig. S2A). Further analysis revealed that this treatment decreased autoantibody titers (Fig. 4C) and reduced the numbers of TFH cells (ICOS+CXCR5+ or ICOS+BTLA+ CD4 cells and IL-21+ CD4 cells), but not B cells (Fig. 4 D and E and Fig. S2B), similar to findings in vitro (Fig. 3C). Moreover, numbers of TH17 (IL-17+), but not TH1 (IFN-γ+) or TH2 (IL-4+), cells were also reduced, consistent with selective in vitro sensitivity of TFH and TH17 cells to NK lysis (Fig. 4E and Fig. S2C).
Fig. 4.
The efficacy of anti-NKG2A F(ab)′2 treatment of CIA. (A Left) CIA was induced in B6.WT mice as described in Materials and Methods. 200 μg of 20d5 F(ab)′2 anti-NKG2A (Ab) or 2A3 F(ab)′2 isotype control Ab (C) was given i.p. at the indicated times (red arrows: d 12, 15, 18, and 22; black arrows indicate CII boost). n = 9 mice per group; Student's t test: *P = 0.02 of two groups. (Center) Area under the curve (AUC) of individual mouse in A Left from the outset of treatment (**P < 0.01, Mann-Whitney test). (Right) Disease incidence is shown. Data represent at least five independent experiments. (B) Representative images of joint histology are shown. Red arrowhead indicates pannus formation. (C) Serum titers of anti-chicken CII IgG, IgG1, and IgG2a are shown. ***P < 0.001; **P < 0.01. (D Left) Representative plots of TFH cells from nondLNs are shown. Comparisons are made between F(ab)′2-treated and control-treated mice. (Right) Statistical analyses are shown. n = 5 (isotype control) and n = 4 [F(ab)′2]; Student's’ t test: *P < 0.05. (E) Levels of intracellular IL-17 and -21 in total CD4 cells from nondLNs were compared and graphed. n = 5 (isotype control) and n = 4 [F(ab)′2]; Student's’ t test: *P < 0.05.
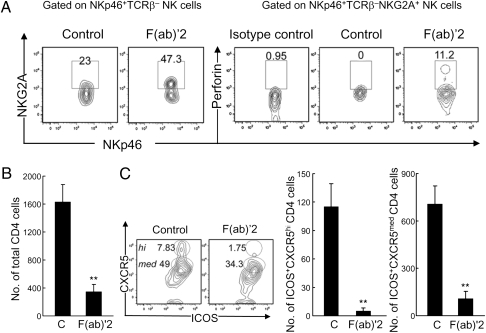
Finally, we investigated the impact of anti-NKG2A F(ab)′2 Ab treatment on joint infiltration by activated NK cells and CD4+ TH cells. Treatment with anti-NKG2A resulted in increased proportions of perforin+ NK cells (Fig. 5A) as well as a substantial reduction in the numbers of CD4+ T cells (Fig. 5B) and a prominent decrease in ICOS+CXCR5+ TFH cells (Fig. 5C).
Fig. 5.
The contribution of anti-NKG2A F(ab)′2 treatment on CD4 cell and NK cell infiltration into joints. (A Left) Increased NKG2A+ NKp46+TCRβ− NK cells in joints of B6 mice after F(ab)′2 treatment. (Right) Increased intracellular perforin expression in intraarticular NKG2A+ NK cells after F(ab)′2 treatment. Rat IGg2a Ab was used as isotype control for perforin staining. (B) Numbers of CD4+ cells in infiltrated joints of F(ab)′2-treated and control (C) B6 mice. (C Left) Representative plots of intraarticular ICOS+CXCR5+CD4+ (TFH) cells from both groups of mice (B) are shown. Numbers of ICOS+CXCR5hiCD4+ cells (Center) and ICOS+CXCR5medCD4+ cells (Right) are shown. Cells were isolated from paws and ankles pooled from three mice per group. Average arthritis score: control = 4; F(ab)′2 = 1. Data represent two separate experiments; error bars denote mean ± SE. Student's’ t test: **P < 0.01.
Discussion
Clinical approaches to the treatment of arthritis have focused mainly on downstream events in this autoimmune disease process and have yielded drugs that inhibit TNF and IL-1 cytokine expression by macrophages and synovial cells (13, 14). Here we describe a strategy based on targeting these pathogenic TH subsets responsible for induction of the two primary components of CIA: autoantibodies (anti-collagen and rheumatoid factor) and intraarticular inflammatory responses.
Previous findings have indicated that TFH cells are particularly well equipped to induce autoantibody responses in collaboration with GC B cells (7, 15). The contribution of TFH cells to the autoantibody response was confirmed by adoptive transfer studies revealing induction of strong anti-collagen Ab responses. Moreover, mild disease was observed in mice supplied with TFH or TH17 cells alone, whereas severe arthritic disease reflected cooperation between TFH and TH17 cells (Fig. 1). These observations and others suggest that the disease may proceed along two cellular pathways: one that depends on TFH-induced autoantibodies and a second that depends mainly on induction of inflammatory responses by TH17 cells. We propose that binding of autoantibodies to collagen in joints activates and triggers local inflammatory cell infiltration (16) and that TH17-dependent cytokine responses amplify this response, resulting in robust and destructive intraarticular disease (e.g., Fig. 1). An important caveat to this model comes from the potential plasticity of these TH subsets after expansion in vivo (17). Additional studies are needed to address the stability of TH17 and TFH cells after long-term sojourn in adoptive hosts.
The contribution of NK cells to arthritis has been unclear, in part because of the diverse activities of this lymphocyte subset (18). Our experimental approach has revealed the importance of NK cells in regulating arthritis in an animal model. The regulatory role of NK cells may depend largely on its cytotoxic function, as judged from its dependence on perforin expression in these studies. Interestingly, NK cells in RA synovium that display impaired cytotoxic activity may contribute to dysregulated immune responses in arthritic patients (19, 20). The susceptibility of pathogenic TFH and TH17 subsets to NK-dependent lysis in vitro prompted us to test the impact of F(ab)′2 anti-NKG2A-dependent up-regulation of NK activity in vivo. Anti-NKG2A F(ab)′2 mAb treatment did not deplete NKG2A+ NK cells or render NK cells hyporesponsive. Instead, blockade of the inhibitory CD94/NKG2A receptor for Qa-1/Qdm enhanced NK cell lysis of TH17 and TFH target cells in vitro and inhibited disease progression in vivo. These studies indicate that anti-NKG2A-dependent interruption of the inhibitory interaction between Qa-1/Qdm on activated CD4 TH cells and NKG2A+ NK cells “released the brakes” on NK cells, resulting in increased elimination of pathogenic CD4+ TH cells and durable inhibition of arthritis.
The basis for preferential NK cell targeting of TH17 and TFH cells, rather than TH1 or TH2 cells, in these studies is unclear. Increased levels of NKG2D ligands by TH17/TFH cells probably do not play a decisive role in the susceptibility of these TH subsets (Fig. S3C), and further studies are required to define the selective sensitivity of the TH17–TFH pair to NK lysis. In sum, these studies indicate that amelioration of arthritis can be achieved through mobilization of NK cells by anti-NKG2A F(ab)′2 treatment and consequent elimination of pathogenic autoreactive T cells. Because CD94/NKG2A and its ligands are highly conserved in rodents and humans, this anti-NKG2A–based approach may be translated into an effective, previously undescribed therapy for human RA.
Materials and Methods
Mice.
C57BL/6 (B6), Rag2−/−, Rag2−/−Prf1−/− (Taconic Farms), and B6.Qa-1 R72A mice (back-crossed for 11 generations; ref. 8) were housed in pathogen-free conditions. All experiments were performed in compliance with federal laws and institutional guidelines as approved by the Dana–Farber Cancer Institute Animal Care and Use Committee.
CIA Induction and Assessment.
Chicken CII (MD Bioscience) was dissolved in 0.01 M acetic acid at a concentration of 4 mg⋅mL−1 by stirring overnight at 4 °C. All mice were males between the age of 8 and 12 wk. To induce CIA, C57BL/6 (B6.WT) mice were injected intradermally at the base of the tail with 150 μg of chicken CII emulsified in complete Freund's adjuvant (supplemented with 4 mg⋅mL−1 Mycobacterium tuberculosis) and boosted at the indicated days with 100 μg of chicken CII emulsified in incomplete Freund's adjuvant. For adoptive transfers, purified CD4 (CD25+CD4+ Treg depleted) and B cells from arthritic mice with differing severity as noted were transferred into Rag2−/−Prf1−/− or Rag2−/− hosts, immunized at d 0, and boosted at the indicated days as described above. Clinical assessment of CIA was performed every 2–3 d each week, and scoring was as follows: 0, normal; 1, mild swelling and/or erythema confined to the midfoot or ankle joint; 2, moderate edematous swelling extending from the ankle to the metatarsal joints; and 3, pronounced swelling encompassing the ankle, foot, and digits. Each limb was graded thus, allowing a maximum score of 12 per mouse.
Measurement of Abs Against CII.
Serum levels of anti-chicken or anti-mouse CII IgG or IgG subclasses were measured by ELISA. Serum was collected at indicated days after first chicken CII immunization. Briefly, 96-well ELISA microplates were coated with chicken or mouse CII (Chondrex) at 5 μg⋅mL−1 dissolved in dilution buffer (Chondrex) at 100 μL per well at 4 °C overnight. 100 μL of diluted serum sample was incubated for 2 h at room temperature. The plates were washed with PBST (0.05% Tween-20 in PBS) five times, followed by addition of peroxidase conjugated goat anti-mouse IgG at 1:50,000 concentration (Sigma) or peroxidase-conjugated anti-mouse IgG1 or anti-mouse IgG2a at 1:1,000 concentration (BD Bioscience) at 100 μL per well. After 1 h of incubation at room temperature and washing, the final color development was achieved by adding 3,3′,5,5′-tetramethylbenzidine substrate (BD Bioscience) to each well at 100 μL per well, and absorbance was measured at 405 nm at appropriate time.
Flow Cytometry.
Spleen and LNs were excised, and single-cell suspensions were prepared. Draining LNs (dLNs) included popliteal and inguinal LNs; nondraining LNs (nondLNs) were axillary, cervical, and mesenteric LNs. For isolation of intraarticular cells, paw pieces were isolated and digested with collagenase/dispase (Roche) for 1 h at 37 °C followed by filtration to yield single-cell suspensions. Cells were incubated with Fc block for 15 min followed by staining with relevant Abs against surface markers. CD45 marker was included to gate leukocytes from joints for further analysis. To detect levels of surface NKG2A, cells from control and F(ab)′2-treated groups were first incubated with rat 20d5 F(ab)′2 Ab (1 μg⋅mL−1) (Novo Nordisk A/S) for 30 min to saturate surface NKG2A and further stained with FITC–anti-rat Ig, κ light chain monoclonal Ab. For intracellular cytokine staining, cells were restimulated with leukocyte activation mixture (BD Bioscience) for 5 h, stained with surface markers, fixed, and permeabilized, followed by incubation with indicated Abs. To stimulate NK cells, cells from each organ (2 × 106 cells per mL) were stimulated with plate-bound anti-NK1.1 (20 μg⋅mL−1) for 1 h followed by the addition of BD GolgiPlug (BD Bioscience) and incubated further for 6–7 h. Intracellular staining of perforin in NK cells was performed as described above. Cells were acquired on a FACSCanto II by using FACSDIva software (BD Biosciences) and analyzed with FlowJo software (Tristar).
In Vitro Differentiation of TH Subsets.
Cells from spleen and LNs were collected from B6 mice preimmunized with 150 μg of chicken CII/CFA. CD4+CD25– cells were purified and enriched by negative selection. 2 × 105 cells per mL CD4 cells were stimulated with 100 μg⋅mL−1 chicken CII in the presence of 2 × 106 irradiated total splenocytes. For the differentiation of CD4 cells to each TH cell phenotype, the following cytokine mixtures were added into the culture: TH1: 5 ng⋅mL−1 rmIL-12 and 10 μg⋅mL−1 anti-IL-4 Ab; TH2: 10 ng⋅mL−1 rmIL-4, 10 μg⋅mL−1 anti-IL-12 Ab, 10 μg⋅mL−1 anti-IFN-γ Ab; TH17: 3 ng⋅mL−1 TGF-β, 20 ng⋅mL−1 rIL-6, 20 ng⋅mL−1 rIL-23, 10 μg⋅mL−1 anti-IL-12 Ab, 10 μg⋅mL−1 anti-IFN-γ Ab, 10 μg⋅mL−1 anti-IL-4 Ab; TFH: 50 ng⋅mL−1 IL-21, 10 μg⋅mL−1 anti-IFN-γ Ab, 10 μg⋅mL−1 anti-IL-4 Ab, 20 ng⋅mL−1 rIL-6, and 20 μg⋅mL−1 anti-TGF-β (1D11) Ab. At d 5, live CD4+ cells were harvested from cultures by percoll gradient centrifugation. Total RNA was extracted, and quantitative RT-PCR was performed to measure levels of transcription factors by using specific TaqMan probes (Applied Biosystems) as indicated.
Proliferation Assay.
NondLN cells were incubated for 48 h with different concentrations of chicken CII in DMEM supplemented with 10% FCS and 50 μM β-mercaptoethanol. 3H-thymidine was incorporated in the last 18 h before quantification with a Micro-β counter (Wallac).
NK Cytotoxicity Assay.
Purified NK cells were incubated with 1,000 U⋅mL−1 hIL-2 (Peprotech) for 5 d and used as effector cells. Target cells were labeled with 50 μCi of Na2[51Cr]O4 for 1 h at 37 °C and washed three times with PBS before mixing (1 × 104 per well) with effector cells in U-bottomed 96-well plates at different E:T ratios as indicated, in triplicate. After 4 h of incubation, cell-free supernatants were collected, and radioactivity was measured by Micro-β counter (Wallac). Percent lysis is calculated by (sample release − spontaneous release)/(maximum release − spontaneous release) × 100.
Statistical Analyses.
Statistical analyses were performed by using Student's t test or Mann–Whitney test as indicated. Error bars denote mean ± SE. P < 0.05 was considered to be statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001).
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Research Grant AI 037562 (to H.C.), a Collaborative Sponsored Research Agreement with Novo Nordisk (H.C.), a gift from The Leroy Schecter Research Foundation (H.C.), a Swedish Research Council Award (X.W.), and a grant from the National Natural Science Foundation of China (to X.W.). J.W.L. is a National Research Service Award Fellow (T32 CA070083).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112188108/-/DCSupplemental.
References
- 1.Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther. 2009;11:229. doi: 10.1186/ar2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho YG, Cho ML, Min SY, Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Asquith DL, Miller AM, McInnes IB, Liew FY. Animal models of rheumatoid arthritis. Eur J Immunol. 2009;39:2040–2044. doi: 10.1002/eji.200939578. [DOI] [PubMed] [Google Scholar]
- 4.Bevaart L, Vervoordeldonk MJ, Tak PP. Evaluation of therapeutic targets in animal models of arthritis: How does it relate to rheumatoid arthritis? Arthritis Rheum. 2010;62:2192–2205. doi: 10.1002/art.27503. [DOI] [PubMed] [Google Scholar]
- 5.Leipe J, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62:2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- 6.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 7.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 8.Lu L, et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leavenworth JW, et al. Analysis of the cellular mechanism underlying inhibition of EAE after treatment with anti-NKG2A F(ab’)2. Proc Natl Acad Sci USA. 2010;107:2562–2567. doi: 10.1073/pnas.0914732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HJ, et al. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci USA. 2011;108:2010–2015. doi: 10.1073/pnas.1018974108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002;46:1763–1772. doi: 10.1002/art.10410. [DOI] [PubMed] [Google Scholar]
- 12.de Matos CT, et al. Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: Role of CD94/NKG2A in control of cytokine secretion. Immunology. 2007;122:291–301. doi: 10.1111/j.1365-2567.2007.02638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuna AA, Megeff C. New drugs for the treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2000;57:225–234. doi: 10.1093/ajhp/57.3.225. [DOI] [PubMed] [Google Scholar]
- 14.Schuna AA. Rituximab for the treatment of rheumatoid arthritis. Pharmacotherapy. 2007;27:1702–1710. doi: 10.1592/phco.27.12.1702. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowley MJ, Nandakumar KS, Holmdahl R. The role of collagen antibodies in mediating arthritis. Mod Rheumatol. 2008;18:429–441. doi: 10.1007/s10165-008-0080-x. [DOI] [PubMed] [Google Scholar]
- 17.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahern DJ, Brennan FM. The role of Natural Killer cells in the pathogenesis of rheumatoid arthritis: Major contributors or essential homeostatic modulators? Immunol Lett. 2011;136:115–121. doi: 10.1016/j.imlet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Dalbeth N, et al. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–6426. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 20.Aramaki T, et al. Prediction of DAS28-CRP remission in patients with rheumatoid arthritis treated with tacrolimus at 6 months by baseline variables. Mod Rheumatol. 2009;19:652–656. doi: 10.1007/s10165-009-0216-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.