Abstract
The control of red blood cell and megakaryocyte development by the regulatory protein GATA1 is a paradigm for transcriptional regulation of gene expression in cell lineage differentiation and maturation. Most GATA1-regulated events require GATA1 to bind FOG1, and essentially all GATA1-activated genes are cooccupied by a TAL1/E2A/LMO2/LDB1 complex; however, it is not known whether FOG1 and TAL1/E2A/LMO2/LDB1 are simultaneously recruited by GATA1. Our structural data reveal that the FOG1-binding domain of GATA1, the N finger, can also directly contact LMO2 and show that, despite the small size (< 50 residues) of the GATA1 N finger, both FOG1 and LMO2 can simultaneously bind this domain. LMO2 in turn can simultaneously contact both GATA1 and the DNA-binding protein TAL1/E2A at bipartite E-box/WGATAR sites. Taken together, our data provide the first structural snapshot of multiprotein complex formation at GATA1-dependent genes and support a model in which FOG1 and TAL1/E2A/LMO2/LDB1 can cooccupy E-box/WGATAR sites to facilitate GATA1-mediated activation of gene activation.
Keywords: haematopoiesis, protein–DNA interactions, protein–protein interactions, transcription factor complex
The transcription factor GATA1 activates or represses the expression of hundreds of genes during erythroid development (1, 2). Although the mechanisms of GATA1-mediated transcriptional regulation are far from fully understood, GATA1 activity often requires the coregulator and direct binding partner FOG1/ZFPM1 (3). Chromatin occupancy studies also show that activation of gene expression by GATA1 almost invariably involves cooccupation at the relevant genomic loci by TAL1/SCL, E2A proteins (E47 or E12), LMO2 and LDB1 (1, 2, 4, 5). GATA1 preferentially targets WGATAR sites on DNA, whereas TAL1/E2A heterodimers target E-box sites (CANNTG); the multiprotein complexes containing these proteins (GATA1, TAL1, E2A, LMO2, and LDB1) bind to bipartite E-box/GATA sites and have been identified in numerous haematopoietic cell lines (2, 6). These complexes may also include one or more additional regulatory proteins such as Pu.1, ETO-2 (5), and SSBP (7), which potentially modulate the activity of the complex.
It has not yet been established if TAL1/E2A/LMO2/LDB1 complexes and FOG1 take part in the same GATA1-containing transcriptional complexes or instead form distinct assemblies. Although chromatin occupancy analysis indicates that FOG1 and TAL1 have essentially the same distribution patterns at active genes (5), it has been proposed that the composition of GATA1-associated protein complexes might vary with time, such that TAL1/LMO2/LDB1 replaces FOG1, or vice versa. Time course chromatin immunoprecipitation studies do not, however, support this model (5).
The structural basis for many of these interactions is unknown. We have previously determined the structure of a complex formed by the N-terminal zinc finger of GATA1 (GATA1NF) and a FOG1 zinc finger (8) and have described the conserved mechanism by which LMO-family proteins such as LMO2 and LMO4 recognize their common partner protein LDB1 (9). Little, however, is currently known about the nature of the interactions made by LMO2 with either GATA1 or TAL1/E2A, although we do know that the LMO2-LDB1 complex binds TAL1/E2A heterodimers with an affinity of approximately 10 nM (10), which is similar to the affinity of the LMO2/LDB1 interaction (11). LMO2 is thought to bridge TAL1/E2A and GATA1, either through simultaneous binding of both TAL1/E2A and GATA1 or via LDB1, which itself self-associates through an N-terminal domain (12).
In this study we show that LMO2 and FOG1 contact different surfaces of GATA1NF. NMR and mutagenesis data further reveal that LMO2 and FOG1 can simultaneously bind to GATA1NF, consistent with a mechanism in which a single LMO2 molecule bridges TAL1/E2A and GATA1 at bipartite E-box/WGATAR sites, and FOG1 and TAL1/E2A/LMO2/LDB1 are simultaneously recruited by GATA1 to activate gene expression. These data provide insight into the mechanisms through which multiprotein transcriptional complexes involving both DNA-binding proteins and coregulators that do not directly contact DNA are built up at gene promoters.
Results
LMO2-LDB1, GATA1, and TAL1/E12 Form Multiprotein Complexes on DNA.
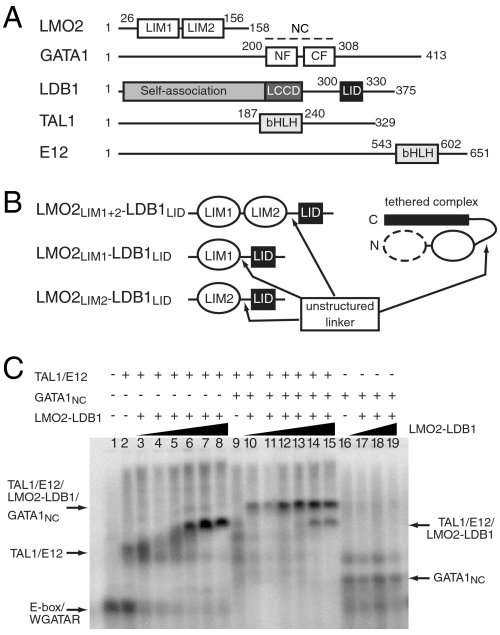
GATA1 contains two GATA-type zinc fingers (Fig. 1A). The C-terminal finger (GATA1CF) binds WGATAR sites, whereas GATA1NF binds FOG1 and can also bind GATC DNA sites and contribute to the binding of GATA1 to double palindromic GATA sites (13, 14). LMO2 comprises two LIM domains, double zinc finger domains that act as protein-interaction motifs (Fig. 1A). Recombinant LMO2 is poorly soluble and tends to aggregate, but is stabilized by the presence of the LIM-interaction domain (LID) of LDB1 (LDB1LID; Fig. 1A). We have therefore engineered a range of stable protein complexes in which either or both LIM domains from LMO2 are tethered to LDB1LID by a flexible linker (LMO2LIM1+2-LDB1LID, LMO2LIM1-LDB1LID, and LMO2LIM2-LDB1LID, Fig. 1B) (11, 15).
Fig. 1.
GATA1 and LMO2-LDB1 form multiprotein complexes on DNA. (A) Schematics of proteins within TAL1/E12/LMO2/LDB1/GATA1 transcriptional complexes. NC comprises the N-terminal (NF) and C-terminal (CF) zinc fingers of GATA1; bHLH, basic helix-loop-helix domain; LCCD, LDB1-CHIP conserved domain; LID, LIM-interaction domain. (B) Topologies of tethered LMO2-LDB1 complexes relevant to this study. (C) EMSA analysis of TAL1bHLH/E12bHLH/LMO2LIM1+2-LDB1LID complex formation on a E-box/WGATAR oligonucleotide in the presence and absence of GATA1NC. TAL1/E12 was at a concentration of 300 nM and GATA1NC was at 500 nM. LMO2LIM1+2-LDB1LID added at concentrations of 16, 32, 50, 100 200, and 400 nM (lanes 3–8 and 10–15), or 0, 50, 100, and 200 nM (lanes 16–19).
To begin to define the GATA1/LMO2 interaction, we carried out GST-pulldown experiments, establishing that a region of GATA1 comprising both zinc fingers (residues 200–308, GATA1NC) was required for the GATA1/LMO2 interaction (Fig. S1). EMSA experiments were then used to demonstrate that LMO2LIM1+2-LDB1LID could simultaneously interact with GATA1 and TAL1/E12. LMO2LIM1+2-LDB1LID was titrated into solutions containing a bipartite E-box/WGATAR oligonucleotide (5) and either a TAL1bHLH/E12bHLH heterodimer alone or TAL1bHLH/E12bHLH together with GATA1NC. The interaction of LMO2 with the DNA-bound proteins is significantly stronger when GATA1NC is also present (Fig. 1C, lanes 3–8; compare lanes 10–15). Therefore a single LMO2LIM1+2-LDB1LID molecule can bridge the two DNA-binding proteins, giving rise to a multiprotein complex with a higher overall stability. It was previously suggested that separate molecules of LMO2 might bind to GATA1 and TAL1/E12 and that the bridge between these complexes on DNA might be provided by the self-association domain of LDB1 (15). The latter domain was absent from our experiments, demonstrating that self-association of LDB1 is not necessary for LMO2 to stabilize the DNA-bound complexes. We also tested the ability of TAL1/E12 to bind to the different LMO2-LDB1LID tethered complexes. EMSA experiments clearly showed that TAL1/E12 efficiently bound the tandem LMO2LIM1+LIM2-LDB1LID construct but could not bind to either of the single LIM-LDB1LID constructs (Fig. S2).
LMO2LIM2 Binds GATA1NF.
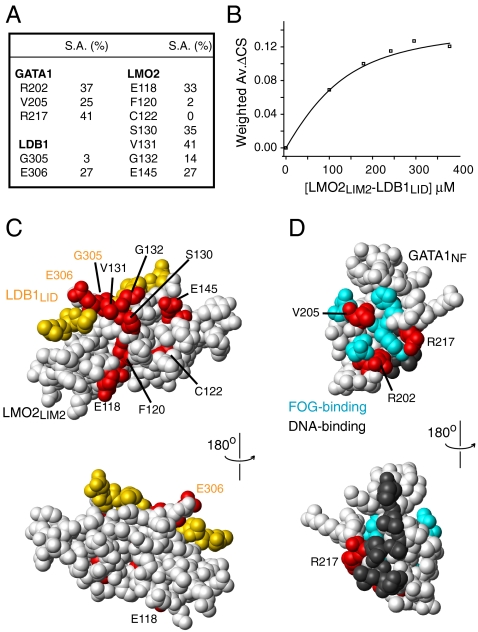
NMR chemical shift perturbation experiments using GATA1 and LMO2-LDB1LID constructs further refined the interacting domains to be GATA1NF and LMO2LIM2 (Fig. S3); the key residues from both proteins are summarized in Fig. 2A. The identification of LMO2LIM2 as the GATA1-binding module is consistent with the published finding that mutation or removal of LMO2LIM2 prevented LMO2 coimmunoprecipitating GATA1 (16). Inspection of the chemical shift changes that occur during GATA1NF/LMO2LIM2-LDB1LID titrations revealed that the interaction is in intermediate to fast exchange on the chemical shift time scale, consistent with a relatively low affinity interaction. We analyzed chemical shift changes for four separate resonances from each protein and derived an association constant for the interaction of (5 ± 2) × 104 M-1 (Fig. 2B).
Fig. 2.
Identification of interface residues in GATA1NF and LMO2LIM2-LDB1LID. (A) Residues from GATA1, LMO2, and LDB1 that showed significant backbone amide chemical shift changes. Also shown is the solvent accessibility for the side chain of each residue as calculated by MolMol (40). (B) Typical binding curve (derived in this case from chemical shift changes for residue R217) following titration of LMO2LIM2-LDB1LID into 15N-GATA1NF. The data are fitted as described previously (41). (C) Space-filling model of the lowest energy structure of the LMO2LIM2-LDB1LID complex (white and yellow, respectively) from PDB ID code 2L3K. Residues that undergo significant (two standard deviations above the mean) chemical shift changes upon addition of GATA1NF are shown in red. LMO2 residues are labeled in black and LDB1 residues in orange. (D) Space-filling model of GATA1NF (white) from PDB ID code 1Y0J with LMO2-interacting residues shown in red, key FOG1-interacting residues shown in cyan and key DNA-binding residues (based on PDB ID code 1GAT) shown in gray.
Residues in either LMO2LIM2-LDB1LID or GATA1NF that showed significant chemical shift changes following addition of the other protein were mapped onto the solution structures of LMO2LIM2-LDB1LID (PDB ID code 2L3K) and GATANF (17). The shifted residues cluster together on both proteins (Fig. 2 C and D). In the case of LMO2LIM2-LDB1LID, a relatively broad surface was identified (Fig. 2C), whereas on GATA1NF there appeared to be some overlap of the LMO2-binding and FOG1-binding interfaces (Fig. 2D). In particular, GATA1V205 appears to be part of both binding surfaces. However, the amide group from V205 is not solvent accessible and no significant side-chain chemical shift changes were observed for this residue upon titration with LMO2LIM2-LDB1LID, making it likely that the observed amide shift was an indirect effect. There was no apparent overlap of the putative DNA-binding and LMO2-binding residues of GATA1NF.
We were unable to detect any intermolecular NOEs for the GATA1NF/LMO2LIM2-LDB1LID complex, probably due to the interaction being in intermediate to fast exchange on the chemical shift time scale. The chemical shift data were instead used to generate a data-driven model of the GATA1NF/LMO2LIM2-LDB1LID complex using the program HADDOCK and the known structures of both GATA1NF and LMO2LIM2-LDB1LID (Fig. 3). Based on a LIGPLOT (18) analysis of the GATA1NF/LMO2LIM2-LDB1LID model, the interface comprises E200, R202, T210, T212, P213, W215, R217, and Y223 from GATA1NF and K127, H128, V131, G132, D133, E145, and Q146 from LMO2LIM2. The interaction appears to be dominated by hydrogen bonding and electrostatic contacts with a smaller contribution from hydrophobic side chains (Figs. S4 and S5). Overall the observed interactions are in good agreement with the backbone chemical shift changes; significantly shifted peaks correspond to residues that are directly involved in the interface, or are proximal to those residues. Only residues from the second zinc-ligating module in LMO2LIM2 directly contact GATA1NF, and no residues from LDB1 are directly implicated in binding, rather LDB1-G305 and LDB1-E306 pack against LMO2-V131, which in turn packs against GATA1-R217, suggesting that the backbone chemical shift changes observed for those LDB1 residues (Fig. 2) were indirect (Fig. 3 A and B). Our model reveals a different binding site for GATA1 on LMO2 than that proposed on the basis of predicted binding hot spots by El Omari et al. (19). Whereas their proposed site centered on the final α-helix in LMO2, our experimental binding data indicate that GATA1 binds the second strand of the first β-hairpin and the loops on either side of the second β-hairpin of the final zinc-binding module of LMO2.
Fig. 3.
Data-driven model of the LMO2LIM2-LDB1LID/GATA1NF complex. (A) Ribbon and (B) surface diagrams showing LDB1LID in yellow with orange labels, LMO2LIM2 in blue, and GATA1NF in red. See also Fig. S5.
LMO2 Competes with DNA for Binding to the GATA1NF.
Although it is GATA1CF that is responsible for binding to WGATAR sites, GATA1NF can contribute to binding of double GATA sites (13) and shows a preference for binding to GATC motifs. Using the coordinates from structures of DNA bound to GATA1CF or GATA3CF (20, 21), we superimposed a double-stranded DNA oligonucleotide onto our model of the GATA1NF/LMO2LIM2-LDB1LID complex, revealing significant clashes between the DNA and the LDB1LID that should disfavor the formation of a LMO2-GATA1NF-DNA complex (Fig. 4A). To test this prediction, we carried out an EMSA using GST-GATA1NF and an oligonucleotide containing a GATC site. A shifted band was observed as expected (Fig. 4B, lane 2), but the addition of LMO2LIM1+2-LDB1LID reduced the intensity of the shifted band substantially, whereas the addition of LMO4LIM1+2-LDB1LID (which does not interact with GATA1NF or TAL1/E12 complexes; Fig. S6) did not, indicating that both LMO2 and DNA cannot bind simultaneously to GST-GATA1NF.
Fig. 4.
LMO2 competes with DNA for binding to GATA1NF. (A) Surface representation of the model of the LMO2LIM2-LDB1LID/GATA1NF complex superimposed with the GATA3CF/DNA structure (PDB ID code 3DFV). Only the DNA (gray) from the GATA3CF/DNA complex is shown. The structures were aligned using the backbone atoms of the GATA fingers. LMO2LIM2 is blue, LDB1LID is yellow, and GATA1NF is red. (B) EMSA showing the effect of LMO2LIM1+2-LDB1LID and LMO4LIM1+2-LDB1LID (concentrations as indicated) on GST-GATA1NF (3 μM) binding to a GATC-containing oligonucleotide. Note that for the LMO4LIM1+2-LDB1LID experiment the lane containing the GATC oligonucleotide is from the same gel with intervening lanes removed for clarity.
GATA1 Simultaneously Binds LMO2 and dFOGF1.
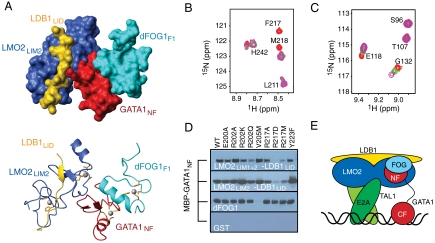
Comparison of the GATA1NF/LMO2LIM2-LDB1LID model with our previous structure of GATA1NF bound to the first zinc finger from Drosophila melanogaster FOG1 (dFOGF1) (8) indicated that LMO2 binds GATA1NF on a surface that is distinct from the dFOGF1-binding surface of the latter protein. A HADDOCK-derived model of the LMO2LIM2-LDB1LID/GATA1NF/FOGF1 complex, calculated using the results obtained from the current study together with the structure of the GATA1NF/dFOGF1 complex, suggested that LMO2 and FOG could simultaneously bind GATA1NF (Fig. 5A).
Fig. 5.
FOG1 and LMO2-LDB1 simultaneously bind GATA1NF in vitro. (A) Surface and ribbon diagrams of a data-driven model of LMO2LIM2-LDB1LID/GATA1NF/dFOGF1 complex. LMO2LIM2 is in blue, LDB1LID in gold, GATA1NF in red, and dFOGF1 in cyan. Zn-coordinating residues are orange sticks and Zn(II) ions are gray spheres. (B) A portion of the 15N-HSQC spectrum of 15N-dFOGF1 alone (red), following addition of GATA1NF (green) and after further addition of LMO2LIM2-LDB1LID (purple). The initial concentration of 15N-dFOG1 was 220 μM. (C) A portion of the 15N-HSQC spectrum of 15N-LMO2LIM2-LDB1LID alone (red), following addition of 1 M equivalent of GATA1NF (green) and following the further addition of 1 M equivalent of dFOGF1 (purple). (D) GST-pulldown assays in which MBP-GATA1NF was pulled down by resin-bound GST-LMO2LIM1+2-LDB1LID (Top), GST-LMO2LIM2-LDB1LID (Second Top), GST-dFOGF1 (Second Bottom) or GST (Bottom). Binding was detected by Western blot using anti-MBP antibodies. (E) Schematic of a LMO2 (blue)/LDB1 (yellow)/TAL1(dark green)/E2A (light green)/GATA1 (red)/FOG1 (light blue) complex bound to DNA (black).
Chemical shift perturbation experiments were used to verify this model. The addition of 1.5 M equivalents of dFOGF1 to a 15N-GATA1NF/LMO2LIM2-LDB1LID complex gave rise to chemical shift changes in the 15N-HSQC spectrum of 15N-GATA1NF for residues that we previously demonstrated to be important for dFOGF1 binding (Fig. S7A) (8). 15N-GATA1NF resonances that had shifted upon binding to LMO2LIM2-LDB1LID in the first step of the experiment remained shifted, consistent with the formation of a three-way GATA1NF-dFOGF1-LMO2LIM2-LDB1LID complex. Similarly, addition of GATA1NF to 15N-dFOGF1 resulted in essentially the same chemical shift changes as observed previously (22). The addition of a twofold molar excess of LMO2LIM2-LDB1LID to this solution did not result in further changes to the 15N-HSQC spectrum of 15N-dFOGF1 (Fig. 5B). Similarly, the addition of LMO2LIM2-LDB1LID to 15N-dFOGF1 in the absence of GATA1NF did not induce any changes in the 15N-HSQC spectrum of dFOGF1 (Fig. S7B). These data confirm that LMO2LIM2-LDB1LID does not perturb the interaction between GATA1NF and dFOGF1 and that LMO2LIM2-LDB1LID does not directly contact dFOGF1. Finally, the addition of up to 1.5-fold molar equivalents of unlabeled dFOGF1 to a GATA1NF/15N-LMO2LIM2-LDB1LID sample resulted in further shifts of the same peaks in the same direction as observed for the titration of GATA1NF into15N-LMO2LIM2-LDB1LID (Fig. 5C), but no additional peaks were seen to shift. These data indicate that dFOGF1 does not perturb the interaction between GATA1NF and LMO2LIM2-LDB1LID and that formation of a ternary complex increases the affinity of the LMO2/GATA1 complex.
To corroborate these data-driven models, we constructed a series of GATA1NF point mutants, pinpointing residues that contact either LMO2LIM2-LDB1LID or dFOGF1. These mutants were tested for their ability to bind LMO2LIM2-LDB1LID and dFOGF1 in GST-pulldown experiments (Fig. 5D and Fig. S8A). GATA1V205M, a disease-causing mutation that was previously shown to abrogate the GATA1/FOG1 interaction (8, 23), bound LMO2-LDB1LID constructs, but not dFOGF1. Conversely, a charge-reversing GATA1R217D mutant could not bind LMO2LIM1+2-LDB1LID, but bound dFOGF1 normally, consistent with the position of R217 at the GATA1NF/LMO2 interface. Mutations of R202 to alanine or lysine did not have significant effects on LMO2 binding, although an R202Q mutant did show a decrease in binding to LMO2LIM1+2-LDB1LID. These results were confirmed by 15N-HSQC titrations in which either GATA1V205M or GATA1R217D was added to 15N-LMO2LIM2-LDB1LID. The addition of GATA1V205M to 15N-LMO2LIM2-LDB1LID caused peak shifts similar to those induced by GATA1NF, whereas GATA1R217D did not cause any significant changes to the spectrum (Fig. S8 C and D).
Discussion
The GATA1NF Is a Focal Point for GATA1 Activity.
Our data provide structural insight into the assembly of higher-order transcriptional complexes at hematopoietic gene promoters, demonstrating that a single molecule of LMO2 nucleates formation of a complex involving TAL1/E2A, LDB1, and GATA1 and that LMO2 and FOG1 can simultaneously bind to GATA1. Surprisingly, our data revealed that it is the N-terminal zinc finger of GATA1 that mediates the interaction between GATA1 and LMO2, demonstrating that this approximately 40-residue domain is responsible for contacting both LMO2 and FOG1. GATA1NF can also participate in DNA binding (14), and the DNA-recognition surface overlaps partially with the LMO2-binding surface. This overlap sets up a situation in which DNA binding by GATA1NF and LMO2 binding are mutually exclusive and indicates that LMO2/LDB1 would not be recruited to GATC sites. Genome-wide occupancy studies reveal that the binding of GATA1 to canonical WGATAR sites is far more prevalent than binding at other motifs, indicating that the recruitment of GATA1 to loci that contain only a GATC site is most likely a relatively rare event (1, 2). However, the GATA1NF also modulates binding to double GATA sites (24), which are highly represented in GATA-occupied sites of up-regulated genes (1, 6). The type of modulation depends on the DNA sequence; the NF can modestly increase binding to a subset of double GATA sites, but has little or even a negative effect on other double GATA sites (24, 25). Members of the TAL1/E2A/LMO2/LDB1 complex are also highly represented in GATA1-occupied sites of up-regulated genes, suggesting that LMO2-LDB1 must be recruited to double GATA sites. Thus, competition of TAL1/E2A/LMO2/LDB1 with DNA for binding to the GATA1NF likely provides an extra level of regulation for the activity of GATA1.
None of the reported disease-causing mutations in GATA1NF (reviewed in ref. 26) lie on the LMO2-binding face. Instead they lie on the DNA-binding face (R216Q, R216W), in or proximal to the FOG1-binding face (V205M, G208S, G208S), or on a part of GATA1NF that has no known role (D218G, D218Y) (8). It is possible that D218 lies on the binding face for a further partner of GATA1. A peptide aptamer that inhibits LMO2 in vivo (in both erythropoietic ES cell differentiation assays and a preclinical mouse transplantation assay for LMO2-associated T-cell leukemia) appears to bind the final zinc-binding module of LMO2 (27). The mechanism of action of this peptide aptamer has not been clearly established, but it may act by blocking LMO2/GATA interactions. Thus, the GATA1-binding site on LMO2 may represent a good target for the design of inhibitors to treat LMO2-associated T-cell lymphomas.
LMO2 Acts as a Bridge Between DNA-Binding Proteins.
LMO-family proteins have long been thought to act as molecular bridges that make connections between DNA-bound transcription factors (28, 29). Our data support a model in which a single molecule of LMO2 bridges the DNA-binding proteins GATA1 and TAL1/E12, thereby creating a stable complex on DNA (Fig. 5E). In our model, the DNA contacts would be made by TAL1/E2A heterodimers and GATA1CF. The GATA1NF binds the C-terminal half of the LIM2 domain of LMO2, leaving LIM1 and the N-terminal half of LMO2 available to contact TAL1/E2A. We had observed that both LIM domains were required to bind TAL1/E2A (Fig. S2), consistent with mutagenic data from El Omari et al., which showed that the hinge region between the two domains is important for the LMO2/TAL1 interaction (19).
Genome-wide occupancy studies have shown that TAL1 can be recruited to WGATAR sites in the absence of an E-box site (6), or when the DNA-binding of TAL1 is abrogated through mutation of the basic domain (5, 6). In these situations, it is likely that localization of TAL1 (probably as a heterodimer) relies on its interaction with the LMO2/LDB1/GATA1 complex, although TAL1 might under such circumstances make contacts with noncanonical DNA sites.
The Assembly of Multifocal Transcriptional Complexes Involves Networks of Strong and Weak Interactions.
LDB1 binds LMO2 and LMO2-LDB1 binds TAL1/E12 with relatively high affinities [KA ≈ 108 M-1 (11)], and EMSAs indicate that TAL1/E12 and GATA1 bind their DNA targets with KA values of up to 109 M-1 (25). Thus, the TAL1/E12/LMO2/LDB1 complex forms a relatively stable complex bound to DNA, as does GATA1 with DNA. In contrast, the strength of the GATA1NF-LMO2LIM2 interaction is relatively modest (KA ≈ 104–105 M-1) and in this regard is reminiscent of the GATA1/FOG1 interaction (8, 30); these low affinities are probably responsible for the lack of detection of higher-order complexes in some reciprocal coimmunoprecipitation assays (31). However, these interactions appear to play important roles in the regulation of multiple target genes, and the question arises as to how these weak and strong interactions complement each other in the formation of stable complexes on chromatin. We suggest a possible answer to this question, based on our biophysical data and extant binding and activation data for these proteins.
Genome-wide ChIP analysis of GATA1 occupancy reveals that a large proportion (58%) of GATA1-activated genes display multiple GATA1-occupied DNA segments (2). Data from several studies show that LDB1 can form homooligomers (13, 32), and LDB proteins are thought to facilitate long-range enhancer–promoter interactions, including GATA1-mediated looping at the β-globin promoter (6, 30, 33). The isolated self-association domain of LDB1 forms trimers, suggesting that LDB1 could connect up to three GATA1-bound sites (12). The in vitro association constant determined for the self-association domain of LDB1 is relatively weak [concentration at 50% trimer approximately 10-5 M(12)], supporting a model in which chromatin linkages formed through LDB1 will be favored at sites where LDB1 is preconcentrated (e.g., through binding of TAL1/E2A/LMO2/LDB1 complexes to multiple sites on DNA) rather than a model in which preformed multimeric protein complexes are actively recruited to DNA. Four of the nine individual zinc fingers of FOG proteins bind GATA1 with a similar modest affinity in vitro [KA ≈ 104–105 M-1 (8, 32)], each contributing to the ability of FOG1 to regulate the transcriptional activity of GATA1 (34). Thus a single molecule of FOG1 can potentially interact with multiple GATA1 molecules bound at separate sites. The combination of relatively weak interactions hints at a “coalescence mechanism” for stable chromatin binding, whereby the binding of individual partners might be relatively transient and have little effect, whereas the presence of multiple copies of each partner on separate promoter/enhancer sites could create networks of weak interactions that synergistically effect changes in the expression of target genes through avidity effects.
Methods
Protein Production and Purification.
The construction, expression, and purification of LMO2-LDB1LID, LMO2LIM2-LDB1LID, and dFOGF1 were described previously (11, 22). GATA1NC (P17679, residues 200–318) and GATA1NF (P17679, residues 200–248) were expressed and purified as described (SI Methods).
Interaction Assays.
35S-GATA1 constructs were produced using a Promega TnT (T7) Coupled Reticulocyte Lysate Transcription/Translation kit. Maltose binding protein (MBP)-GATA1 constructs were expressed in Escherichia coli, purified by amylose affinity chromatography, and bound to GST-fusion proteins immobilized on glutathione-sepharose beads, and bound protein was visualized using either a PhosphorImager (Molecular Dynamics) or Western blotting using an anti-MBP monoclonal antibody (Sigma-Aldrich). Folding of GATA1NF mutants showing a reduced interaction with LMO2LIM1+2-LDB1LID was assessed by 1D 1H NMR (Fig. S8B). EMSAs were carried out as described in ref. 10. Refer to SI Methods for full details.
NMR Spectroscopy.
For LMO2-LDB1LID/GATA1 chemical shift perturbation experiments, the concentration of the labeled protein was maintained and up to 5 M equivalents (5×) of unlabeled protein were added to 15N-LMO2-LDB1LID (approximately 0.2 mM), 15N-GATA1NF (0.1 mM) or 15N-GATA1NC (0.2 mM). 1H, 15N HSQC spectra were recorded after each addition. Overall weighted average chemical-shift changes were calculated using the equation Δavg = (((δH)2 + (δN × 0.154)2)/2)1/2 (35). For additional experiments using dFOGFI, either LMO2LIM2-LDB1LID (1×) or GATA1NF (1×) then LMO2LIM2-LDB1LID (up to 2×) were added to 15N-dFOG1FI (0.2 mM); dFOG1FI (1.5×) or GATA1NF (1×) then dFOG1FI (up to 1.5×) were added to 15N-LMO2LIM2-LDB1LID (0.2 mM); and, LMO2LIM2-LDB1LID (1×) or LMO2LIM2-LDB1LID (1×) then dFOG1FI (1.5×) were added to 15N-GATA1NF (0.2 mM).
HADDOCK Modeling.
The LMO2LIM2-LDB1LID structure and the lowest energy GATA1NF/dFOG1FI complex structure (PDB ID code 1Y0J (8)) were used as starting structures for docking calculations using HADDOCK 2.0 (36, 37), which is based on CNS 1.1 (38). The absence of large-scale changes to the NMR spectra of either protein implies no significant conformational rearrangements take place upon formation of the complex, and so the backbones of both proteins were fixed during the calculations. Residues 202 and 217 of GATA1NF and 130–132 and 145 of LMO2LIM2-LDB1LID were designated as active based on surface accessibility (> 30% exposed to solvent) and chemical shift perturbation data (> 2 SD above the mean change). Although LDB1306 met these criteria, no consistent structures could be obtained if this residue was designated as active. Neighboring residues with side-chain surface accessibility > 30% were defined as passive (129, 133, 134, 146, and 148 of LMO2LIM2 and 201, 203, and 216 of GATA1NF). The LDB1LID/LMO2LIM2 interface was constrained based on our structural data (PDB ID code 2L3K). A total of 200 final structures (from 1,000 after rigid body docking) were subjected to cluster analysis and the 10 structures with the best HADDOCK score (39) were used to represent the structures of each complex.
Supplementary Material
Acknowledgments.
The authors thank Gerd Blobel for comments on the manuscript. J.P.M. and J.M.M. are Senior Research Fellows of the Australian National Health and Medical Research Council (NHMRC). This work was supported by grants from the NHMRC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The LMO2LIM2-LDB1LID/GATA1NF and LMO2LIM2-LDB1LID/GATA1NF/dFOG1 complexes have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2L6Y and 2L6Z, respectively)
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105898108/-/DCSupplemental.
References
- 1.Yu M, et al. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol Cell. 2009;36:682–695. doi: 10.1016/j.molcel.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng Y, et al. Erythroid GATA1 function revealed by genome-wide analysis of transcription factor occupancy, histone modifications, and mRNA expression. Genome Res. 2009;19:2172–2184. doi: 10.1101/gr.098921.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang AP, et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90:109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara T, et al. Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol Cell. 2009;36:667–681. doi: 10.1016/j.molcel.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripic T, et al. SCL and associated proteins distinguish active from repressive GATA transcription factor complexes. Blood. 2009;113:2191–2201. doi: 10.1182/blood-2008-07-169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soler E, et al. The genome-wide dynamics of the binding of Ldb1 complexes during erythroid differentiation. Genes Dev. 2010;24:277–289. doi: 10.1101/gad.551810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, et al. Single-stranded DNA-binding proteins regulate the abundance of LIM domain and LIM domain-binding proteins. Genes Dev. 2007;21:942–955. doi: 10.1101/gad.1528507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liew CK, et al. Zinc fingers as protein recognition motifs: Structural basis for the GATA-1/friend of GATA interaction. Proc Natl Acad Sci USA. 2005;102:583–588. doi: 10.1073/pnas.0407511102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deane JE, et al. Tandem LIM domains provide synergistic binding in the LMO4∶Ldb1 complex. EMBO J. 2004;23:3589–3598. doi: 10.1038/sj.emboj.7600376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan DP, Duncan JL, Lee C, Kuchel PW, Matthews JM. Assembly of the oncogenic DNA-binding complex LMO2-Ldb1-TAL1-E12. Proteins. 2008;70:1461–1474. doi: 10.1002/prot.21638. [DOI] [PubMed] [Google Scholar]
- 11.Ryan DP, et al. Identification of the key LMO2-binding determinants on Ldb1. J Mol Biol. 2006;359:66–75. doi: 10.1016/j.jmb.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 12.Cross AJ, Jeffries CM, Trewhella J, Matthews JM. LIM domain binding proteins 1 and 2 have different oligomeric states. J Mol Biol. 2010;399:133–144. doi: 10.1016/j.jmb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Trainor CD, et al. A palindromic regulatory site within vertebrate GATA-1 promoters requires both zinc fingers of the GATA-1 DNA-binding domain for high-affinity interaction. Mol Cell Biol. 1996;16:2238–2247. doi: 10.1128/mcb.16.5.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton A, Mackay J, Crossley M. The N-terminal zinc finger of the erythroid transcription factor GATA-1 binds GATC motifs in DNA. J Biol Chem. 2001;276:35794–35801. doi: 10.1074/jbc.M106256200. [DOI] [PubMed] [Google Scholar]
- 15.Jurata LW, Gill GN. Structure and function of LIM domains. Curr Top Microbiol Immunol. 1998;228:75–113. doi: 10.1007/978-3-642-80481-6_4. [DOI] [PubMed] [Google Scholar]
- 16.Terano T, Zhong Y, Toyokuni S, Hiai H, Yamada Y. Transcriptional control of fetal liver hematopoiesis: Dominant negative effect of the overexpression of the LIM domain mutants of LMO2. Exp Hematol. 2005;33:641–651. doi: 10.1016/j.exphem.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski K, Czolij R, King GF, Crossley M, Mackay JP. The solution structure of the N-terminal zinc finger of GATA-1 reveals a specific binding face for the transcriptional co-factor FOG. J Biomol NMR. 1999;13:249–262. doi: 10.1023/a:1008309602929. [DOI] [PubMed] [Google Scholar]
- 18.Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- 19.El Omari K, et al. Structure of the leukemia oncogene LMO2: Implications for the assembly of a hematopoietic transcription factor complex. Blood. 2010;117:2146–2156. doi: 10.1182/blood-2010-07-293357. [DOI] [PubMed] [Google Scholar]
- 20.Omichinski JG, et al. NMR structure of a specific DNA complex of Zn-containing DNA binding domain of GATA-1. Science. 1993;261:438–446. doi: 10.1126/science.8332909. [DOI] [PubMed] [Google Scholar]
- 21.Bates DL, Chen Y, Kim G, Guo L, Chen L. Crystal structures of multiple GATA zinc fingers bound to DNA reveal new insights into DNA recognition and self-association by GATA. J Mol Biol. 2008;381:1292–1306. doi: 10.1016/j.jmb.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liew CK, et al. Solution structures of two CCHC zinc fingers from the FOG family protein U-shaped that mediate protein-protein interactions. Structure. 2000;8:1157–1166. doi: 10.1016/s0969-2126(00)00527-x. [DOI] [PubMed] [Google Scholar]
- 23.Nichols KE, et al. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat Genet. 2000;24:266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trainor CD, Ghirlando R, Simpson MA. GATA zinc finger interactions modulate DNA binding and transactivation. J Biol Chem. 2000;275:28157–28166. doi: 10.1074/jbc.M000020200. [DOI] [PubMed] [Google Scholar]
- 25.Omichinski JG, et al. A small single-“finger” peptide from the erythroid transcription factor GATA-1 binds specifically to DNA as a zinc or iron complex. Proc Natl Acad Sci USA. 1993;90:1676–1680. doi: 10.1073/pnas.90.5.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciovacco WA, Raskind WH, Kacena MA. Human phenotypes associated with GATA-1 mutations. Gene. 2008;427:1–6. doi: 10.1016/j.gene.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appert A, et al. Targeting LMO2 with a peptide aptamer establishes a necessary function in overt T-cell neoplasia. Cancer Res. 2009;69:4784–4790. doi: 10.1158/0008-5472.CAN-08-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visvader JE, Mao XH, Fujiwara Y, Hahm K, Orkin SH. The LIM-domain binding protein Ldb1 and its partner LMO2 act as negative regulators of erythroid differentiation. Proc Natl Acad Sci USA. 1997;94:13707–13712. doi: 10.1073/pnas.94.25.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadman IA, et al. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 1997;16:3145–3157. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song SH, Hou C, Dean A. A positive role for NLI/Ldb1 in long-range beta-globin locus control region function. Mol Cell. 2007;28:810–822. doi: 10.1016/j.molcel.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez P, et al. GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J. 2005;24:2354–2366. doi: 10.1038/sj.emboj.7600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowalski K, et al. Characterization of the conserved interaction between GATA and FOG family proteins. J Biol Chem. 2002;277:35720–35729. doi: 10.1074/jbc.M204663200. [DOI] [PubMed] [Google Scholar]
- 33.Morcillo P, Rosen C, Baylies MK, Dorsett D. Chip, a widely expressed chromosomal protein required for segmentation and activity of a remote wing margin enhancer in Drosophila. Genes Dev. 1997;11:2729–2740. doi: 10.1101/gad.11.20.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox AH, et al. Transcriptional cofactors of the FOG family interact with GATA proteins by means of multiple zinc fingers. EMBO J. 1999;18:2812–2822. doi: 10.1093/emboj/18.10.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayed A, et al. Latent and active p53 are identical in conformation. Nat Struct Biol. 2001;8:756–760. doi: 10.1038/nsb0901-756. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez C, Boelens R, Bonvin AM. HADDOCK: A protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125:1731–1737. doi: 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- 37.van Dijk M, van Dijk ADJ, Hsu V, Boelens R, Bonvin AMJJ. Information-driven protein-DNA docking using HADDOCK: It is a matter of flexibility. Nucleic Acids Res. 2006;34:3317–3325. doi: 10.1093/nar/gkl412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 39.de Vries SJ, et al. HADDOCK versus HADDOCK: New features and performance of HADDOCK2.0 on the CAPRI targets. Proteins. 2007;69:726–733. doi: 10.1002/prot.21723. [DOI] [PubMed] [Google Scholar]
- 40.Koradi R, Billeter M, Wuthrich K. MOLMOL: A program for display and analysis of macromolecular structures. J Mol Graph. 1996;14:51–55. 29–32. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 41.Farmer BT, 2nd, et al. Localizing the NADP+ binding site on the MurB enzyme by NMR. Nat Struct Biol. 1996;3:995–997. doi: 10.1038/nsb1296-995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.