Abstract
The US3 protein kinase of herpes simplex virus 1 plays a key role in blocking apoptosis induced by viral gene products or exogenous agents. The US3 protein kinase is similar to protein kinase A with respect to substrate range and specificity. We report that in the yeast two-hybrid system a domain of US3 essential for antiapoptotic activity reacted with programmed cell death protein 4 (PDCD4). We report that US3 interacts with PDCD4, that PDCD4 is posttranslationally modified in infected cells both in a US3-dependent and -independent fashion, and that depletion of PDCD4 by siRNA blocked apoptosis induced by a Δα4 mutant virus. In infected cells, PDCD4 accumulates in the nucleus, whereas US3 accumulates in the cytoplasm. Studies designed to elucidate the convergence of these proteins led to the discovery that US3 protein kinase cycles between the nucleus and cytoplasm and that US3 retains PDCD4 in infected cell nuclei.
Keywords: innate immunity, nuclear cytoplasmic shuttling
One of the most important host cell defenses against infection with herpes simplex virus 1 (HSV-1) is programmed cell death—a vain attempt of self-sacrifice to prevent the spread of infection. The significance of this host defense is apparent from the number of viral gene products whose function is, at least in part, directed to block apoptosis. These viral proteins include glycoprotein D, glycoprotein J, and US3 protein kinase (US3 PK) (1–7). These gene products block apoptosis induced by Fas ligand, osmotic shock induced by sorbitol, virus-dependent induction of massive lysosomal discharge, overexpression of proapoptotic members of the Bcl-2 family of proteins, overexpression of caspase 3, or by the synthesis of proapoptotic viral proteins as, for example, in cells infected with the mutant d120 lacking the α4 gene encoding ICP4, the major regulatory protein of the virus (1–8). This mutant is defective in postα (immediate early) gene expression and is a strong inducer of apoptosis (1). This report concerns the US3 PK and a potential antiapoptotic target of the enzyme.
The gene encoding the US3 PK contains two nested transcriptional units. The largest encodes the US3 PK containing residues 1–481. The second transcriptional unit yields an mRNA that encodes a protein designated US3.5 containing residues 77–481 of the US3 PK. In tests involving a limited repertoire of apoptosis-inducing factors, US3 PK blocked apoptosis, whereas the US3.5 PK did not even though both proteins are enzymatically active (9). The substrate range and specificity of the US3 and US3.5 PKs are similar to those of protein kinase A (10). The US3 PK is active as a dimer; a heterodimer consisting of polypeptides 1–181 and 164–481 actively blocks apoptosis, whereas the individual polypeptides do not (11). The antiapoptotic activity requires two domains of US3 PK: the enzymatically active site near the C terminus and a domain near the N terminus that presumably acts as a ligand to the substrates that regulate or cause apoptosis of the infected cell. In an attempt to identify such a ligand, we have used the sequence encoding residues 1–188 as bait in a yeast two-hybrid system. In this report, we present evidence that the N terminus of US3 PK interacts with programmed cell death protein 4 (PDCD4) and that this protein is involved in apoptosis induced by the d120 mutant of HSV-1.
PDCD4 is a 469-residue tumor suppressor protein with binding sites for RNA and several proteins including RNA helicase eIF4A. A key function related to interaction with eIF4A is interference with assembly of the cap-dependent translation initiation complex (12–14). PDCD4 cycles between nucleus and cytoplasm (15). The sequence of PDCD4 predicts numerous phosphorylation sites. Of these sites, phosphorylation of Ser67, and particularly Ser457 by Akt/protein kinase B (Akt/PKB), correlates with nuclear import, destabilization, and decreased inhibition of activator protein-1 (AP-1) mediated transcription (16). Regulation of PDCD4 and its functions depend on numerous factors and are cell type-dependent (14). Although PDCD4 plays a role in apoptosis, the precise mechanism is unknown.
In this report, we show that US3 interacts with PDCD4, that in infected cells the protein is posttranslationally modified in a US3-dependent and -independent manner, and that it attenuates apoptosis induced by a HSV-1 mutant. In an attempt to reconcile the localization of PDCD4 in the nucleus with the primary localization of US3 PK in the cytoplasm, we discovered that US3 PK cycles between the cytoplasm and the nucleus. Moreover, we found that PDCD4 protein tends to localize in the cytoplasm with time after infection with the ΔUS3 mutant virus but not in wild-type virus infected cells.
Results
US3 and US3.5 PKs Interact with PDCD4.
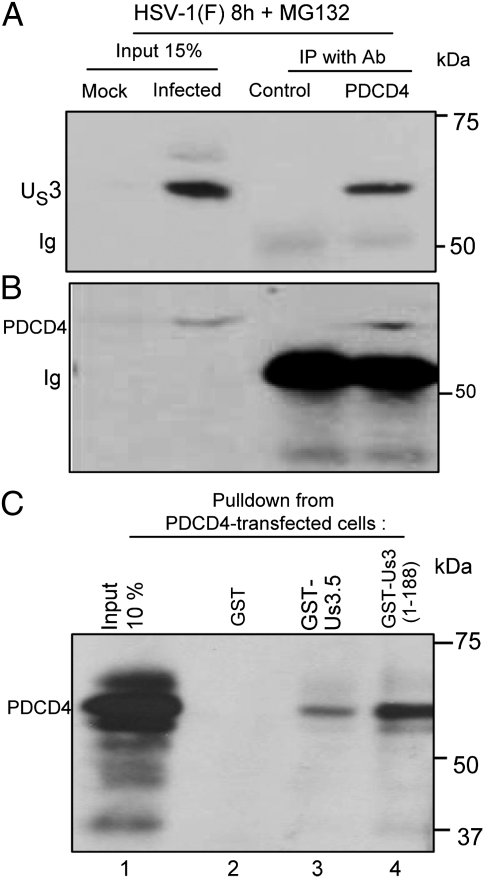
The interaction of US3 PK and PDCD4 was demonstrated in two series of experiments (Fig. 1). In the first, lysates of HeLa cells harvested 8 h after exposure to 10 pfu of HSV-1(F) per cell were reacted with normal rabbit serum or polyclonal rabbit antibody to PDCD4. The immune complexes were collected, solubilized, and electrophoretically separated on a denaturing gel, then transferred to a nitrocellulose sheet and reacted with antibody to US3. As shown in Fig. 1A, lanes 3 and 4, antibody to PDCD4 but not normal rabbit serum pulled down the US3 protein. A duplicate blot was reacted with antibody to PDCD4. As expected, the antibody to PDCD4 reacted in immunoblots with the PDCD4 precipitated by the PDCD4 antibody (Fig. 1B). In the second series of experiments, lysates of HeLa cells harvested 36 h after transfection with PDCD4 were reacted with GST, GST fused to US3.5, or GST fused to residues 1–188 of the US3 PK. After overnight incubation, the GST and the chimeric GST proteins were collected with Glutathione-Sepharose 4B beads, solubilized, electrophoretically separated on denaturing gels, transferred to a nitrocellulose sheet, and reacted with antibody to PDCD4. As shown in Fig. 1C, PDCD4 was pulled down by GST- US3.5 and by GST- US3 (1-188) chimeric proteins but not by GST.
Fig. 1.
US3 interacts with PDCD4. HeLa cells were mock-infected or infected with HSV-1(F). At 3 h after infection, the cells were replenished with medium containing MG132. The proteasomal inhibitor was added to preclude loss of PDCD4 through degradation. The cells were harvested at 8 h after infection and processed as described in Materials and Methods. (A) Aliquots of lysates of infected cells was reacted with normal rabbit serum (lane 3) or anti-PDCD4 rabbit polyclonal serum (lane 4). The immune precipitates were collected, solubilized subjected to electrophoresis in denaturing polyacrylamide gels, and reacted with antibody to US3 PK. (B) The infected cells lysated were reacted with normal rabbit serum (lane 3) or anti-PDCD4 serum (lane 4). In this instance, the electrophoretically separated immune complexes collected and processed as above were reacted with antibody to PDCD4. (C) Lysates of HeLa cells transfected with a plasmid encoding PDCD4 were reacted for 12 h with GST-fused US3.5 protein (residues 77–481, lane 3), the N-terminal domain of US3 (residues 1–188, lane 4), or GST protein (lane 2). The protein complexes were harvested with Glutathione-Sepharose 4B beads, rinsed, solubilized, subjected to electrophoresis in denaturing gels, and reacted with antibody against PDCD4.
We conclude that the US3 PK and, in particular, the polypeptide containing residues 77–188 interacts with PDCD4.
Depletion of PDCD4 with Specific siRNA Attenuated Apoptosis in Cells Infected with d120 Mutant of HSV-1.
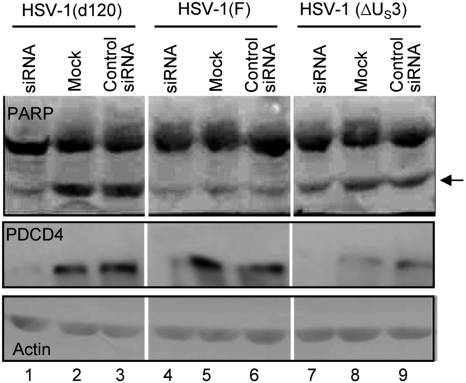
Studies published elsewhere have shown that cells infected with the d120 mutant undergo marked changes in morphology and gene expression resulting in programmed cell death (1, 17). Apoptosis induced by this mutant can be blocked by expression of US3 PK (5). The objective of this series of experiments was to determine the role of PDCD4 in apoptosis induced by the d120 mutant. HEp-2 cells were mock-transfected or transfected with siRNA or control RNA as described in Materials and Methods. At 70 h after transfection, the cells were infected with wild-type virus, ΔUS3 mutant, or the d120 mutant. As noted earlier, the d120 mutant induces apoptosis in virtually all cells tested, whereas US3 is one of several antiapoptotic genes encoded by HSV-1 and, in transfected cells, it blocks apoptosis induced by the d120 mutant virus (1–5). The cells were harvested 12 h after infection and probed with antibodies to PARP, a common target of proapoptotic caspases, and PDCD4, respectively. The results (Fig. 2) were as follows: (i) PDCD4 was not detected in cells transfected with siRNA (lanes 1, 4, and 7). (ii) Significantly lower amounts of cleaved PARP product were detected in cells transfected with siRNA and then infected with d120 mutant than in cells mock-treated or transfected with control RNA before infection (compare lanes 1, 2, and 3). (ii) The ΔUS3 mutant virus did not cause significant levels of programmed cell death, and in cells infected with this mutant, the amounts of cleaved PARP were less abundant than in cells infected with d120 mutant virus (compare lanes 1–3 with corresponding lanes 7–9). (iv) The amounts of PARP cleavage product in cells mock-treated or transfected with control RNA and then infected within wild-type virus were significantly lower than those detected in corresponding cells infected with the d120 mutant (compare lanes 2 and 3 with 5 and 6).
Fig. 2.
Depletion of PDCD4 in cells transfected with siRNA attenuates apoptosis induced by the d120 mutant virus. Replicate cultures of HeLa cells were mock-infected or exposed to 5 pfu of HSV-1(F) or mutant viruses d120 or ΔUS3 per cell, respectively, 70 h after transfection with siRNA specific for PDCD4 or control RNA. The cells were harvested 12 h after infection, solubilized, subjected to electrophoresis in denaturing gels, and reacted with antibody to PARP, PDCD4, or actin. The arrow points to the cleavage product of PARP characteristic of apoptosis.
The results suggest that PDCD4 plays a role in the induction of apoptosis in the d120 mutant infected cell.
PDCD4 Is Posttranslationally Modified in Both US3-Dependent and -Independent Manner.
In this series of experiments, HeLa cells were mock-infected or infected with 10 pfu of HSV-1(F), ΔUS3, or d120 mutant viruses per cell. The cells were harvested at 3, 7, or 10 h after infection, solubilized, subjected to electrophoresis in denaturing gels, transferred to a nitrocellulose membrane, and reacted with antibodies to PDCD4 or to phosporylated Ser67 of PDCD4 [P(S67)-PDCD4]. The results shown in Fig. 3 were as follows: the amounts of PDCD4 decreased at 7 and 10 h after infection concurrently with the appearance of a slower migrating form of the protein (Fig. 3A, lanes 5–12). The slow migrating form was more intense in cells infected with the ΔUS3 mutant or wild-type virus than in cells infected with the d120 mutant (Fig. 3A, compare lanes 5, 7, 9, and 11 with lanes 8 and 12). The appearance of the slow migrating form of PDCD4 noted here was independent of the US3 protein kinase. Its origin or properties are not known. Fig. 3B shows the reactivity of an antibody specific for P(S67)-PDCD4. This antibody reacted with one band (lane 11) in electrophoretically separated lysates of cells harvested 10 h after infection with wild-type virus. The results suggest in infected cells phosphorylation of S67 is mediated by the US3 PK.
Fig. 3.
Accumulation of PDCD4 decreases in amounts and is posttranslationally modified in HSV-1–infected cells. Replicate cultures of HeLa cells were mock-infected or exposed to 10 pfu of HSV-1(F) or mutant viruses d120 or ΔUS3 per cell, respectively. The cells were harvested at 3, 7, or 10 h after infection, lysed in the presence of protease and phosphatase inhibitors, subjected to electrophoresis in denaturing gels, and reacted with antibodies to PDCD4, a rabbit antibody to phosphorylated serine 67 of PDCD4 [P(S67)-PDCD4], or actin. The arrows in A indicate PDCD4 and a slower migrating form of PDCD4 prominent in wild-type or ΔUS3 mutant virus infected cells. In B, the arrow points to a band reactive with the anti-P-Ser67 in cells infected with wild-type virus.
US3 PK Cycles Between Cytoplasm and Nucleus.
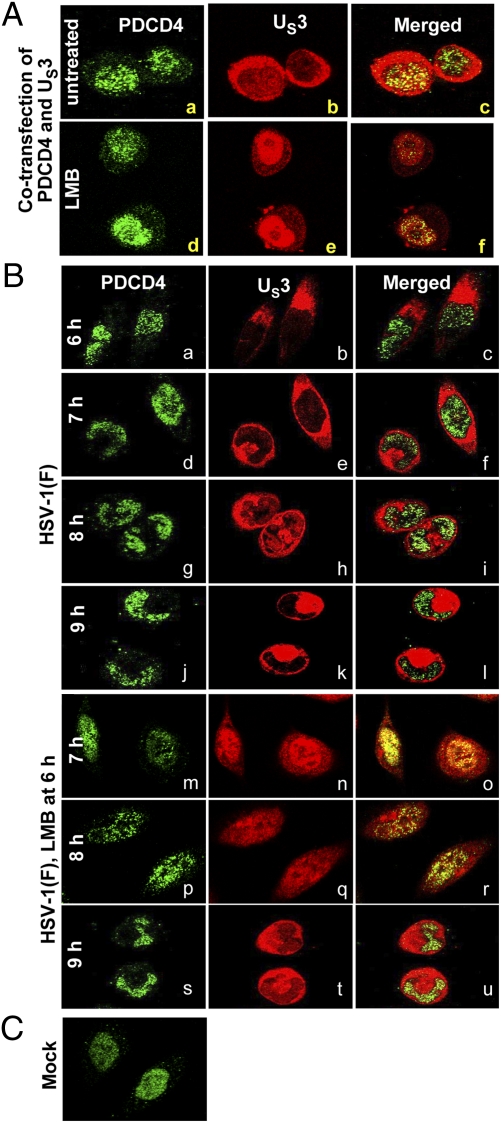
As noted in the introduction, PDCD4 has been reported to cycle between the nucleus and cytoplasm (15). In our studies, PDCD4 invariably accumulated in the nucleus of mock-infected cells (e.g., Fig. 4C) or cells infected with wild-type virus (Fig. 4B, a, d, g, and j), whereas the bulk of US3 was localized in the cytoplasm (Fig. 4B, b, e, h, and k). One explanation for the modification of PDCD4 by US3 PK would be if one of the components cycled between the nucleus and the cytoplasm. To test this hypothesis, we used leptomycin B (LMB) to arrest the export of proteins from the nucleus. LMB is a specific nuclear export inhibitor. It alkylates and inhibits chromosomal region maintenance/exportin 1 (CRM1) required for transport of proteins containing a nuclear export signal (18).
Fig. 4.
US3 shuttles between the nucleus and cytoplasm. (A) HeLa cells grown in 4-well slides were cotransfected with US3 and PDCD4 (0.1 μg of each plasmid per well). At 24 h after transfection, the medium was replaced with fresh medium of medium containing 10 μM LMB for 1 h. The cells were fixed and reacted with mouse monoclonal antibody to PDCD4 (1:200) and rabbit polyclonal antibody to US3 PK (1:1,000). (B) HeLa cells grown in 4-well slides were mock-infected or infected with 10 pfu of HSV-1(F) per cell. At 6 h after infection, the medium in a replicate set of slides was replaced with fresh medium containing 10 μM LMB. The cell cultures were fixed at 6, 7, 8, or 9 h after infection and reacted with antibodies to PDCD4 or US3 as above. For quantification of the distribution of US3 PK, digital images of adjacent fields were collected and the distribution of US3 PK within the infected cells was tabulated. The green fluorescence in A a, d and B a, d, g, j, m, p, and s shows the intracellular localization of PDCD4. The red fluorescence in A b, e, and B b, e, h, k, n, q, and t shows the intracellular localization of US3.
Two series of experiments reported here indicate that in infected cells the US3 cycles between the cytoplasm and the nucleus. In the first, HeLa cells grown on 4-well sides were cotransfected with PDCD4 and US3. At 24 h after transfection, the cells were mock-treated or exposed to 10 μM LMB. After 1 h, the cells were fixed and reacted with antibodies to PDCD4 and US3, respectively. As shown in Fig. 4A, in the presence of LMB, the US3 PK accumulated in the nucleus (compare b and c with e and f). As expected, LMB did no affect the nuclear localization of PDCD4.
To verify that US3 is also entrapped by LMB in the nucleus of infected cells, replicate HeLa cell cultures in 4-well slides were mock-treated or exposed to LMB at 6 h, then fixed and reacted with antibodies at 7, 8, and 9 h after infection. To quantify our observation, we counted 200–300 cells in 10 adjacent fields exhibiting US3 in nucleus. The results (Fig. 4B) were as follows: At 1 h after exposure to LMB, 97% of the cells exhibited US3 exclusively in the nucleus (Fig. 4B, m–o). At 2 h after exposure to LMB, the number of cells exhibiting nuclear US3 was reduced to 47% (Fig. 4B, p–r). The number of cells exhibiting nuclear US3 was reduced to 4% at 3 h after exposure to LMB (Fig. 4B, s–u). In untreated cells (Fig. 4B, d–l), US3 was present in the cytoplasm of nearly all cells.
We conclude from these studies that under conditions tested, PDCD4 is retained in the nucleus, whereas US3 PK cycles between the nucleus and the cytoplasm. On the basis of studies on transfected cells, we conclude that the cycling of US3 PK does not depend on viral gene products.
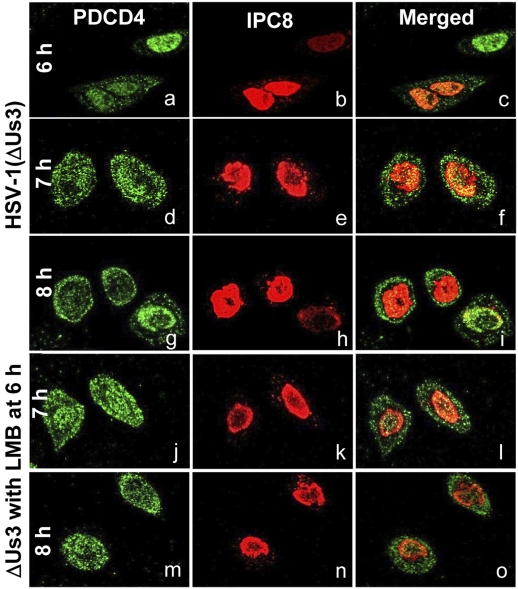
US3 PK Is Required for Retention of PDCD4 in the Nucleus.
In this series of experiments, HeLa cells were exposed to 10 pfu of the ΔUS3 mutant virus per cell. At 6 h after infection, the cells were mock-treated or exposed to 10 μM LMB. The cells were fixed and stained with antibodies to PDCD4 or ICP8 at 6, 7, or 8 h after infection. The significant finding of these studies (Fig. 5) is that PDCD4 was present in both nucleus and cytoplasm. Thus, at 6, 7, and 8 h after infection and mock treatment, the number of cells exhibiting PDCD4 in cytoplasm were 47, 62, and 86%, respectively. In LMB-treated cells, the cells exhibiting PDCD4 in the cytoplasm comprised 65% and 84% of total at 7 and 8 h after infection, corresponding to 1 and 2 h of exposure to LMB. Thus, PDCD4 was exported, increasing amounts with time after infection with the ΔUS3 mutant virus. LMB had no apparent effect on the localization of PDCD4, indicating that this protein did not cycle between the nucleus and cytoplasm in these cells. The results suggest that nuclear retention of PDCD4 at relatively late times after infection depended on the US3 PK.
Fig. 5.
PDCD4 accumulates in the cytoplasm of cells with time after infection with ΔUS3 mutant virus. HeLa cells grown in 4-well slides were exposed to 10 pfu of ΔUS3 mutant virus per cell. At 6 h after infection, the medium in a replicate set of wells was replaced with medium containing 10 μM LMB. The cells were fixed at 6, 7, or 8 h after infection and stained with antibodies to PDCD4 and ICP8. ICP8 localizes in the nucleus and served to demarcate this structure. The green fluorescence in a, d, g, j, and m shows the intracellular localization of PDCD4. The red fluorescence in b, e, h, k, and n shows the intracellular localization of ICP8.
Discussion
The salient points of this report are that (i) the US3 PK physically interacts with PDCD4 a tumor suppressor gene reported to regulate apoptosis. Depletion of PDCD4 by siRNA attenuated the induction of apoptosis by the d120 mutant. (ii) Retention of PDCD4 in the nucleus of infected cells requires the presence of the US3 PK, and (iii) US3 PK cycles between the cytoplasm and nucleus. The significance of the results reported here rest on the following considerations.
Programmed cell death or apoptosis plays an important role in many biologic processes from embryonic development to normal tissue turnover. Malignant transformation of cells frequently is accompanied by a loss of ability to undergo apoptosis. Virus replication disrupts regulatory cells processes and, not surprisingly, cells attempt to undergo apoptosis as a result. As noted in the introduction, an attempt to undergo apoptosis is an important host response to infection and, in response, viruses have evolved functions that block it. The US3 PK is a key HSV-1 gene product involved in blocking apoptosis by exogenous agents (e.g., sorbitol) or noxious viral gene products expressed in the absence of ICP4 (1, 2, 5–8). The components of the US3 PK necessary to block apoptosis are the N-terminal domain that presumably binds to the cellular protein that triggers apoptosis and the catalytic domain that is near the C terminus (9, 11). In the current studies, we used the noncatalytic N-terminal domain as bait and identified one ligand reported to regulate apoptosis. We have shown that both the intact protein and the N-terminal domain of the US3 PK interact with PDCD4. This protein is posttranslationally modified in infected cells by both US3 PK-dependent and -independent manners. It is of particular interest that the US3 PK-dependent modification identified in this study is the phosphorylation of Ser67 discussed in more details below. Finally, we have shown that cleavage of PARP, a common indicator of apoptosis, is reduced in d120 mutant virus-infected cells in which PDCD4 had been depleted by siRNA. The questions that remain unresolved are the nature of other virus-induced modifications of PDCD4, whether they play a role in the accelerated turnover in infected cells, and the mechanism by which PDCD4 induces apoptosis.
The localization of PDCD4 has been reported to vary in a cell type-dependent manner (14). One explanation for this variability is that PDCD4 cycles between the nucleus and cytoplasm (15). As noted in the introduction, PDCD4 is phosphorylated at Ser67 and Ser457 by Akt/PKB, but only phosphorylation of Ser457 correlates with nuclear import of PDCD4 (16). Nuclear localization of S67 as an indication of phosphorylation by Akt/PKB is consistent with the nuclear localization of PDCD4 in wild-type virus-infected cells. Potential discrepancies arise from two considerations. First, phosphorylated Ser67 accumulates relatively late infection. Second, this laboratory published evidence that Akt/PKB is active throughout infection with ΔUS3 mutant virus, but only at early times in cells infected with wild-type virus (19). One hypothesis amenable to future exploration is that US3 protein kinase usurps the function or activates other kinases that act on the same site.
Lastly, the least expected finding is that US3 PK cycles between the cytoplasm and nucleus. US3 PK has been reported to phosphorylate nuclear lamella (20, 21), and given the vast number of its substrates, its presence in the nucleus could be anticipated, although by the time of immunofluorescence, the bulk of the protein localizes in the cytoplasm. The near quantitative translocation of US3 PK to the nucleus in the presence of LMB does not depend on viral gene products. Cycling between nucleus and cytoplasm is generally a property of chaperones. It remains to be seen whether in addition to modifying the function of proteins by phosphorylation US3 PK acts as a companion for nuclear-cytoplasmic transit.
Materials and Methods
Cell Lines, Viruses, and Antibodies.
HEp-2 and HeLa cells were obtained from ATCC. The properties of the wild-type virus HSV-1(F), the ΔUS3 mutant (R7041), and the antibodies against US3 were reported (22, 23). The d120 mutant lacking the gene encoding ICP4 and anti-ICP8 antibodies were kind gifts from N. de Luca (University of Pittsburgh) and D. Knipe (Harvard University), respectively. The anti-PDCD4 mouse monoclonal antibody (used at 1:1,000 dilution), the PDCD4 rabbit polyclonal antibody (used at 1:1,000 dilution), and the rabbit antibody to phosphorylated Ser67 of PDCD4 [P(S67)-PDCD4] (used at 1:600 dilution) were from Abcam (Japan). The anti-poly (ADP ribose) polymerase (PARP) rabbit antibody was from Cell Signaling Technology.
Preparation of Cell Lysates and Immunoblots Blots.
In all experiments, the cells were mock-infected or exposed to 10 pfu of virus per cell in mixture 199 supplemented with 1% calf serum. After 90 min, the inoculum was replaced with DMEM supplement with 5% serum. The cells were harvested at times indicated in Results by scraping, collected by centrifugation, rinsed with PBS containing protease inhibitor mixture and PhosSTOP phosphatase inhibitor (Roche), and dissolved in 200 μL of lysis buffer (20 mM Tris·HCl, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM NaVO4, and 1 μg/mL leupeptin at pH 7.5) in the presence of the two inhibitors and finally disrupted by sonication. Solubilization of protein harvested from infected cells, electrophoresis in denaturing polyacrylamide gels transfer to nitrocellulose serum, pretreatment with 5% BSA, and reaction with appropriate antibodies were done. The protein bands were detected with secondary antibodies conjugated to HRP or alkaline phosphatase, and β-actin served as a loading control.
Immunoprecipitation.
Mock-infected or infected HeLa cells were exposed to 5 μM of inhibitor MG132 at 3 h after infection were harvested as above, lysed in lysis buffer, disrupted by sonication, and clarified by centrifugation at 6,000 rpm in the 3325 rotor of the Haeraeus Biofuge Pico Centrifuge for 10 min. The supernatant fluids were reacted with appropriate antibodies at 4 °C for 12 h. The immune complexes were captured with protein A conjugated to beads. The beads were rinsed five times with the lysis buffer. The bound proteins were denatured in 2× loading buffer at 95 °C for 5 min, separated on SDS-polyacrylamide gels, and analyzed by immunobloting with appropriate primary and secondary antibodies.
GST Pull-Down of Interactive Proteins.
HeLa-transfected PDCD4 protein was reacted at 4 °C for 12 h with 4 mg/mL purified GST-fused US3.5, the N-terminal domain of US3 (residues 1–188) protein, or GST protein in the presence of Glutathione-Sepharose 4B beads. The beads were rinsed extensively with PBS, and the proteins were eluted with 20 mM reduced glutathione. The eluted proteins were subjected to electrophoresis in denaturing 10% polyacrylamide gels, transferred onto a nitrocellulose membrane, and reacted first mouse monoclonal antibody PDCD4 and subsequently with a HRP-conjugated goat anti-mouse IgG.
Depletion of PDCD4 and Analyses of Apoptosis.
The siRNA sequences supplied by Invitrogen were AAGGUGGCUGGAACAUCUAUU and AATAGATGTTCCAGCCACCTT. HEp-2 cells were grown to ≈30% confluence were transfected with 200 pmol of siRNA per 25-cm2 flask by using Lipofectamine 2000 according to manufacturer's protocols. Replicate cultures were infected with 5 pfu of viruses per cell 70 h after transfection and harvested 12 h after infection, processed as described above and probed with antibodies to PDCD4, PARP, or actin. Accumulation of cleaved PARP served as an indicator of apoptosis.
Immunofluorescence Studies.
HeLa cells were seeded on 4-well slides, incubated with 10 pfu of viruses per cell. At 6 h after infection, the replicate well were mock-infected or exposed to 10 μM LMB. The cells were fixed with 4% paraformaldehyde for 30 min at 6, 7, 8, or 9 h after infection, reacted with antibodies to mouse PDCD4 (1:200) and rabbit US3 (1:1,000) or ICP8 (1:2,000) in buffer A (1% BSA, 0.1% Triton X-100, 10% horse serum in PBS solution) for 1 h at 37 °C, then rinsed in buffer A and reacted with antibodies conjugated to Alexa Fluor 594 goat anti-rabbit or Alexa Fluor 488 goat anti-mouse. After incubation for 1 h, the cells were rinsed, mounted, and examined in a Zeiss confocal microscope. All images were taken with the Zeiss microscope.
Transfection of Cells with pcDNA3.1 Plasmids Encoding PDCD4 and US3 PK.
A eukaryotic expression plasmid pcDNA3.1 containing the PDCD4 gene (kindly provided by Hsinsheng Yang, University of Kentucky) or the US3 gene described (5) were cotransfected into HeLa cells (0.1 μg per well for each plasmid) with the aid of Lipofectamine 2000. The cells were either mock-treated or exposed to LMB at 70 h after transfection, then fixed and processed as above.
Acknowledgments
These studies were aided by National Cancer Institute Grant R01 CA088860.
Footnotes
The authors declare no conflict of interest.
References
- 1.Leopardi R, Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou G, Galvan V, Campadelli-Fiume G, Roizman B. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J Virol. 2000;74:11782–11791. doi: 10.1128/jvi.74.24.11782-11791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerome KR, et al. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or Fas. J Immunol. 2001;167:3928–3935. doi: 10.4049/jimmunol.167.7.3928. [DOI] [PubMed] [Google Scholar]
- 5.Munger J, Chee AV, Roizman B. The U(S)3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J Virol. 2001;75:5491–5497. doi: 10.1128/JVI.75.12.5491-5497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benetti L, Munger J, Roizman B. The herpes simplex virus 1 US3 protein kinase blocks caspase-dependent double cleavage and activation of the proapoptotic protein BAD. J Virol. 2003;77:6567–6573. doi: 10.1128/JVI.77.11.6567-6573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benetti L, Roizman B. In transduced cells, the US3 protein kinase of herpes simplex virus 1 precludes activation and induction of apoptosis by transfected procaspase 3. J Virol. 2007;81:10242–10248. doi: 10.1128/JVI.00820-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murata T, et al. Herpes simplex virus type 2 US3 blocks apoptosis induced by sorbitol treatment. Microbes Infect. 2002;4:707–712. doi: 10.1016/s1286-4579(02)01590-3. [DOI] [PubMed] [Google Scholar]
- 9.Poon AP, Benetti L, Roizman B. U(S)3 and U(S)3.5 protein kinases of herpes simplex virus 1 differ with respect to their functions in blocking apoptosis and in virion maturation and egress. J Virol. 2006;80:3752–3764. doi: 10.1128/JVI.80.8.3752-3764.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benetti L, Roizman B. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc Natl Acad Sci USA. 2004;101:9411–9416. doi: 10.1073/pnas.0403160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poon AP, Roizman B. Mapping of key functions of the herpes simplex virus 1 U(S)3 protein kinase: The U(S)3 protein can form functional heteromultimeric structures derived from overlapping truncated polypeptides. J Virol. 2007;81:1980–1989. doi: 10.1128/JVI.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang HS, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki C, et al. PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. Proc Natl Acad Sci USA. 2008;105:3274–3279. doi: 10.1073/pnas.0712235105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lankat-Buttgereit B, Göke R. The tumour suppressor Pdcd4: Recent advances in the elucidation of function and regulation. Biol Cell. 2009;101:309–317. doi: 10.1042/BC20080191. [DOI] [PubMed] [Google Scholar]
- 15.Böhm M, et al. The transformation suppressor protein Pdcd4 shuttles between nucleus and cytoplasm and binds RNA. Oncogene. 2003;22:4905–4910. doi: 10.1038/sj.onc.1206710. [DOI] [PubMed] [Google Scholar]
- 16.Palamarchuk A, et al. Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res. 2005;65:11282–11286. doi: 10.1158/0008-5472.CAN-05-3469. [DOI] [PubMed] [Google Scholar]
- 17.Galvan V, Brandimarti R, Roizman B. Herpes simplex virus 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J Virol. 1999;73:3219–3226. doi: 10.1128/jvi.73.4.3219-3226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudo N, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benetti L, Roizman B. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J Virol. 2006;80:3341–3348. doi: 10.1128/JVI.80.7.3341-3348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryckman BJ, Roller RJ. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J Virol. 2004;78:399–412. doi: 10.1128/JVI.78.1.399-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjerke SL, Roller RJ. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology. 2006;347:261–276. doi: 10.1016/j.virol.2005.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 23.Purves FC, Longnecker RM, Leader DP, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]