Abstract
Approximately 8–20% of breast cancer patients receiving neoadjuvant chemotherapy fail to achieve a measurable response and endure toxic side effects without benefit. Most clinical and imaging measures of response are obtained several weeks after the start of therapy. Here, we report that functional hemodynamic and metabolic information acquired using a noninvasive optical imaging method on the first day after neoadjuvant chemotherapy treatment can discriminate nonresponding from responding patients. Diffuse optical spectroscopic imaging was used to measure absolute concentrations of oxyhemoglobin, deoxyhemoglobin, water, and lipid in tumor and normal breast tissue of 24 tumors in 23 patients with untreated primary breast cancer. Measurements were made before chemotherapy, on day 1 after the first infusion, and frequently during the first week of therapy. Various multidrug, multicycle regimens were used to treat patients. Diffuse optical spectroscopic imaging measurements were compared with final postsurgical pathologic response. A statistically significant increase, or flare, in oxyhemoglobin was observed in partial responding (n = 11) and pathologic complete responding tumors (n = 8) on day 1, whereas nonresponders (n = 5) showed no flare and a subsequent decrease in oxyhemoglobin on day 1. Oxyhemoglobin flare on day 1 was adequate to discriminate nonresponding tumors from responding tumors. Very early measures of chemotherapy response are clinically convenient and offer the potential to alter treatment strategies, resulting in improved patient outcomes.
Keywords: in vivo imaging, near-infrared, diffuse optics, tissue spectroscopy, therapeutic monitoring
An increasing number of patients diagnosed with locally advanced breast cancer undergo preoperative neoadjuvant chemotherapy (NAC) (1). Although disease-free and overall survival is approximately identical compared with postoperative therapy, primary NAC has been shown to downstage tumor grade and reduce tumor volume, leading to more breast-conserving surgeries (2–4). Patients who experience a pathological complete response (pCR) are associated with longer disease-free and overall survival (2, 5, 6). Unfortunately, between 8% and 20% of patients will have no clinical or pathologic response and will not benefit from months of treatment (2, 7). Noninvasive markers to predict response very early during therapy would help physicians make evidence-based changes to treatment strategies, potentially minimizing side effects and maximizing therapeutic outcome.
Current methods for measuring response, including palpation, mammography, ultrasound, and MRI, have shown limited success (8–11). These methods largely rely on anatomic information, which is generally insensitive to early, functional changes caused by chemotherapy. Alternatively, functional imaging technologies, including contrast-enhanced MRI, magnetic resonance spectroscopy, and 18F-fluorodeoxy-glucose PET (FDG-PET), have shown some predictive capability by monitoring tumor metabolism or perfusion during treatment (12–18). For example, reductions in tumor FDG uptake measured after one cycle of chemotherapy have been shown to be predictive of a favorable outcome (19). Practical limitations, however, may prevent these techniques from being widely applied in clinical practice for this use (20). Current functional imaging modalities require exogenous contrast agents that may be poorly tolerated by some patients and are performed at significant expense (20, 21).
Diffuse optical spectroscopic imaging (DOSI) provides quantitative, functional information from breast tumors in a noninvasive manner using multiwavelength near IR light. Tissue concentrations (ct) of oxygenated hemoglobin (ctO2Hb), deoxygenated hemoglobin (ctHHb), water (ctH2O), and lipids as well as tissue oxygen saturation are measured using tomographic imaging instruments (22, 23) and hand-held probes (24–26). Because near IR light is nonionizing, it is possible to use DOSI to monitor physiological changes on a frequent basis without exposing tissue to potentially harmful radiation.
Laboratory and commercial DOSI instruments have been used to characterize both normal and tumor breast tissue (25, 27–29), breast tissue metabolic changes after breast core biopsy (30), and response to neoadjuvant chemotherapy (22, 31–37). Previous neoadjuvant studies have focused on complete pathologic (pCR) response as a primary clinical endpoint and have shown that reductions in ctO2Hb, ctHHb, and ctH2O, apparent as early as the first week of primary therapy and continuing until surgery, are predictive of pathologic response (32, 35). These response metrics seem to be consistent between tumors despite chemotherapy regimen and mechanism of action (38).
We present here clinical evidence that functional measurements made with DOSI observed the first day after administration of preoperative chemotherapy are predictive of therapy response in patients with primary breast cancer. In contrast to previous NAC studies, we focus here on identifying nonresponding (NR) patients within 1 d of their first infusion. Although the clinical endpoint NR has not been studied as extensively as pCR, the ability to identify these patients very early in treatment has unique implications for rapidly informing treatment strategy alterations.
Results
Table S1 indicates subject and tumor characteristics; 8 of 24 tumors achieved pCR, 11 tumors were classified as partial response (PR), and 5 tumors were classified as NR.
Responding Tumors Show a Flare in ctO2Hb on Day 1 of Treatment.
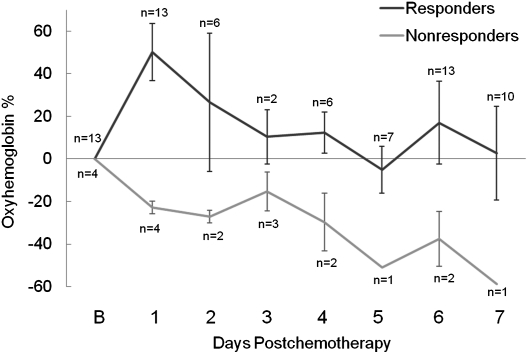
To determine time points at which diagnostically relevant functional changes occur during the first week of treatment, values of oxyhemoglobin, deoxyhemoglobin, water, and lipids were analyzed in subjects who were measured at least 3 d during the first week of treatment. Table S1 indicates the 17 subjects who met this criterion, and it includes four NR, seven PR, and six pCR tumors. In these subjects, the percent change from baseline of ctO2Hb was the only optical metric that discriminated responding subjects from nonresponding subjects, and the maximum difference in the mean oxyhemoglobin values between responders and nonresponders occurred on day 1 after the start of therapy. Fig. 1 shows the observed mean percent change from baseline of ctO2Hb over the first week in responding and nonresponding groups.
Fig. 1.
Percent change in ctO2Hb during the first 7 d of chemotherapy in responding and nonresponding tumors. The number of tumors measured at each day is indicated. The maximum separation between these groups occurred on day 1. Error bars represent SE.
Generalized estimating equations (GEE) models that incorporated therapy response, treatment regimen, measurement day, and interaction terms were used to fit the outcomes of oxyhemoglobin, deoxyhemoglobin, water, and lipid changes from baseline. Tumor and normal tissue measurements were modeled separately over the first 7 d of treatment for all 23 subjects. Sensitivity analyses were performed to assess the effect of outlying data points on the model outcomes (SI Results). Mean values for percent change from baseline and 95% confidence intervals for the means as predicted by the models for oxyhemoglobin in both tumor and normal tissue are shown in Table 1. All other outcomes are shown in Table S2 and Table S3. At day 1, the differences between the predicted means for NR vs. PR (68.0%) and NR vs. pCR (68.9%) in oxyhemoglobin were larger than corresponding differences at other measurement days, and the 95% confidence intervals for the NR group did not overlap those intervals computed for the PR and pCR groups. The strongest statistical significance for any of the outcomes was found for oxyhemoglobin at day 1 for NR vs. PR (nominal P value = 2.0 × 10−12, multiple comparisons corrected P value = 2.7 × 10−11) and NR vs. pCR (nominal P value = 2.5 × 10−6, corrected P value = 3.6 × 10−5).
Table 1.
Mean values and 95% confidence limits for percent change from baseline for 7 d after the start of chemotherapy in oxyhemoglobin in tumor and normal tissue as predicted by a GEE model
| Day | pCR (n = 8) | PR (n = 11) | NR (n = 5) |
| Tumor |
|||
| 1 | 44.25 (17.89, 70.6) | 43.42 (24.12, 62.71) | −24.61 (−34.71, −14.51) |
| 2 | −1.4 (−20.46, 17.67) | 22.76 (−8.43, 53.96) | −30.22 (−42.86, −17.59) |
| 3 | 23.01 (6.63, 39.4) | 14.81 (−14.59, 44.21) | −20.63 (−41.6, 0.34) |
| 4 | −7.52 (−46.74, 31.7) | 24.75 (−13.23, 62.72) | −29.22 (−46.29, −12.15) |
| 5 | −15.68 (−40.26, 8.9) | 16.6 (−17.3, 50.5) | −41.62 (−55.48, −27.77) |
| 6 | −4.05 (−21.81, 13.71) | 26.2 (−19.9, 72.3) | −37.78 (−55.63, −19.94) |
| 7 | −21.55 (−44.96, 1.85) | 1.21 (−40.13, 42.54) | −49.38 (−63.24, −35.52) |
| Normal |
|||
| 1 | 8.5 (−7.85, 24.85) | 26.19 (−2.57, 54.96) | −4.33 (−10.5, 1.83) |
| 2 | −2.43 (−15.09, 10.23) | 3.17 (−11.28, 17.61) | −14.42 (−25.79, −3.05) |
| 3 | 4.57 (−2.03, 11.17) | −9.74 (−24.19, 4.7) | −13.2 (−22.7, −3.7) |
| 4 | −15.05 (−30.15, 0.05) | 19.39 (−12.92, 51.7) | −19.78 (−32.3, −7.26) |
| 5 | −13.62 (−32.85, 5.6) | 7.71 (0.62, 14.8) | −25.33 (−29.2, −21.46) |
| 6 | −3.09 (−17.57, 11.39) | 6.12 (−8.05, 20.29) | −25.01 (−31.05, −18.97) |
| 7 | −16.23 (−36.71, 4.26) | −11.19 (−24.51, 2.14) | −29.78 (−33.65, −25.91) |
Treatment type (cytotoxic, cytotoxic and bevacizumab, or cytotoxic and trastuzumab) did not contribute information to the models of percent change from baseline in tumor oxyhemoglobin (score statistic P value = 0.38), deoxyhemoglobin (P = 0.28), lipids (P = 0.069), or water (P = 0.46). Additionally, histology (invasive ductal carcinoma vs. invasive lobular carcinoma), Scarff–Bloom–Richardson grade, c-erbB2 status, estrogen receptor status, progesterone receptor status, age, and body mass index did not show any significant effects on the models (score statistic 0.071 < P < 0.99 for all) except for age in the outcome of lipids (score statistic P value = 0.039).
In contrast to the tumor tissue, models of normal tissue revealed that the NR group was not simultaneously statistically different from both the PR and pCR groups for any of the measurement days for any outcome. To further explore the paired tumor and normal measurements, the difference between the percent change from baseline for the normal breast and the percent change from baseline for the tumor breast tissue was computed and used as the outcome variable for a GEE model. A near zero estimate for this outcome variable is expected if paired normal and tumor tissue experience the same trends (both in time and magnitude). For oxyhemoglobin, deoxyhemoglobin, water, and lipids, there were multiple measurement days in which the outcome variable was statistically different from zero, which was indicated by the confidence intervals of estimates (Table S4). Despite this result, it is still possible that normal measurements mimicked the temporal behavior of tumor measurements but at a reduced magnitude. For example, for the model outcome of oxyhemoglobin in normal tissue, there was an increase in the PR and pCR groups on day 1 (26.2% and 8.5%, respectively) (Table 1) and a decrease in the NR group (−4.3%), although a pairwise analysis between response groups did not indicate statistically significant differences after correcting for multiple comparisons.
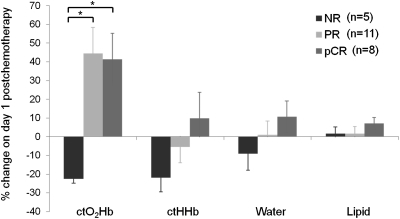
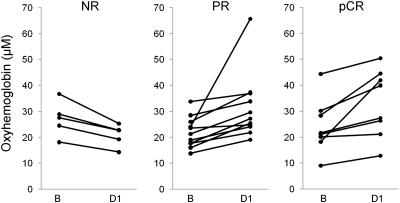
Because the most significant differences between response groups occurred on day 1 after treatment, which was indicated by the GEE analysis, the following results focus on this time point. Fig. 2 shows the observed mean percent change of ctO2Hb, ctHHb, water, and lipids in all 23 study subjects (24 tumors) on day 1. An increase in ctO2Hb was observed in both PR (44.5% ± 46.1% SD) and pCR (41.4% ± 39.1% SD) groups, and a decrease was observed in the NR (−22.5% ± 5.10% SD) group. This trend was mirrored in the contralateral normal measurements, with increases in ctO2Hb in the PR (22.6% ± 43.1% SD) and pCR (12.6% ± 22.0% SD) groups and only small deviations from baseline in the NR (−0.4% ± 9.7% SD) group. Fig. 3 shows absolute molar concentrations of ctO2Hb in tumors at baseline and day 1 for NR, PR, and pCR groups.
Fig. 2.
Percent change in ctO2Hb, ctHHb, water, and lipids on day 1 compared with baseline. Based on longitudinal GEE models, statistically significant differences, noted with asterisks, were found for NR vs. PR (nominal P value = 3.6 × 10−16, multiple comparisons corrected P value = 5.0 × 10−15) and NR vs. pCR (nominal P value = 1.6 × 10−13, corrected P value = 2.2 × 10−12), which were adjusted for differences in tissue type and treatment. Error bars represent SE.
Fig. 3.
Absolute values of tumor ctO2Hb at baseline and day 1 for the different response groups. Baseline and day 1 values in the NR group were 27.2 μM (±6.8 SD) and 20.9 μM (±4.3 SD), respectively, and this represents an average change of −22.5%. Baseline and day 1 values in the PR group were 22.0 μM (±5.9 SD) and 31.5 μM (±12.8 SD), respectively, with an average change of 44.5%. Baseline and day 1 values in the pCR group were 24.1 μM (±10.4 SD) and 33.1 μM (±13.0 SD), respectively, with an average change of 41.4%.
Observed values of tumor ctHHb change were lowest in the NR group (−21.9% ± 17.1% SD) and trended higher in the PR (−5.6% ± 27.5% SD) and pCR groups (9.8% ± 39.3% SD). No trend was observed in ctHHb in contralateral normal tissue, and mean percent change at day 1 was close to zero for all three response groups. Values of water and lipids were not useful to discriminate response groups on day 1 of treatment.
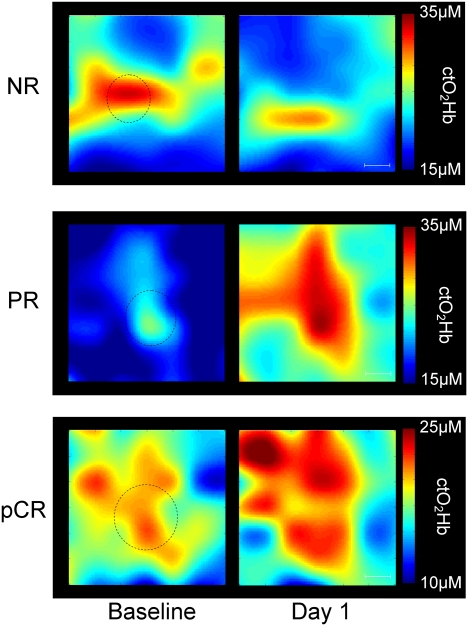
Fig. 4 shows representative ctO2Hb maps at baseline and day 1 for three different study subjects, one NR, one PR, and one pCR. All of the maps show a 6 × 6-cm measurement area that includes tumor and a surrounding normal margin. The approximate tumor location, determined by ultrasound and palpation, is indicated by a dotted circle. In each example, baseline ctO2Hb values in the region corresponding to tumor were elevated over the surrounding normal tissue, an observation that was previously shown (25).
Fig. 4.
ctO2Hb maps from three different subjects at baseline and day 1 after the start of neoadjuvant chemotherapy. Each map shows a 6 × 6-cm measurement area that includes the tumor and a surrounding normal margin. (Scale bar: 1 cm.) The circles represent the location and approximate anatomic size of the tumors determined by ultrasound. (Top) An example of a 17-mm tumor that did not respond to chemotherapy. Mean tumor ctO2Hb dropped 21.6% at day 1, and spatial extent decreased by 54.3%. (Middle) An example of a partial response. Tumor was 20 mm before chemotherapy. Mean tumor ctO2Hb increased 53.1% at day 1, and spatial extent increased by 142.1%. (Bottom) An example of a pathologic complete response. Tumor was 30 mm before chemotherapy. Mean tumor ctO2Hb increased 5.6% at day 1, and spatial extent increased by 4.5%.
ctO2Hb Magnitude and Spatial Extent Discriminate Nonresponders on Day 1 of Therapy.
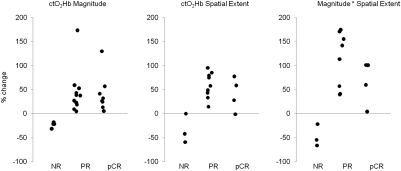
Fig. 5 Left shows a scatter plot of tumor ctO2Hb change from baseline. Perfect separation of NR tumors from both PR and pCR tumors is achieved using the single feature of ctO2Hb change at day 1.
Fig. 5.
Percent change in ctO2Hb magnitude (concentration), spatial extent, and magnitude × spatial extent for NR, PR, and pCR tumors. In all three cases, perfect separation was achieved between nonresponding and responding tumors.
The spatial extent of elevated oxyhemoglobin was calculated for 16 subjects with sufficiently large measurement areas. The area of elevated ctO2Hb expanded in PR (57.4% ± 27.7% SD) and pCR (47.7% ± 33.6% SD) subjects and decreased in NR subjects (−33.4% ± 30.3% SD). The change in ctO2Hb spatial extent is shown in Fig. 5 Middle. When magnitude and spatial extent were combined into a single metric, PR subjects experienced a 139.0% ± 114.3% SD increase, pCR subjects experienced an 88.3% ± 62.5% SD increase, and NR subjects experienced a −47.3% ± 23.1% SD decrease in this metric. This combined metric was able to perfectly discriminate NR from PR and pCR, and this finding is shown in Fig. 5 Right.
Discussion
We have shown that significant functional changes occur after the first day of preoperative chemotherapy and that these changes are correlated with therapy response. The presence of a flare (increase in magnitude and spatial extent above baseline) in oxyhemoglobin values was indicative of either a partial clinical response or a pathologic complete response. These two patient groups represent individuals who are likely to benefit from improved rates of breast-conserving surgery and/or improved overall and disease-free survival (2–4, 6).
Zhou et al. (33), in collaboration with our group, previously published a case study in which early vascular response was observed in a patient receiving neoadjuvant cytotoxic therapy who had achieved a partial overall response. A transient increase followed by a decrease in blood flow and metabolic rate of oxygen consumption measured using diffuse correlation spectroscopy and DOSI was observed on day 3 of therapy (this subject was not measured on day 1 or 2 of therapy). Others have shown similar findings of early functional changes in animal models using FDG-PET. In three separate studies, an early transient increase in tumor FDG uptake was observed during the first week after chemotherapy, independent of tumor growth. This observation was followed by a rapid decrease in FDG uptake and subsequent tumor regression (39–41). The cause of the observed flare was uncertain; it was hypothesized that exposure to chemotherapy transiently induced glucose hypermetabolism in tumors, and cancer cells, inefficiently adapted to the induced metabolic stress, underwent apoptotic cell death through metabolic catastrophe (42, 43).
The biologic origin of the oxyhemoglobin flare in responding subjects observed in this study may stem from one or both of the following factors: (i) a rapid decrease in cellular metabolism caused by cytotoxic chemotherapy-induced cell damage and a subsequent decrease in oxyhemoglobin conversion to deoxyhemoglobin, and/or (ii) increased perfusion to tissue. Previous investigations have shown significant apoptotic activity and decreased proliferation within 24 h after anthracycline therapy (44–46). A drop in deoxyhemoglobin would also be expected to accompany decreased metabolism; however, only a small drop was observed in the PR group, and an increase was observed in the pCR group.
The response may be better explained by perfusion changes caused by an acute inflammatory response induced by cell damage and death. Previous studies have shown that clinical outcome in breast cancer patients is associated with elevations of proinflammatory serum biomarkers after taxane treatment (47–50). Tumor cell exposure to anthracyclines and some platin drugs has been shown to induce immunostimulatory apoptosis and presentation of damage-associated molecular patterns, including calreticulin, heat-shock proteins, and high-mobility group box 1 proteins, before, during, or after apoptosis (51–54). These damage-associated molecular patterns signal the release of proinflammatory cytokines and inflammatory molecules, including IL-1, IL-6, TNFα, nitric oxide, and histamine, which stimulate innate and adaptive immunity and induce vascular changes. The vascular hallmarks of acute inflammation include increased vascular permeability and vessel dilation, which transiently increases perfusion to the injured area over the course of hours to days (55–57). Although DOSI does not directly measure blood flow, the concomitant increase in total hemoglobin observed during oxyhemoglobin flare does suggest an increase in perfusion.
NR patients may not elicit the same immune response because of a higher proportion of chemoresistant or nonimmunostimulatory tumor cells. Additionally, NR patients may have severely limited perfusion and be especially devoid of vascular reactivity because of loss of smooth muscle cell coverage and innervations (58). This loss could also inhibit drug-induced cell/vascular damage and subsequent inflammatory flare.
Measurements from the contralateral normal breasts support the idea of a systemic or tissue-specific vascular/inflammatory response. Although not statistically significant, responding subjects seemed to exhibit an oxyhemoglobin flare in the contralateral normal breast, whereas nonresponding subjects showed almost no change at day 1. The magnitude of the flare in the normal breast was reduced compared with tumor measurements. The magnitude of oxyhemoglobin flare in both tumor and contralateral normal tissue may reflect individual subject vascular and immune responsiveness to cellular stress, which might be closely related to chemosensitivity. It is also of note that, because DOSI measurements represent the average optical properties of the measurement volume, it is possible that surrounding normal tissue may contribute to the observed flare in tumor locations.
This study is limited because of the small number of subjects and lack of additional confirmatory methods. The additional measurements of glucose metabolism, blood flow, and inflammatory markers would help to elucidate the underlying biological basis of flare response. Another limitation of this study is that several different therapy regimens were explored. It is of note, however, that the flare reaction seemed to be indicative of response regardless of age, body mass index, and tumor subtypes, and no differences were seen between treatment regimens. It should be emphasized that this study was retrospective and correlative in nature, and future prospective validations are necessary to confirm the predictive nature of oxyhemoglobin flare.
In conclusion, we observed a significant tumor ctO2Hb flare reaction on the first day after neoadjuvant chemotherapy in responding subjects. This flare was adequate to perfectly discriminate nonresponding subjects from both partial and pathologic complete responders. Furthermore, the majority of responding subjects showed ctO2Hb flare in the contralateral normal breast, whereas nonresponding subjects did not. The implementation of these measurements in the clinic may be used to monitor and strategically alter treatment strategies for breast cancer patients by rapidly identifying likely nonresponders. This implementation could lead to the development of new approaches for managing patients and expediently assessing chemosensitivity for individual subjects.
Materials and Methods
A detailed description of materials and methods is available in SI Materials and Methods.
Briefly, DOSI, which is a near IR optical imaging technique, was used to measure tissue concentrations of oxyhemoglobin, deoxyhemoglobin, water, and lipids in breast cancer patients undergoing neoadjuvant chemotherapy (Fig. S1). Changes in the magnitude and spatial dynamics of these quantities in tumor and normal breast tissue were statistically compared with overall response to chemotherapy in 23 subjects with 24 tumors. The GEE method was used to analyze the correlation between values for oxyhemoglobin, deoxyhemoglobin, water, and lipids measured on different days for individual subjects. Treatment response was stratified into a tertiary classification scheme of pCR, PR, and NR. Subjects with no residual carcinoma after therapy were considered pCR. Subjects with a 50% or greater reduction in tumor size determined from the maximum tumor dimensions were considered PR, and subjects with a less than 50% reduction were considered NR. All subjects provided informed consent and participated in this study under a clinical protocol approved by the Institutional Review Board at the University of California, Irvine (02-2306).
Supplementary Material
Acknowledgments
The authors wish to thank Montana Compton for her assistance, as well as the subjects who generously volunteered their time for this study. This work was supported by National Institutes of Health Grants P41-RR01192 (Laser Microbeam and Medical Program), U54-CA105480 (Network for Translational Research in Optical Imaging), U54-CA136400, R01-CA142989, NCI-2P30CA62203 (University of California, Irvine Cancer Center Support Grant), and NCI-T32CA009054 (University of California, Irvine Institutional Training Grant). Beckman Laser Institute programmatic support from Beckman Foundation and Air Force Research Laboratory Agreement Number FA9550-04-1-0101 is acknowledged.
Footnotes
Conflict of interest statement: B.T. and A.C. report patents, which are owned by the University of California, that are related to the technology and analysis methods described in this study. The diffuse optical spectroscopic imaging instrumentation used in this study was constructed in a university laboratory using federal grant support (National Institutes of Health). The University of California has licensed diffuse optical spectroscopic imaging technology and analysis methods to two companies, FirstScan, Inc. and Volighten, Inc., for different fields of use, including breast cancer (FirstScan, Inc.). This research was completed without participation, knowledge, or financial support of either company, and data were acquired and processed from patients by coauthors unaffiliated with either entity. The Institutional Review Board and Conflict of Interest Office of the University of California, Irvine, have reviewed both patent and corporate disclosures and did not find any concerns.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013103108/-/DCSupplemental.
References
- 1.Kaufmann M, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: New perspectives 2006. Ann Oncol. 2007;18:1927–1934. doi: 10.1093/annonc/mdm201. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi P, et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, et al. Research issues affecting preoperative systemic therapy for operable breast cancer. J Clin Oncol. 2008;26:806–813. doi: 10.1200/JCO.2007.15.2983. [DOI] [PubMed] [Google Scholar]
- 4.Gralow JR, et al. Preoperative therapy in invasive breast cancer: Pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–819. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- 5.Fisher ER, et al. Pathobiology of preoperative chemotherapy: Findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–695. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 6.Symmans WF, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 7.Caudle AS, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:1821–1828. doi: 10.1200/JCO.2009.25.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh E, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 9.Mankoff DA, et al. Monitoring the response of patients with locally advanced breast carcinoma to neoadjuvant chemotherapy using [technetium 99m]-sestamibi scintimammography. Cancer. 1999;85:2410–2423. [PubMed] [Google Scholar]
- 10.Tardivon AA, Ollivier L, El Khoury C, Thibault F. Monitoring therapeutic efficacy in breast carcinomas. Eur Radiol. 2006;16:2549–2558. doi: 10.1007/s00330-006-0317-z. [DOI] [PubMed] [Google Scholar]
- 11.Chen JH, et al. Impact of MRI-evaluated neoadjuvant chemotherapy response on change of surgical recommendation in breast cancer. Ann Surg. 2009;249:448–454. doi: 10.1097/SLA.0b013e31819a6e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith IC, et al. Positron emission tomography using [(18)F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000;18:1676–1688. doi: 10.1200/JCO.2000.18.8.1676. [DOI] [PubMed] [Google Scholar]
- 13.Emmering J, et al. Preoperative [18F] FDG-PET after chemotherapy in locally advanced breast cancer: Prognostic value as compared with histopathology. Ann Oncol. 2008;19:1573–1577. doi: 10.1093/annonc/mdn185. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau C, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–5372. doi: 10.1200/JCO.2006.05.7406. [DOI] [PubMed] [Google Scholar]
- 15.Martoni AA, et al. Early (18)F-2-fluoro-2-deoxy-d-glucose positron emission tomography may identify a subset of patients with estrogen receptor-positive breast cancer who will not respond optimally to preoperative chemotherapy. Cancer. 2010;116:805–813. doi: 10.1002/cncr.24820. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz-Dose J, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–541. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 17.Schelling M, et al. Positron emission tomography using [(18)F]Fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–1695. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- 18.Baek HM, et al. Predicting pathologic response to neoadjuvant chemotherapy in breast cancer by using MR imaging and quantitative 1H MR spectroscopy. Radiology. 2009;251:653–662. doi: 10.1148/radiol.2512080553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berriolo-Riedinger A, et al. [18F]FDG-PET predicts complete pathological response of breast cancer to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2007;34:1915–1924. doi: 10.1007/s00259-007-0459-5. [DOI] [PubMed] [Google Scholar]
- 20.Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess. 2007;11:iii–267. doi: 10.3310/hta11440. [DOI] [PubMed] [Google Scholar]
- 21.Gilbert FJ. Breast cancer screening in high risk women. Cancer Imaging. 2008;8:S6–S9. doi: 10.1102/1470-7330.2008.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe R, et al. Diffuse optical tomography of breast cancer during neoadjuvant chemotherapy: A case study with comparison to MRI. Med Phys. 2005;32:1128–1139. doi: 10.1118/1.1869612. [DOI] [PubMed] [Google Scholar]
- 23.Pogue BW, et al. Quantitative hemoglobin tomography with diffuse near-infrared spectroscopy: Pilot results in the breast. Radiology. 2001;218:261–266. doi: 10.1148/radiology.218.1.r01ja51261. [DOI] [PubMed] [Google Scholar]
- 24.Bevilacqua F, Berger AJ, Cerussi AE, Jakubowski D, Tromberg BJ. Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl Opt. 2000;39:6498–6507. doi: 10.1364/ao.39.006498. [DOI] [PubMed] [Google Scholar]
- 25.Cerussi A, et al. In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy. J Biomed Opt. 2006;11:044005. doi: 10.1117/1.2337546. [DOI] [PubMed] [Google Scholar]
- 26.Tromberg BJ, et al. Imaging in breast cancer: Diffuse optics in breast cancer: Detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy. Breast Cancer Res. 2005;7:279–285. doi: 10.1186/bcr1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah N, et al. Spatial variations in optical and physiological properties of healthy breast tissue. J Biomed Opt. 2004;9:534–540. doi: 10.1117/1.1695560. [DOI] [PubMed] [Google Scholar]
- 28.Durduran T, et al. Bulk optical properties of healthy female breast tissue. Phys Med Biol. 2002;47:2847–2861. doi: 10.1088/0031-9155/47/16/302. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Q, et al. Early-stage invasive breast cancers: Potential role of optical tomography with US localization methods. Radiology. 2010;256:367–378. doi: 10.1148/radiol.10091237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanamai W, et al. Diffuse optical spectroscopy measurements of healing in breast tissue after core biopsy: Case study. J Biomed Opt. 2009;14:014024. doi: 10.1117/1.3028012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakubowski DB, et al. Monitoring neoadjuvant chemotherapy in breast cancer using quantitative diffuse optical spectroscopy: A case study. J Biomed Opt. 2004;9:230–238. doi: 10.1117/1.1629681. [DOI] [PubMed] [Google Scholar]
- 32.Cerussi A, et al. Predicting response to breast cancer neoadjuvant chemotherapy using diffuse optical spectroscopy. Proc Natl Acad Sci USA. 2007;104:4014–4019. doi: 10.1073/pnas.0611058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou C, et al. Diffuse optical monitoring of blood flow and oxygenation in human breast cancer during early stages of neoadjuvant chemotherapy. J Biomed Opt. 2007;12:051903. doi: 10.1117/1.2798595. [DOI] [PubMed] [Google Scholar]
- 34.Cerussi AE, et al. Frequent optical imaging during breast cancer neoadjuvant chemotherapy reveals dynamic tumor physiology in an individual patient. Acad Radiol. 2010;17:1031–1039. doi: 10.1016/j.acra.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soliman H, et al. Functional imaging using diffuse optical spectroscopy of neoadjuvant chemotherapy response in women with locally advanced breast cancer. Clin Cancer Res. 2010;16:2605–2614. doi: 10.1158/1078-0432.CCR-09-1510. [DOI] [PubMed] [Google Scholar]
- 36.Jiang S, et al. Evaluation of breast tumor response to neoadjuvant chemotherapy with tomographic diffuse optical spectroscopy: Case studies of tumor region-of-interest changes. Radiology. 2009;252:551–560. doi: 10.1148/radiol.2522081202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Q, et al. Noninvasive monitoring of breast cancer during neoadjuvant chemotherapy using optical tomography with ultrasound localization. Neoplasia. 2008;10:1028–1040. doi: 10.1593/neo.08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tromberg BJ, Cerussi AE. Imaging breast cancer chemotherapy response with light. Commentary on Soliman et al., p. 2605. Clin Cancer Res. 2010;16:2486–2488. doi: 10.1158/1078-0432.CCR-10-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aliaga A, et al. A small animal positron emission tomography study of the effect of chemotherapy and hormonal therapy on the uptake of 2-deoxy-2-[F-18]fluoro-D-glucose in murine models of breast cancer. Mol Imaging Biol. 2007;9:144–150. doi: 10.1007/s11307-007-0091-6. [DOI] [PubMed] [Google Scholar]
- 40.Aide N, et al. Early evaluation of the effects of chemotherapy with longitudinal FDG small-animal PET in human testicular cancer xenografts: Early flare response does not reflect refractory disease. Eur J Nucl Med Mol Imaging. 2009;36:396–405. doi: 10.1007/s00259-008-0984-x. [DOI] [PubMed] [Google Scholar]
- 41.Bjurberg M, et al. Early changes in 2-deoxy-2-[18F]fluoro-D-glucose metabolism in squamous-cell carcinoma during chemotherapy in vivo and in vitro. Cancer Biother Radiopharm. 2009;24:327–332. doi: 10.1089/cbr.2008.0556. [DOI] [PubMed] [Google Scholar]
- 42.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin S, DiPaola RS, Mathew R, White E. Metabolic catastrophe as a means to cancer cell death. J Cell Sci. 2007;120:379–383. doi: 10.1242/jcs.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Archer CD, et al. Early changes in apoptosis and proliferation following primary chemotherapy for breast cancer. British Journal of Cancer. 2003;89:1035–1041. doi: 10.1038/sj.bjc.6601173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchholz TA, et al. Chemotherapy-induced apoptosis and Bcl-2 levels correlate with breast cancer response to chemotherapy. Cancer J. 2003;9:33–41. doi: 10.1097/00130404-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Buchholz TA, et al. The nuclear transcription factor kappaB/bcl-2 pathway correlates with pathologic complete response to doxorubicin-based neoadjuvant chemotherapy in human breast cancer. Clin Cancer Res. 2005;11:8398–8402. doi: 10.1158/1078-0432.CCR-05-0885. [DOI] [PubMed] [Google Scholar]
- 47.Mills PJ, et al. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain Behav Immun. 2008;22:98–104. doi: 10.1016/j.bbi.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nolen BM, et al. Serum biomarker profiles and response to neoadjuvant chemotherapy for locally advanced breast cancer. Breast Cancer Res. 2008;10:R45. doi: 10.1186/bcr2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pusztai L, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 53.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 54.Apetoh L, Mignot G, Panaretakis T, Kroemer G, Zitvogel L. Immunogenicity of anthracyclines: Moving towards more personalized medicine. Trends Mol Med. 2008;14:141–151. doi: 10.1016/j.molmed.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Ryan GB, Majno G. Acute inflammation. A review. Am J Pathol. 1977;86:183–276. [PMC free article] [PubMed] [Google Scholar]
- 56.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 57.Wilhelm DL. Mechanisms responsible for increased vascular permeability in acute inflammation. Agents Actions. 1973;3:297–306. doi: 10.1007/BF01986484. [DOI] [PubMed] [Google Scholar]
- 58.Teicher BA, Ellis LM, editors. Antiangiogenic Agents in Cancer Therapy Series: Cancer Drug Discovery and Development. 2nd Ed. Totowa, NJ: Humana Press; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.