Abstract
Ocean acidification is predicted to impact all areas of the oceans and affect a diversity of marine organisms. However, the diversity of responses among species prevents clear predictions about the impact of acidification at the ecosystem level. Here, we used shallow water CO2 vents in the Mediterranean Sea as a model system to examine emergent ecosystem responses to ocean acidification in rocky reef communities. We assessed in situ benthic invertebrate communities in three distinct pH zones (ambient, low, and extreme low), which differed in both the mean and variability of seawater pH along a continuous gradient. We found fewer taxa, reduced taxonomic evenness, and lower biomass in the extreme low pH zones. However, the number of individuals did not differ among pH zones, suggesting that there is density compensation through population blooms of small acidification-tolerant taxa. Furthermore, the trophic structure of the invertebrate community shifted to fewer trophic groups and dominance by generalists in extreme low pH, suggesting that there may be a simplification of food webs with ocean acidification. Despite high variation in individual species’ responses, our findings indicate that ocean acidification decreases the diversity, biomass, and trophic complexity of benthic marine communities. These results suggest that a loss of biodiversity and ecosystem function is expected under extreme acidification scenarios.
Keywords: global change, natural gradient, emergent effects
Understanding how accelerating environmental change will affect biodiversity and ecosystem function is crucial for effective management (1). Environmental change can cause a restructuring of ecological communities and a reduction of ecosystem function through the loss of stress-intolerant species (2). Variation in sensitivity to environmental change could potentially buffer the effects of species loss on ecosystems through compensatory dynamics among functionally similar species (3–5). However, compensation is much less likely to stabilize ecosystem function if numerous species have similar responses to the environmental change (6). In such cases, entire functional groups may be affected negatively by environmental stress (7).
Increased anthropogenic CO2 is predicted to be a major driver of environmental change in the coming century in both terrestrial and marine ecosystems (8, 9). Large variation among species has been documented in biological responses to CO2-induced ocean acidification in marine ecosystems (10, 11). However, many organisms that build calcareous structures have shown reduced calcification, growth, and survival (10, 12, 13), suggesting that a wide diversity of species with calcified structures may respond similarly to ocean acidification. To date, most studies of acidification effects have been conducted on a species by species basis. To better understand the potential for ecological shifts and compensation among species (14) to stabilize ecosystem function, we need to examine the responses of multispecies assemblages to ocean acidification.
The ecosystem surrounding near shore volcanic CO2 vents in the Mediterranean Sea is a primary example of a naturally acidified marine ecosystem (15) that is not confounded with other environmental factors such as temperature or upwelling (16, 17). The vents are caused by a subterranean source of CO2 and other trace gases (no sulfur) that are bubbled into a shallow stretch of coastal ocean with sloping rocky reefs (18). Shallow rocky reef communities are exposed to increased acidification with natural temporal fluctuations (15). This variability in carbonate chemistry allows for an examination of the ecosystem effects of decreasing pH conditions, such as those effects predicted at the close of this century (19). Previous studies at these CO2 vents found decreased abundances of conspicuous calcifying taxa in the reduced pH environments (15); however, the effects of the decreased abundance of calcifying taxa on community structure and ecosystem properties were not considered.
Here, we examine changes of a multispecies assemblage of marine invertebrates in the face of varying ocean acidification. Specifically, we ask three questions. (i) Does community composition and structure of mobile invertebrates change with ocean acidification? (ii) Is there evidence for density compensation among taxa? (iii) Does density compensation lead to compensation in aggregate invertebrate biomass and trophic structure? In contrast to previous studies at the vent site (15), we focus on an entire assemblage rather than selected focal taxa. The results of our analyses indicate that there is an absence of numerous taxa in extreme low pH, compensated for by increased abundances of acidification-tolerant taxa (primarily crustaceans). However, we found a corresponding decrease in the aggregate invertebrate biomass and a simplification of the trophic structure in extreme low pH conditions, suggesting that density compensation among tolerant taxa does not fully buffer against changes in ecosystem properties and function.
Results
Carbonate Chemistry Associated with CO2 Vents.
Submarine CO2 vents occur at 0.5–3 m depth at sites on the northern and southern sides of a small island (Castello Aragonese d’Ischia: 40° 43.84 N, 13° 57.08 E) directly adjacent to steep, rocky reefs. Water samples and in situ monitoring of seawater pH confirmed three distinct carbonate chemistry zones associated with vent activity, with reduced mean pH and increased temporal variability associated with vent activity (Fig. 1 and Table 1). Although the variability in pH is similar among low and extreme low pH zones, the variability in concentration of hydrogen ions (and other carbonate parameters) is much higher in the extreme low pH zones because of the logarithmic scale of pH. Reductions in seawater pH were driven by increased dissolved inorganic carbon (DIC) concentrations at relatively constant total alkalinities (TA), temperatures, and salinities across zones and sites, which also caused reductions in aragonite and calcite saturation states (Table 2). Deployment of two sensors at either end of the southern low pH zone suggests that pH values are correlated within pH zones (r213 = 0.72, P < 0.001) (Fig. S1).
Fig. 1.
Representative time series of hourly seawater pHT values for (A) northern and (B) southern sites at Castello Aragonese d’Ischia in the ambient (blue), low (yellow), and extreme low (red) pH zones. Time series for (A) the northern site is from September 13 to October 8, 2010, and time series for (B) the southern site is from May 12 to June 14, 2010.
Table 1.
Seawater pH statistics for pH zones associated with CO2 vents at Ischia, Italy, calculated from hourly measurements taken by in situ pH sensors for separate deployments of the sensors
| pH Zone |
|||
| Season/site | Ambient | Low | Extreme low |
| Winter/south | |||
| Mean | — | 7.71 ± 0.39 | 6.56 ± 0.49 |
| CV | — | 0.05 | 0.08 |
| N | — | 646 | 646 |
| Spring/south | |||
| Mean | 8.07 ± 0.09 | 7.75 ± 0.31 | 6.51 ± 0.42 |
| CV | 0.01 | 0.04 | 0.06 |
| N | 792 | 1,801 | 1,801 |
| Fall/north | |||
| Mean | 7.96 ± 0.06 | 7.47 ± 0.19 | 7.21 ± 0.34 |
| CV | 0.01 | 0.03 | 0.05 |
| N | 602 | 602 | 602 |
| Fall/south | |||
| Mean | 8.04 ± 0.09 | 7.84 ± 0.24 | 6.78 ± 0.67 |
| CV | 0.01 | 0.03 | 0.10 |
| N | 712 | 216 | 712 |
Statistics are mean ± SD. CV, coefficient of variation.
Table 2.
Measured and estimated environmental and geochemical variables for pH zones
| Parameter | Ambient | Low | Extreme low | N |
| North | ||||
| Salinity(‰) | 37.9± 0.3 | 37.8±0.4 | 37.9± 0.4 | 3 |
| TA (μmol/kg) | 2,563± 3 | 2,560± 7 | 2,559± 13 | 3 |
| DIC (μmol/kg) | 1,768± 96 | 2,395± 66 | 2,579± 207 | 3 |
| Temperature (°C) | 23.4± 0.7 | 23.8± 0.7 | 23.4± 0.74 | 604 |
| pH (total) | 8.0± 0.1 | 7.8± 0.2 | 7.2± 0.4 | 604 |
| pCO2 (μatm) | 567 ± 100 | 1,075 ± 943 | 6,558 ± 21,347 | 604 |
| Ωaragonite | 3.13 ± 0.35 | 2.32 ± 0.73 | 0.84 ± 0.54 | 604 |
| Ωcalcite | 4.75 ± 0.53 | 3.52 ± 1.11 | 1.27 ± 0.82 | 604 |
| South | ||||
| Salinity(‰) | 37.6± 0.3 | 37.7± 0.2 | 37.7± 0.2 | 4–10 |
| TA (μmol/kg) | 2,563± 2 | 2,560± 7 | 2,563± 13 | 4–10 |
| DIC (μmol/kg) | 2,281± 73 | 2,318± 99 | 3,849± 790 | 4–10 |
| Temperature (°C) | 19.6± 1.5 | 17.5± 2.8 | 17.5± 2.8 | 1,503–3,162 |
| pH (total) | 8.1± 0.1 | 7.8± 0.3 | 6.6± 0.5 | 1,503–3,162 |
| pCO2 (μatm) | 440 ± 192 | 1,581 ± 2,711 | 23,989 ± 16,638 | 1,503–3,162 |
| Ωaragonite | 3.33 ± 0.44 | 1.95 ± 0.85 | 0.32 ± 0.62 | 1,503–3,162 |
| Ωcalcite | 5.11 ± 0.67 | 3.00 ± 1.31 | 0.49 ± 0.96 | 1,503–3,162 |
Values are means (± SD). Parameters in bold were measured from discrete water samples. Parameters in italics were measured with in situ sensors. The values from in situ sensors represent the means of hourly measurements from multiple sensor deployments (Table 1). Parameters in plain text were calculated by applying the salinity and TA values shown in bold to the hourly temperature and pH measurements. DIC measurements were used to calibrate the initial pH measurements and were not used in the calculations of other parameters.
Continuous monitoring of seawater pH allowed us to quantify the temporal variability in pH to more fully characterize the chemical environments surrounding the benthic communities. In the low pH zones in the north and south sites, 47% and 32% of the hourly pH measurements were below 7.8 (the predicted average global sea surface pH value for the year 2100) (20), respectively, whereas ∼91% and 93% of the measurements were below 7.8 in the extreme low pH zones, respectively. We also quantified the number of extreme events (defined here as a pH value of 0.4 units less than the monthly mean pH). This statistic was developed to reflect physiological extremes rather than statistical extremes. We chose a 0.4-pH reduction to define an extreme event based on numerous studies reporting detrimental biological effects at this level (11), and similar conclusions derive from setting higher or lower cutoffs. The number and average length of these extreme events increased in the low and extreme low pH zones (Table 3).
Table 3.
Differences in extreme events among pH zones
| pH Zone |
|||
| Statistic | Ambient | Low | Extreme low |
| North | |||
| Number of extreme events per week | 0 | 7.5 | 24.8 |
| Mean duration (h) | — | 1.6 | 2.3 |
| Maximum duration (h) | — | 4 | 16 |
| Time series (d) | 25 | 25 | 25 |
| South | |||
| Number of extreme events per week | 0.9 | 6.3 | 12.8 |
| Mean duration (h) | 1.4 | 2.9 | 2.9 |
| Maximum duration (h) | 2 | 15 | 12 |
| Time series (d) | 62 | 69 | 90 |
Time series from separate pH sensor deployments within a pH zone (Table 1) were combined to calculate extreme events.
Invertebrate Communities.
Over 15,000 individual invertebrates representing 82 taxonomic families were collected from the rocky reef benthic communities surrounding CO2 vents. The invertebrates collected included crustaceans (amphipods, decapods, isopods, and tanaids), echinoderms, mollusks (bivalves and gastropods), polychaetes, and sipunculids. Individuals were classified to the lowest taxonomic group feasible (59% were classified to species) (Table S1), hereafter termed the operational taxonomic unit (OTU).
The community composition, defined as the OTUs present/absent, differed significantly between the extreme low and ambient pH communities at both sites, but it differed in the extreme low and low pH communities only at the southern site [Permutational Multivariate Analysis of Variance (PERMANOVA) site × pH: pseudo-f2,23 = 1.88, P = 0.006] (Tables 2 and 3 and Fig. S1). We did not detect a significant difference in the community composition between the low and ambient pH communities at either site, but the results were near significance in both cases (P = 0.057–0.059) (Table 3). Numerous taxa were absent in the extreme low pH zones. After rare taxa were removed from the analysis (defined as OTUs present in less than four samples, which accounted for less than 3% of the total number of individuals in those samples), 6% and 31% of the OTUs found in samples from the ambient pH zones were absent in all samples from the low and extreme low pH zones, respectively. Heavily calcified organisms (primarily gastropods, bivalves, and serpulid polychaetes) accounted for 39% of the OTUs absent in extreme low pH, and decapod crustaceans account for another 22%. All of the common OTUs found in the extreme low pH zone samples were also found in the low or ambient pH zones.
The community structure, defined as the relative abundance of individuals within each OTU present in the sample, also varied significantly between sites and pH zones (PERMANOVA site × pH: pseudo-f2,23 = 1.58, P = 0.03) (Tables 2 and 3 and Fig. S2). The community structure followed the patterns found in the community composition, with significant differences between extreme low and ambient pH communities and near significant differences between low and ambient pH communities (P = 0.054–0.057). Abundances of heavily calcified taxa were similar between samples in the low and ambient pH zones but were greatly reduced in extreme low pH zones (Fig. 2), with the exception of juveniles from the family Mytilidae, which were found in high abundance in two samples in the extreme low pH zones. Conversely, the highest abundances of some taxa (i.e., amphipods, tanaids, and some polychaetes) were in samples from the extreme low pH zones (Fig. 2). Although there was variation in the response of OTUs within taxonomic classes, some generalities were observed (Table 4). Numerous amphipod OTUs increased in abundance (26%) or did not show a strong response to reduced pH (26%), whereas the abundance of numerous gastropod OTUs was unaffected in low pH and greatly reduced in extreme low pH (26%) (Table 4). Furthermore, the abundances of all of the common decapod OTUs were reduced in extreme low pH. The variation in responses of OTUs within other taxonomic classes was more pronounced.
Fig. 2.
Density of individuals within the most abundant taxonomic classes in each pH zone. Densities are means ± SEM (n = 8) for the combined northern and southern sites. Taxonomic classes are organized from the most heavily calcified on the left to the least calcified on the right. *, a significant difference in densities between pH zones (α = 0.05); **, a significant interaction (site × pH; α = 0.05).
Table 4.
Total number and percentage of OTUs within each taxonomic group exhibiting one of four responses to decreasing pH
| Response to reduced pH |
||||||
| Taxa | A (Decrease) | B (Decrease in extreme low only) | C (No trend) | D (Increase) | E (Rare) | Total |
| Gastropod | 1 (0.04) | 7 (0.26) | 1 (0.04) | 0 (0) | 18 (0.67) | 27 |
| Bivalve | 0 (0) | 3 (0.30) | 2 (0.20) | 0 (0) | 5 (0.50) | 10 |
| Decapod | 4 (0.22) | 7 (0.39) | 0 (0) | 0 (0) | 7 (0.39) | 18 |
| Amphipod | 3 (0.10) | 2 (0.07) | 8 (0.26) | 8 (0.26) | 10 (0.32) | 31 |
| Tanaid | 2 (0.29) | 0 (0) | 2 (0.29) | 1 (0.14) | 2 (0.29) | 7 |
| Isopod | 3 (0.38) | 1 (0.13) | 1 (0.13) | 1 (0.13) | 2 (0.25) | 8 |
| Polychaete | 20 (0.47) | 0 (0) | 5 (0.12) | 3 (0.07) | 15 (0.35) | 43 |
| Sipunculid | 3 (0.75) | 0 (0) | 0 (0) | 0 (0) | 1 (0.25) | 4 |
The responses to reduced pH are split into five categories: A, a gradual decrease in abundance from ambient to extreme low pH; B, a sudden decrease in abundance in low or extreme low pH (i.e., a threshold response); C, no trend in the abundance; D, increased abundance from ambient to extreme low pH; E, the taxa were too rare to illustrate a trend. The number of OTUs is indicated, and the proportion is given in parentheses.
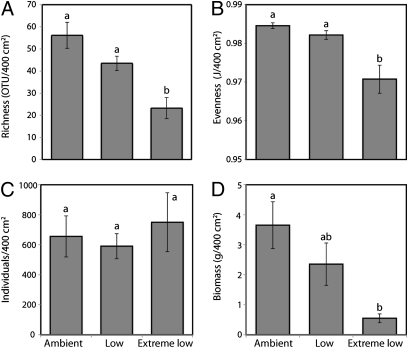
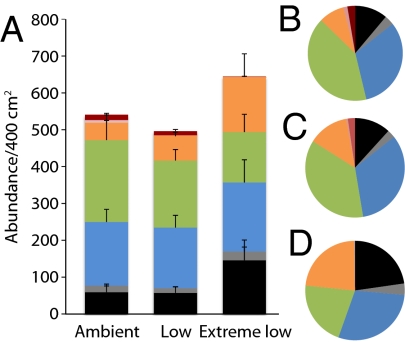
The number of taxa (OTUs) and the evenness of these taxa (using Pielou’s J, which is defined as the ratio of the actual Shannon–Weiner diversity index to the Shannon–Weiner diversity index if all species were equally abundant in the community) were reduced in extreme low pH zones (richness ANOVA: f2,18 = 16.35, P = 0.0001; evenness Kruskal–Wallis test: χ2 = 8.30, df = 2, P = 0.02) (Fig. 3 and Table 4). However, the mean number of individuals did not differ between sites or pH zones (ANOVA: f2,18 = 0.31, P = 0.7) because of density compensation by amphipods, tanaids, and some polychaetes at low and extreme low pH. However, the aggregate invertebrate biomass was significantly reduced in the extreme low pH zones (Kruskal–Wallis test: χ2 = 13.38, df = 2, P = 0.001). The reduction in biomass was primarily caused by the absence of heavily calcified, high biomass taxa (gastropods, bivalves, and serpulid polychaetes) in the extreme low pH zones (Fig. 4). Furthermore, we found significant differences in the trophic structure of the community in ambient and extreme low pH zones (PERMANOVA pH: pseudo-f2,23 = 5.84, P = 0.003) (Table 2). There was a simplification of the trophic structure in extreme low pH, with carnivores and scavengers rare or absent in extreme low pH, a marked decrease in herbivores, and a corresponding increase of detritivores and omnivores (Fig. 5).
Fig. 3.
Invertebrate community indices from ambient, low, and extreme low pH zones for combined northern and southern sites (means ± SEM, n = 8). (A) Taxonomic richness (number of OTUs per 400 cm2), (B) evenness (Pielou’s J), (C) total number of individuals per 400 cm2, and (D) aggregate invertebrate biomass (grams per 400 cm2). Lowercase letters denote differences among pH zones.
Fig. 4.
Contributions of taxonomic groups to (A) abundance and (B) aggregate community biomass (means ± SEM, n = 8). Taxonomic groups include mollusks (black), crustaceans (light gray), and polychaetes (dark gray).
Fig. 5.
Changes in trophic structure among pH zones. Trophic groups: black, detritivores; gray, facultative detritivore/suspension feeders; blue, suspension feeders; green, herbivores; orange, omnivores; pink, scavengers; red, carnivores. (A) Means ± SEM (n = 8) for each trophic group for combined northern and southern sites. (B–D) Mean percentage of each trophic group in the invertebrate communities collected (n = 8) in (B) ambient, (C) low, and (D) extreme low pH zones.
Discussion
Our results highlight divergent and compensatory biological responses to ocean acidification. Invertebrate taxonomic richness was reduced in extreme low pH zones primarily because of the absence of mollusks and decapods, which is consistent with previous studies at the vents that found a reduction in key calcifying species in reduced pH zones (15, 20). Conversely, we found that some crustaceans and polychaetes exhibited their highest densities in the extreme low pH zones, which resulted in similar densities of invertebrates across pH zones. Increased dominance by a few species under conditions of acidification also resulted in lower taxonomic evenness in the extreme low pH zones. Despite this increase in dominance, the changes in community structure were not driven by a few tolerant species, and some generalities in responses were apparent among broad taxonomic classes. The abundance of numerous amphipod genera increased or did not change in extreme low pH, whereas many mollusks exhibited no change in abundance in low pH and were absent in extreme low pH. Building on the previous study of benthic invertebrate abundance at the vent site (15), our results highlight compensatory changes in an entire assemblage of benthic invertebrates.
Although high tolerance among some crustaceans is predicted because of their internal acid–base regulation and low degree of calcification (10, 21), a compensatory increase in the abundance of some amphipods and tanaids could be caused by competitive release or decreased predation rates (22). Although the exact prey of many of the carnivores studied here is unknown, it is possible that some of the carnivorous polychaetes that suffered reductions in abundance in low and extreme low pH zones prey on amphipods and tanaids. Alternatively, the response of the tolerant crustaceans could be driven by interactions with macroalgae. Previous studies found that, although the percent cover of calcified algae decreases in extreme low pH zones, the percent cover of fleshy macroalgae increases (15, 23). Because of the association between small crustaceans and algal turfs and canopies, the increased abundance of small crustaceans in extreme low pH zones could be because of increased availability of habitat and food associated with changes in the percent cover of fleshy macroalgae. Manipulative field experiments are needed to elucidate which of these mechanisms underlies community reorganization under acidified conditions.
Despite density compensation among invertebrates, aggregate invertebrate biomass was reduced in extreme low pH zones. Mollusks contribute approximately one-half of the aggregate invertebrate biomass in the ambient pH zone samples, and the effect of their absence on biomass was not offset by the increased abundance of small-bodied, low-biomass crustacean and polychaete species. These results suggest that density compensation in this system does not stabilize biomass, because a diverse group of taxa responded similarly to acidification (6). Aggregate community biomass is an important ecosystem property linked with ecosystem functions such as nutrient and energy flux (7, 8, 24). The reduction in aggregate invertebrate biomass could indicate a reduction in energy transfer from primary producers to higher trophic levels with acidification. Although our biomass estimates include metabolically inactive calcified tissue that is not used by consumers, the calcified tissue is energetically costly for the invertebrates to produce and represents past metabolic activity (25). Although a reduction in the energy used to build unpalatable shells could increase the efficiency of energy transfer from primary producers to secondary consumers, it is unclear if this efficiency offsets the general reduction in herbivore abundance in extreme low pH. A reduction in energy transfer among trophic levels could be exacerbated by the simplification of the trophic structure in acidified conditions, which could decrease the efficiency of energy transfer among trophic levels.
On average, the pH values in the extreme low pH zones are lower and more variable than model projections for the near future (i.e., 2100) (19). Instead, this experiment’s extreme low pH zones approximate the most extreme acidification scenarios for the year 2300 (26). However, most projections for acidification are from global models that do not characterize small-scale variability in pH. Monitoring in near shore ecosystems suggests that organisms will be exposed to pH values more extreme and variable than those values predicted by global models (14, 27, 28). The extreme low zones that we used in this experiment are, therefore, useful for defining a range of responses to potential acidification scenarios. In contrast, the mean pH values in the low pH zones are directly comparable with projections for near future acidification. Variability in pH in the low pH zones is comparable with the degree of natural variation observed in other systems. For example, fluctuations of 0.72 and 0.67 pH units have been measured over short time frames near coral reefs (29) and in eastern boundary upwelling zones (30), respectively. Thus, the low pH zones may lend insight into the impacts of near future acidification in near shore environments.
Most taxa found in ambient pH zones were also present in low pH zones, although the mean pH in the low zones was comparable with the mean pH of laboratory studies reporting reduced physiological processes (10). Although we cannot infer causality in this observational study, it is possible that the natural variability in carbonate chemistry in this system could drive higher tolerance by rescue of physiological processes at transiently high pH. Repeated exposure to low pH could also support adaptation or acclimation to acidification. As our understanding of the natural variability of pH in other ecosystems grows, it will be useful to compare the variability in those ecosystems with the variability of the vents.
The abrupt declines in ecosystem properties in the extreme low pH zones suggest that the ecosystem impacts of acidification could be nonlinear (30). If ecosystems have an acidification threshold, then the ecosystem responses to global ocean acidification may be sudden. Abrupt ecological shifts are often difficult to predict but are associated with reduced ecological resilience (31). If the ecosystem impacts of acidification are driven by threshold dynamics, monitoring marine ecosystems for indicators of reduced resilience (including slower recovery rates) as acidification progresses will be important for ecosystem management.
The community changes in the reduced pH zones do not capture acidification’s effects on the reproductive success of some species. Acidification could cause tradeoffs in energy allocation between maintenance and reproduction that could reduce reproductive output and affect community structure/function. Unlike global ocean acidification, the vent communities are open to larvae from nonacidified source populations. However, for invertebrates that are direct developers, such as amphipods, tanaids, and sabellid and syllid (Exogoninae) polychaetes, all life stages are exposed to the same environmental conditions. Therefore, the effect of acidification on the reproductive success of these taxa is captured in the community changes at the vents.
Because of rapid environmental change worldwide, there is considerable interest in how species loss and altered communities will affect ecosystem function. Our study builds on previous field studies of responses of individual taxa (15), community structure (14), and larval settlement (22) to illustrate how the relative abundances of various taxa affect ecosystem properties under conditions of ocean acidification. Our results suggest that divergent and compensatory responses of marine species to extreme ocean acidification do not offset the effects on aggregate biomass or trophic structure, and they suggest that acidification will likely affect ecosystem function and the services that ecosystems provide.
Methods
The submarine CO2 vents occur at 0.5–3.0 m depth on the sides of a small island directly adjacent to steep, rocky reefs. We followed the designation of pH zones used by Hall-Spencer et al. (15) along continuous 150-m stretches of rocky reefs, moving from areas of high vent activity (extreme low pH zone) to moderate vent activity (low pH zone) to no visible vent activity (ambient pH zone) in both the northern and southern sites. Each zone was 20 m in length and was separated from the next zone by at least 20–25 m. Vent activity was sampled by counting the number of vents in randomly placed 1-m2 quadrants along a 20-m transect (n = 9).
To quantify variation in pH among the three zones, we used in situ modified Honeywell Durafet pH sensors (32) to record pH and temperature hourly. One sensor was deployed in each of the three experimental zones in the southern site from May 12 to June 14, 2010, and one sensor was deployed in each of the three experimental zones in the northern site from September 13 to October 8, 2010. Two sensors were deployed in the southern site for two additional time series (Table 2). One sensor was deployed in a control site over 3 km away from the vent site from October 12 to 21, 2010, and two sensors were deployed at either end of the low pH zone in the southern site during the same period (Fig. S2). Discrete water samples were collected within 0.25 m of the pH sensors during the initial deployment and retrieval of the sensors using standard operating protocols (33). Discrete water samples used to apply a vicarious calibration to the sensor were processed for TA using an open cell potentiometric titration, DIC with an automated 5011 CO2 coulometer (UIC, Inc.), and salinity with a Guildline Autosal salinometer. The measured values were standardized to a certified reference material provided by Andrew Dickson (Scripps Institution of Oceanography, La Jolla, CA). TA and DIC measurements were used to calculate seawater pHT at in situ temperature using the equation constants by Lueker et al. (34) in the program Seacarb v. 2.3.2 (35), and pHT values were used to calibrate the in situ sensors. Discrete sample measurements were not used to correct for drift in any of the time series, because drift was thought to be smaller than sampling error because of the extreme gradients in pH. Based on replicate bottle samples, sampling error (and hence, sensor accuracy) is estimated to be ∼0.05 pH units.
Benthic invertebrates were collected from 20-cm2 haphazardly selected plots in each experimental zone (n = 4). Two plots were collected within the first 5 m of a designated zone and were at least 3 m apart. The second two plots were collected within the last 5 m of the designated zone and were at least 3 m apart. Plots were 1–1.5 m deep on continuous, rocky substrate ranging from 50° to 85° in orientation from horizontal. Loose materials and mobile invertebrates (<5 cm) were collected using an airlift suction sampler that was placed over the plot for ∼30 s and were funneled into a fine mesh bag (∼150-μm mesh size). The remaining benthos was scraped off the substrate with a chisel. The samples were placed in 4% buffered formalin for 24 h and then transferred to a 70% ethanol solution for storage. A dissecting microscope was used to inspect all of the collected material to separate the invertebrates from the algae. The invertebrates were classified to the lowest taxonomic resolution possible by specialized taxonomists. The biomass was measured as wet weight (after a few minutes on an absorbent paper) for each OTU to 0.001 g. The wet weight included the shells, tests, or tubes for calcifying taxa.
Variation in the mean number of individuals and OTUs among pH zones was tested with two-way crossed ANOVA, with site and pH zone as fixed factors, after tests for normality and homogeneity of variance. We used a Kruskal–Wallis nonparametric test for differences in mean for those comparisons that did not meet the assumptions for normality and equal variance (evenness and biomass). We used Tukey’s Honestly Significant Difference (HSD) test for comparisons among pH zones.
Variation in community composition between pH zones and sites was analyzed on a Bray–Curtis (BC) similarity matrix of presence/absence of OTUs, whereas the community structure was analyzed on a BC similarity matrix of fourth root-transformed data of the total abundance of individuals within OTUs. Because our OTUs were heterogeneous (i.e., a mix of family, genus, and species), we reran this analysis on the abundance of individuals within taxonomic families. The results did not differ statistically, and we present the data from the OTU analysis. Variation in trophic structure was analyzed on a BC similarity matrix of fourth root-transformed data. Nonmetric multidimensional scaling plots were built from the similarity matrices, and we used two-way crossed PERMANOVA with 9,999 permutations, with site and pH zone as fixed factors, to test for differences in community composition, community structure, and trophic structure. We used PERMANOVA to run pairwise t tests among pH zones for composition and structure based on permutations of the residuals under a reduced model. We used two-way crossed ANOVA to test for variation in abundance among pH zones for each taxonomic class (α= 0.05) for those taxa that met assumptions of parametric statistics, and we used Kruskal–Wallis tests for the remaining taxa. Trends in the response of OTUs were assessed visually using bar graphs, and they were not tested for statistical significance because of low power and a large number of comparisons.
Supplementary Material
Acknowledgments
We thank G. Somero and S. Palumbi for their comments, which greatly improved this manuscript. We also thank the staff from the Ischia benthic ecology group of the Stazione Zoologica Anton Dohrn for their support, and we are especially grateful to M. C. Buia, B. Iacono, M. Lorenti, L. Porzio, and Capitan V. Rando for their field assistance. M. Lorenti, V. Zupo, M. B. Scipione, and A. Callea helped with classification of invertebrates, and B. Peterson constructed the pH sensors. We also thank A. Paytan, E. Derse-Crook, L. Teneva, and D. Mucciarone for their laboratory assistance with seawater chemistry. Diagrams were used courtesy of Integration and Application Network and A. Whitesides. This research was supported by a Stanford University Vice Provost for Graduate Education: Strengthening the Core grant (to K.J.K.), a National Science Foundation Graduate Research Fellowship (to K.J.K.), Stanford University setup funds and a Chambers Fellowship (to F.M.), National Science Foundation Grant 0844394 (to T.R.M.), and the Stazione Zoologica Anton Dohrn.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107789108/-/DCSupplemental.
References
- 1.Millennium Ecosystem Assessment . Ecosystems and Human Well-Being: Current State and Trends. Washington, DC: Island Press; 2006. [Google Scholar]
- 2.Fischlin A, et al. Ecosystems, their properties, goods, and services. In: Parry ML, et al., editors. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007. pp. 211–272. [Google Scholar]
- 3.McNaughton SJ. Diversity and stability of ecological communities: A comment on the role of empiricism in ecology. Am Nat. 1977;111:515–525. [Google Scholar]
- 4.Tilman D. Population versus ecosystem stability. Ecology. 1996;77:350–363. [Google Scholar]
- 5.Ives AR, Gross K, Klug JL. Stability and variability in competitive communities. Science. 1999;286:542–544. doi: 10.1126/science.286.5439.542. [DOI] [PubMed] [Google Scholar]
- 6.Fischer JM, Frost TM, Ives AR. Compensatory dynamics in zooplankton community responses to acidification: Measurement and mechanisms. Ecol Appl. 2001;11:1060–1072. [Google Scholar]
- 7.Vinebrooke RD, et al. Trophic dependence of ecosystem resistance and species compensation in experimentally acidified lake 302S (Canada) Ecosystems. 2003;6:101–113. [Google Scholar]
- 8.Gattuso JP, Buddemeier RW. Ocean biogeochemistry. Calcification and CO2. Nature. 2000;407:311–313. doi: 10.1038/35030280. [DOI] [PubMed] [Google Scholar]
- 9.Intergovermental Panel on Climate Change . In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, et al., editors. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 10.Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009;37:1131–1134. [Google Scholar]
- 11.Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett. 2010;13:1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 12.Gattuso JP, Frankignoulle EM, Wollast R. Carbon and carbonate metabolism in coastal aquatic systems. Annu Rev Ecol Syst. 1998;29:405–434. [Google Scholar]
- 13.Riebesell U, et al. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- 14.Wootton JT, Pfister CA, Forester JD. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc Natl Acad Sci USA. 2008;105:18848–18853. doi: 10.1073/pnas.0810079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall-Spencer JM, et al. Volcanic carbon dioxide vents show ecological effects of ocean acidification. Nature. 2008;454:95–99. doi: 10.1038/nature07051. [DOI] [PubMed] [Google Scholar]
- 16.Manzello DP, et al. Poorly cemented coral reefs of the eastern tropical Pacific: Possible insights into reef development in a high-CO2 world. Proc Natl Acad Sci USA. 2008;105:10450–10455. doi: 10.1073/pnas.0712167105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomsen J, et al. Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences. 2010;7:3879–3891. [Google Scholar]
- 18.Tedesco D. Chemical and isotopic investigations of fumarolic gases from Ischia Island (Italy): Evidence of magmatic and crustal contribution. J Volcanol Geotherm Res. 1996;74:233–242. [Google Scholar]
- 19.Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- 20.Cigliano M, Gambi MC, Rodolfo-Metalpa R, Patti FP, Hall-Spencer JM. Effects of ocean acidification on invertebrate settlement at volcanic CO2 vents. Mar Biol. 2010;157:2489–2502. [Google Scholar]
- 21.Melzner F, et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeosciences. 2009;6:2313–2331. [Google Scholar]
- 22.Micheli F, et al. The dual nature of community variability. Oikos. 1999;85:161–169. [Google Scholar]
- 23.Porzio L, Buia MC, Hall-Spencer JM. Effects of ocean acidification on macroalgal communities. JEMBE. 2011;400:278–287. [Google Scholar]
- 24.Naeem S, Li S. Biodiversity enhances ecosystem reliability. Nature. 1997;390:507–509. [Google Scholar]
- 25.Palmer AR. Calcification in marine molluscs: How costly is it? Proc Natl Acad Sci USA. 1992;89:1379–1382. doi: 10.1073/pnas.89.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldeira K, Wickett ME. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res Oceans. 2005 110, C09S04, 10.1029/2004JC002671. [Google Scholar]
- 27.Feely RA, Sabine CL, Hernandez-Ayon JM, Ianson D, Hales B. Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science. 2008;320:1490–1492. doi: 10.1126/science.1155676. [DOI] [PubMed] [Google Scholar]
- 28.Yu P, Matson PG, Martz TR, Hofmann GE. The ocean acidification seascape and its relationship to the performance of calcifying marine invertebrates: Laboratory experiments on the development of urchin larvae framed by environmentally-relevant pCO2/pH. JEMBE. 2011;400:288–295. [Google Scholar]
- 29.Ohde S, van Woesik R. Carbon dioxide flux and metabolic processes of a coral reef, Okinawa. Bull Mar Sci. 1999;65:559–576. [Google Scholar]
- 30.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. Catastrophic shifts in ecosystems. Nature. 2001;413:591–596. doi: 10.1038/35098000. [DOI] [PubMed] [Google Scholar]
- 31.Scheffer M, et al. Early-warning signals for critical transitions. Nature. 2009;461:53–59. doi: 10.1038/nature08227. [DOI] [PubMed] [Google Scholar]
- 32.Martz T, Connery J, Johnson K. Testing the Honeywell Durafet for seawater pH applications. Limnol Oceanogr Methods. 2010;8:172–184. [Google Scholar]
- 33.Dickson AG, Sabine CL, Christian JR, editors. Guide to Best Practices for Ocean CO2 Measurements. PICES Special Publication 3. Sidney, BC, Canada: North Pacific Marine Science Organization; 2007. Available at: http://cdiac.ornl.gov/oceans/Handbook_2007.html. [Google Scholar]
- 34.Lueker TJ, Dickson A, Keeling CD. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2: Validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar Chem. 2000;70:105–119. [Google Scholar]
- 35.Lavigne H, Gattuso JP. Seacarb: Seawater Carbonate Chemistry with R. R Package Version 2.3.5. 2010. Available at http://CRAN.R-project.org/package=seacarb. Accessed November 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.