Abstract
Many animal species communicate with their mates through acoustic signals, but this communication seems to become a struggle in urbanized areas because of increasing anthropogenic noise levels. Several bird species have been reported to increase song frequency by which they reduce the masking impact of spectrally overlapping noise. However, it remains unclear whether such behavioral flexibility provides a sufficient solution to noisy urban conditions or whether there are hidden costs. Species may rely on low frequencies to attract and impress females, and the use of high frequencies may, therefore, come at the cost of reduced attractiveness. We studied the potential tradeoff between signal strength and signal detection in a successful urban bird species, the great tit (Parus major). We show that the use of low-frequency songs by males is related to female fertility as well as sexual fidelity. We experimentally show that urban noise conditions impair male–female communication and that signal efficiency depends on song frequency in the presence of noise. Our data reveal a response advantage for high-frequency songs during sexual signaling in noisy conditions, whereas low-frequency songs are likely to be preferred. These data are critical for our understanding of the impact of anthropogenic noise on wild-ranging birds, because they provide evidence for low-frequency songs being linked to reproductive success and to be affected by noise-dependent signal efficiency.
Keywords: signal evolution, noise pollution, frequency-dependent masking, extra-pair paternity, signal trade-off
The use of acoustic signals to attract and stimulate sexual partners is a widespread phenomenon in the animal kingdom, and many species rely to some extent on auditory contact for reproductive success (1). However, rapid worldwide urbanization (2) and the associated rise in noise pollution makes efficient acoustic communication increasingly difficult in areas in and around cities and in proximity of highways, airports, and industrial areas (3–5). Most anthropogenic noise is related to traffic or industrial machinery, and it is typically biased to low frequencies (3, 6). Interestingly, several urban bird species have been found to reduce the spectral overlap with anthropogenic noise by shifting songs up to higher frequencies (7–9), which is presumed to aid communication and thereby, increase reproductive performances (6, 7).
The ability to adjust song frequency on a short evolutionary timescale may be an important factor determining avian breeding success in noisy urban environments (5, 10). Anthropogenic noise has been reported to have a detrimental impact on bird breeding density and reproductive output (11–13), with particularly negative effects for species vocalizing at low frequencies (14). The effect can be partly explained by a lack of song frequency flexibility in those species that do not learn their vocalizations (e.g., pigeons and cuckoos) (11, 14). However, even species that have been shown to immediately adjust song frequency in the presence of experimental noise (15–18) may suffer reduced breeding success when potential benefits of a spectral adjustment are not sufficient to avoid negative masking impact (19) or when spectral adjustment comes at a cost of reduced attractiveness (5).
Low frequencies can play a crucial role in female attraction, because they have the potential to convey a message of male quality (20, 21); they also transmit relatively well through vegetation and probably, into nest cavities (22, 23). However, the rising noise levels of our modern society may turn these concordant advantages into a tradeoff between frequencies that are optimal for signal strength in terms of mate attraction or optimal for signal detection in terms of masking avoidance. Noisy human activities may interfere in this way with what may have been a stable factor in signal efficiency over long periods of evolutionary time.
Two major gaps in assessing the impact of urban noise on fitness and the advantage of song frequency flexibility are (i) a lack of insight into whether singing low matters in avian mate attraction and (ii) a lack of evidence from the field for frequency-dependent signal efficiency related to the presence of anthropogenic noise.
Although spectral characteristics have been correlated to male qualities that could affect female choice (20, 24) and song-related sexual infidelity has been reported for female birds (25–27), we lack data that indicate a reproductive advantage for singing low-frequency songs. Assuming higher quality to be related to potentially costly low-frequency songs, we may expect male performance to peak when it counts most for the bird—during the few days a year when eggs are fertilized (28, 29). Similarly, although within and between population patterns can show consistently higher frequency use at noisy sites, such as in great tits (7, 30, 31), and although we recently revealed the underlying mechanism of active spectral avoidance in this species experimentally (17), we lack data on communicative consequences in the field. Any evidence showing a noise impact on the perception of communicative sounds in birds has, so far, only come from studies under laboratory conditions (32–34), outside a context meaningful to signal efficiency and reproductive success.
Here, we studied acoustic courtship interactions in a natural woodland area among male and female great tits during the courtship ritual at dawn. We studied breeding great tit pairs at their nest box, which allowed us to document close-range male–female interactions. We used pairs of microphones simultaneously, one inside and one outside the nest box, to record male song behavior and female response behavior (Fig. 1A) (35), starting when nests were near completion. We explored the role of singing low-frequency song types in male–female communication during the dawn chorus. We analyzed male song behavior in relation to the laying sequence and tested whether male song frequencies were related to female fertility as well as female sexual fidelity. Subsequently, we conducted a field experiment in which we played songs from a male's repertoire to his female inside the nest box. Females are known to discriminate accurately under these acoustically difficult conditions (23, 36), which allows us to test for an effect of experimental noise exposure on the efficiency in triggering a female response, specifically for low- vs. high-frequency songs.
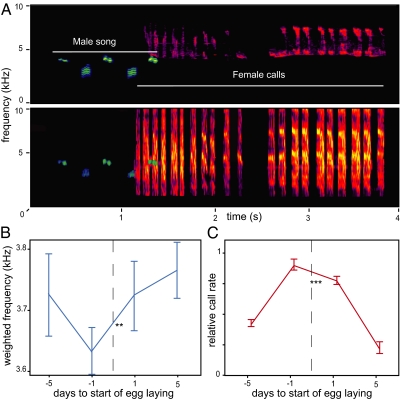
Fig. 1.
The dawn chorus ritual of great tits (Parus major) peaks with female fertility. (A) Sonogram of a stereo recording shows the acoustic interaction between a male (song in blue) and a female (calls in red). Males continuously sing or call close to the nest box during dawn, and females can call in response. Females call mostly at the start of male dawn singing, during song type switches, and shortly before emergence from the nest box. Upper and Lower show recordings made with the outside and inside microphones, respectively. (B) Within individual variation of great tit song behavior is related to egg laying (GLMM; **P < 0.001), and males sing lowest when fertility is highest (egg day −1). (C) Female calling changes with egg laying (GLMM; ***P < 0.001), and females call most on days around the start of laying. The graphs show means ± 1 SE. The x axes show days related to start of laying (egg day 0; indicated by barred line), and y axes show male average weighted frequency of low notes and female calling (number of calls produced during dawn chorus, normalized per female).
Results
Singing Low Peaks with Female Fertility.
Males vary in how low the different song types in their repertoire are as well as how often they use the relatively low song types (accumulating into spectral performance). Song spectral performance varied over time within individuals and peaked with the moment of highest fertility [generalized linear mixed model (GLMM): egg day2; χ2 = 18.76, degrees of freedom (df) = 3, P < 0.001], because individual males sang lowest just before the start of egg laying (Fig. 1B). In contrast, males did not change the spectral frequency of their song types in relation to egg laying (GLMM: egg day2; χ2 = 1.43, df = 3, P = 0.70), which implies that great tit males selectively used low-frequency song types, especially when interacting with their fertile mates. Other song features did not peak with fertility (song type duration: P = 0.27, repertoire size: P = 0.31), although start of dawn singing increased with progress in the laying stage (χ2 = 8.75, df = 3, P = 0.033) (Fig. S1). Female calling activity level peaked synchronously with male spectral song performance at the start of egg laying (χ2 = 18.34, df = 3, P < 0.001) and rapidly dropped after the first few eggs had been laid (Fig. 1C). Furthermore, females left the nest box earlier before than after egg laying (GLMM: egg day; χ2 = 19.71, df = 1, P < 0.001) (Fig. S1).
Low-Singing Males Get Cuckolded Less Often.
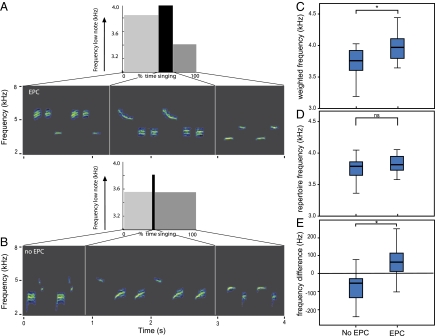
We tested whether performing with low-frequency songs at the peak in fertility was related to female sexual fidelity and found that noncuckolded males sang lower songs compared with cuckolded males [linear mixed models (LMM); F1,21 = 6.84, P = 0.018] (Fig. 2). Noncuckolded males did not have lower-frequency song types (Fig. 2D) but used the low-frequency song types from their repertoire for a larger proportion of time (Fig. 2E). Interestingly, female fidelity was also related to nest box emergence [generalized linear model (GLM); χ2 = 7.14, df = 1, P = 0.008]. Unfaithful females, at the peak of fertility, left their nest box earlier (17.5 ± 4.8 min before sunrise; mean ± SD) compared with females who did not engage in extra-pair copulations (0.04 ± 5.71 min after sunrise).
Fig. 2.
Males singing low-frequency songs suffer less paternity loss. (A and B) Examples of song type repertoires and song type use for two neighboring males in relation to paternity loss. (A) The cuckolded male (EPC) has similar song types compared with (B) the noncuckolded male (no EPC), and the neighbors mainly differ in the percentage of time during which they use their low- and high-frequency song types. Sonograms show their repertoires consisting of three song types, and the graphs show the peak frequency of the lowest note in relation to the percentage of time that the individual is using a particular song type. (C and D) Box-whisker plots showing differences in song behavior between paternity groups. (C) Non-cuckolded males sing, on average, lower than cuckolded males during the dawn chorus at the peak of female fertility (LMM; F1,21 = 6.84, *P = 0.018). (D) Differences cannot be ascribed to noncuckolded males having lower song types in their repertoire (LMM; F1,21 = 1.64, P = 0.22; peak frequencies of low notes averaged over song types of an individual's repertoire). (E) Differences are the result of using the lower song types more often [LMM; F1,21 = 7.39, *P = 0.014; difference between average weighted song frequency (C) and frequency averaged over repertoire (D) per individual.]
Low Songs Lose Signal Efficiency in Anthropogenic Noise.
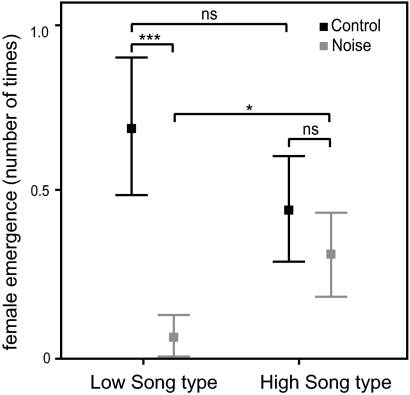
We measured female response to playback of high- and low-frequency song types from the repertoire of their own mate under noisy and control conditions (Fig. S2 shows an example of signal-to-noise ratios of both song types under both noise conditions). Females are known to emerge from the nest box in response to dawn singing, after which they typically immediately copulate with their mates (28), and female calling is related to fertility (this study); therefore, we used these two measures, emerging and calling from the nest box, as a proxy for female preference. Both song types (high and low) were played on 2 subsequent d with and without noise exposure inside the nest box. Female emergence from the nest box differed across tests (GLMM; χ2 = 8.63, df = 3, P = 0.035) (Fig. 3), depending on noise and the song type played. Females emerged less to low-frequency song types with noise than without noise (pairwise comparison; low noise–low control: P < 0.001), whereas female emergence response to high-frequency song types was unaffected by our noise exposure (high noise–high control: P = 0.39). Females did not emerge more often during playback of low-frequency song types than high-frequency song types under control conditions (low control–high control: P = 0.20) but emerged significantly more often in response to playback of high-frequency song types than low-frequency song types during noise exposure (high noise–low noise: P = 0.044), Only 9 of 16 females called before nest box emergence, but calling nonetheless showed a similar trend in response pattern—less response to low-frequency song types under noisy than control conditions (P = 0.08) and noise-independent response levels to high song types (P = 0.78).
Fig. 3.
Reduced female response to low male songs in noise. Females were played the lowest and highest song type from their mate's repertoire on days with and without experimental noise exposure. Shown is the number of trials during which females emerged from their nest box as the response measure. Under noisy conditions, female response to song changed for the low-frequency song types (GLMM; low noise vs. low control: ***P < 0.001) but not for the high-frequency song types (P = 0.39). This finding resulted in high-frequency songs being more effective compared with low-frequency songs in noise (*P = 0.044). Females had no prior experience with noise and were adjusted to noise conditions for ∼24 h before the start of the playback.
Discussion
Our findings show that male great tits sing their lowest songs at the peak of female fertility with a reward of sexual fidelity. The females involved in extra-pair copulations in our study also seem to sneak away before sunrise, which is suggestive for an active mate choice decision (cf. 37). Acoustic variation among singing birds concerns the most reliable information on male qualities under poor light conditions, and sexual selection for low-frequency songs yields, therefore, a feasible explanation for the well-timed spectral performance of males. We also show that signal efficiency depends on song frequency in the presence of anthropogenic noise. Low-frequency songs show reduced effectiveness in triggering female responses in noise and thereby, are less effective than high-frequency songs, showing that it pays urban birds to increase song frequencies when confronted with noisy conditions.
There are several not mutually exclusive explanations for the relationship between song frequency and female fidelity. Female songbirds make song-based reproductive decisions during the dawn chorus (26, 27), and female great tits in our study could have used spectral variation for male quality assessment when the production of low frequencies is, for example, physically constrained or bears retaliation costs (20, 38). Alternatively, low-frequency songs may be under indirect sexual selection, because low frequencies can covary with more complex spectral features (38). For instance, females could prefer broad-banded song types that can be physically demanding to produce (38) and will, on average, be lower in frequency compared with small-banded song types. Low-frequency songs could also be favored by natural selection pressures such as the transmission properties of the acoustic environment (22), including the complex acoustic structure of a nest box (23). Lower frequencies may experience transmission-dependent increase in signal-to-noise ratios under normal circumstances, and whether high- or low-frequency songs are favored under anthropogenic noise will then depend on the relative strengths of these two environmental selection pressures.
However, another interpretation of the female preference for males shifting their singing efforts to the low-frequency song types in their repertoire could be that different song types serve different functions. Low-frequency song types could play an explicit role in male–female communication, whereas high-frequency song types could be more important in male–male communication. Several bird species are known to have different song types for intra- and intersexual signaling (39), and song frequency of great tits has been shown to be positively related to male density (40). During the dawn chorus, great tit males also interact with neighboring males, flying back and forth between territory boundaries and matching song types. If males selectively sing low-frequency songs to females and high-frequency songs to males and if the amount of interaction time spent with females varies with laying stage and mate guarding, this finding could also explain the observed patterns in frequency use in relation to female fertility and sexual fidelity. Alternatively, low-frequency songs could be used in song contest among males to guard paternity and be indirectly related to female preference if females use the outcome of the contest in extra-pair mating decisions (26).
Our playback results show experimental evidence for a noise-dependent advantage of high-frequency songs under natural field conditions. Low-frequency songs suffer reduced efficiency in male–female communication under noisy conditions, favoring the use of high-frequency songs. This finding is in line with experimental data showing that great tits actively avoid spectral overlap with background noise (17). In these earlier experiments, male great tits were not only shown to switch to high-frequency song types during exposure with low-frequency city noise, but they were also shown to do the opposite during exposure to high-frequency inverse city noise. Many species have now been observed to raise song frequencies up in urban noise in both natural and experimental setting (7, 8, 15), and although the benefits in terms of masking release have been debated recently (19), our results show that, in great tits, such a change will substantially improve male–female communication. Such a strategy of reducing spectral overlap with background noise can act concomitantly with other signaling strategies, such as raising song amplitude (41), or it can be used as an alternative for those species for which raising amplitude above a certain level is too energetically demanding.
The evolutionarily novel urban conditions may affect both natural and sexual selection pressures acting on bird song. If low-frequency songs are under sexual selection through female preference and if high-frequency songs are under natural selection through noise-dependent signal efficacy, we may expect a modern tradeoff with crucial fitness consequences—use low-frequency songs to stimulate females or use high-frequency songs to avoid masking noise.
If a signal is not detected, it can also not be discriminated from other signals, and therefore, for species in which females make sound-based reproductive decisions, we would expect signal detection to prevail over signal strength under high-noise conditions. A focus on detection of conspecifics rather than discrimination among them, which has been observed in female frogs exposed to natural chorus noise (42), can result in a preference shift from the low to the high frequencies. Consequently, the tradeoff will limit high-quality males in urban areas to distinguish themselves spectrally from competitors. An interesting follow-up study could be to find out whether there are alternative vocal parameters in which high-quality males can excel that may explain urban divergence through sensory drive to, for example, higher and faster songs (30, 38). Great tits living in noisy territories in cities have already been found to respond more strongly to songs recorded in similar territories (31), and although familiarity remains to be excluded as a factor (43), these findings suggest that urban noise conditions have the potential to alter sexual selection pressures.
Our findings contribute to the expanding field of research that links the presence of roads, traffic, and traffic noise to reduced bird breeding densities (44–47). Noise can mask acoustic signals and is known, for example, to cause a decline in the number of breeding bird territories (6, 11). Individuals that have to settle for noisy locations may suffer from reduced pairing and thus, reduced reproductive success (12, 44), or they may end up with low-quality or at least, less-productive mates, laying smaller clutches and raising fewer offspring close to noisy highways (13). The masking impact by traffic noise will be highest for those species that use low frequencies to attract females, which is shown by our field playback experiment, and this finding can explain why species vocalizing at lower frequencies suffer most from anthropogenic noise pollution (11, 14, 48).
In conclusion, we have shown that evolutionarily novel urban conditions can undermine the selective advantage of using low-frequency song types. Furthermore, we found the use of low-frequency song types to be related to reproductive success through female fidelity, which shows that low-frequency singing is under direct or indirect sexual selection. These findings also show that benefits of masking release through singing higher frequencies in cities are constrained by a potential loss in signal strength and point to the existence of a modern tradeoff. It would be interesting to examine further how anthropogenic noise can alter the strength, direction, or specific target of selection pressures acting on bird song. Studies on urban acoustics will likely continue to provide both scientific opportunity and conservation concern. They generate new insights on environmental causes for evolutionary change, but they should also raise awareness of the consequences of noisy human behavior.
Materials and Methods
The study was conducted in a nest box population of great tits situated in the National Park Dwingelderveld in the Netherlands between April and May in 2009 and 2010. Territories were mapped, and nest boxes were checked for nest building every other day. Behavioral recording and nest box extension (see below) began when nests were near completion to minimize nest desertion because of our activities. All males and females were included only one time in this study. The Leiden Committee for animal experimentation approved the study under number 08073.
Acoustic Recordings and Measurements.
We used SongMeters (16-bit, 24-kHz sample rate; Wildlife Acoustics Inc.) to automatically record male and female acoustic behavior. A microphone placed inside the nest box was used to record female calls, whereas the other microphone outside recorded the male's dawn song. Both microphones were used to assess time of female emergence by the sounds of her claws on the nest box and movement of the wings when taking off. We recorded the dawn ritual (1 h before until 1 h after sunrise) across the laying phase. We identified song types of the social male and determined start of dawn singing, total time spent singing, song type repertoire size, time of female emergence, and total number of calls produced by females with the program Audacity 1.3. Bout duration and low-note frequency were measured for each song type independently (17) to determine song spectral performance. Low-note frequency was multiplied with bout duration and divided by the total amount of time spent singing to get an average weighted measure of song frequency per day.
Paternity Analysis.
Chicks were sampled for blood on the second day and parents were sampled on the seventh day posthatching for DNA extraction. To assign paternity, we used the six microsatellite loci (described in ref. 49). Loci were PCR-amplified using a QIAGEN Multiplex PCR Kit and the manufacturer's protocol. Allele lengths were determined (described in ref. 50). Cervus 3.0 (51) calculated the mean exclusion power of the six markers to be 0.99 for the first (female) parent and 0.99 for the second (male) parent (given the genotype of the first parent). For each chick, we assessed whether it was sired by the social mate. Paternity of the social mate was excluded and the offspring was assigned as extra-pair if there were at least two mismatches between the social father's and offspring's genotype.
Experimental Noise Exposure.
We extended the normal nest box by removing the roof and adding a second box on top (made of the same material) that was inaccessible to the birds but had a hole in the bottom. We inserted a speaker at a height of 15 cm within this second box to allow playback of noise mimicking conditions as if the nest box was situated 50–100 m from a major highway (13) and avoid near field effects at the position of the female. Fig. S2 shows an example of experimental and natural noise profiles.
Noise playback of artificially generated low-frequency traffic noise (low pass-filtered white noise in the range of 1–10 kHz, with a decreases of 6.5 dB/kHz) (17) was carried out using full-range speakers (2.5 in; Peerless) connected to an mp3 player with a battery pack hidden under the leaf litter. Noise level was gradually increased to ∼68.0 dB (A-weighted; SPL) at the position of the nest, and females were familiarized with the noise in their nest box for 24 h.
Stimuli Preparation and Playback Procedure.
We determined the highest and lowest song type from a male's repertoire based on peak frequency of the low note (average difference of 591.1 ± 285.7 Hz; mean ± SD). We selected a high-quality recording of a strophe of a single song type for each female tested with songs from the repertoire of her own social mate and created a stimulus file 30 s in length (36). Both high- and low-frequency song type stimuli were bandpass-filtered from 2 to 10 kHz, normalized for amplitude, and played from a speaker (SC 4ND; Visaton) on a pole positioned at ∼1.5 m and an angle of 45° from the nest entrance. Great tits typically sing at a distance of 8–16 m from the nest box, which results in a song amplitude of ∼60 dB(A) at the position of the female. We played the songs that had been recorded at the position of the nest box at an amplitude of ∼62 dB (A-weighted; measured 1 m away from the speaker) to get similar song amplitudes at the position of the female and to avoid detection by the focal male (Fig. S2 shows an example of song type signal to noise ratios inside the nest box under noisy and control conditions). The song amplitude at the position of the female always exceeded the detection thresholds for great tits in noise (52) to allow discrimination among song types. Playback experiments were carried out during daytime to avoid male interference. We carried out four experiments per female using both high- and low-frequency song types on 2 different d (with and without noise). In each experiment, females received four 30-s trials of the same song type. Both high- and low-frequency song types were played to females on the same day, with a minimum of 30 min between the two experiments. The order of song type and noise presentation was balanced across females. Nest boxes were observed from a hide, an experiment started when females had been inside the nest box for at least 15 min, and a trial only started when males were away from the nest box and not singing (36). All but one female received the playbacks on 2 consecutive d. We scored whether females emerged or called during a trial.
Data Analyses.
All multivariate analyses were carried out in SPSS 17.0, and data were transformed when necessary to meet model assumptions. We used different subsets of males and females for the observational analyses depending on the availability of suitable recordings and paternity data.
We related male and female behavior to start of laying (egg day = 0) when fertility is presumed to be high. We selected a subset of pairs (n = 15) for which we had suitable recordings before (egg days −5 and −1) and during laying (egg days 1 and 5). We tested whether within individual vocal performance peaked at fertility using a generalized linear mixed model (GLMM) with a power link function, a normal error distribution (or Poisson distribution for number of calls), individual as subject, and nest box site and egg day as fixed factors. We assigned a unique code to each song type of an individual male and tested whether the frequency averaged over song type changed across egg laying in a GLMM with individual song type as subject and site and egg day as fixed factors.
We used a subset of individuals (n = 22) for which we had control recordings at the peak of fertility (egg day −1) to test whether cuckolded males [males with extra-pair chicks in their nest (EPC)] differed in male song frequency using LMM, with date as random factor and site and EPC as fixed factors. We compared weighted song frequency with frequency averaged over song type to assess whether singing by EPC males differed in repertoire composition, repertoire use, or both. We used the same subset to compare female nest box emergence among EPC groups on egg day −1 in a GLM with site and EPC as fixed factors.
We used a balanced playback design (n = 16) to test for a differential impact of noise on female response to high- and low-frequency song types, controlling for order of stimulus presentation and day of noise exposure. Female response (number of trials emerged or called) to male playback of high- and low-frequency song types was tested in a GLMM with a Poisson error distribution, log link function, and noise treatment, and song type (high or low), stimulus order, and day were fixed factors.
Supplementary Material
Acknowledgments
We thank K. Riebel, J. Podos, H. Brumm, and three anonymous referees for critically reading previous versions of the manuscript and providing valuable feedback. We thank Staatsbosbeheer and Natuurmonumenten for allowing us to work in their forest reserves. We are very grateful to C. Both for being able to work at his research sites and giving his valuable feedback during field work. We thank L. van Dijk for assistance in the field.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109091108/-/DCSupplemental.
References
- 1.Bradbury JW, Vehrencamp SL. Principles of Animal Communication. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 2.Grimm NB, et al. Global change and the ecology of cities. Science. 2008;319:756–760. doi: 10.1126/science.1150195. [DOI] [PubMed] [Google Scholar]
- 3.Barber JR, Crooks KR, Fristrup KM. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol Evol. 2010;25:180–189. doi: 10.1016/j.tree.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Slabbekoorn H, et al. A noisy spring: The impact of globally rising underwater sound levels on fish. Trends Ecol Evol. 2010;25:419–427. doi: 10.1016/j.tree.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Slabbekoorn H, Ripmeester EAP. Birdsong and anthropogenic noise: Implications and applications for conservation. Mol Ecol. 2008;17:72–83. doi: 10.1111/j.1365-294X.2007.03487.x. [DOI] [PubMed] [Google Scholar]
- 6.Brumm H, Slabbekoorn H. Advances in the Study of Behavior. Vol. 35. San Diego, CA: Elsevier Academic Press; 2005. Acoustic communication in noise; pp. 151–209. [Google Scholar]
- 7.Slabbekoorn H, Peet M. Ecology: Birds sing at a higher pitch in urban noise. Nature. 2003;424:267. doi: 10.1038/424267a. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Cardoso GC. Which birds adjust the frequency of vocalizations in urban noise? Anim Behav. 2009;79:863–867. [Google Scholar]
- 9.Potvin DA, Parris KM, Mulder RA. Geographically pervasive effects of urban noise on frequency and syllable rate of songs and calls in silvereyes (Zosterops lateralis) Proc R Soc Lond B Biol Sci. 2011;278:2464–2469. doi: 10.1098/rspb.2010.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patricelli GL, Blickley JL. Avian communication in urban noise: Causes and consequences of vocal adjustment. Auk. 2006;123:639–649. [Google Scholar]
- 11.Francis CD, Ortega CP, Cruz A. Noise changes avian communities and species interactions. Curr Biol. 2009;19:1415–1419. doi: 10.1016/j.cub.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 12.Bayne EM, Habib L, Boutin S. Impacts of chronic anthropogenic noise from energy-sector activity on abundance of songbirds in the boreal forest. Conserv Biol. 2008;22:1186–1193. doi: 10.1111/j.1523-1739.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- 13.Halfwerk W, Holleman LJM, Lessells CM, Slabbekoorn H. Negative impact of traffic noise on avian reproductive success. J Appl Ecol. 2011;48:210–219. [Google Scholar]
- 14.Goodwin SE, Shriver WG. Effects of traffic noise on occupancy patterns of forest birds. Conserv Biol. 2011;25:406–411. doi: 10.1111/j.1523-1739.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- 15.Gross K, Pasinelli G, Kunc HP. Behavioral plasticity allows short-term adjustment to a novel environment. Am Nat. 2010;176:456–464. doi: 10.1086/655428. [DOI] [PubMed] [Google Scholar]
- 16.Verzijden MN, Ripmeester EAP, Ohms VR, Snelderwaard P, Slabbekoorn H. Immediate spectral flexibility in singing chiffchaffs during experimental exposure to highway noise. J Exp Biol. 2010;213:2575–2581. doi: 10.1242/jeb.038299. [DOI] [PubMed] [Google Scholar]
- 17.Halfwerk W, Slabbekoorn H. A behavioral mechanism explaining noise-dependent frequency use in urban birdsong. Anim Behav. 2009;78:1301–1307. [Google Scholar]
- 18.Bermúdez-Cuamatzin E, Ríos-Chelén AA, Gil D, Garcia CM. Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol Lett. 2011;7:36–38. doi: 10.1098/rsbl.2010.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemeth E, Brumm H. Birds and anthropogenic noise: Are urban songs adaptive? Am Nat. 2010;176:465–475. doi: 10.1086/656275. [DOI] [PubMed] [Google Scholar]
- 20.Gil D, Gahr M. The honesty of bird song: Multiple constraints for multiple traits. Trends Ecol Evol. 2002;17:133–141. [Google Scholar]
- 21.Davies NB, Halliday TR. Deep croaks and fighting assessment in toads Bufo bufo. Nature. 1978;274:683–685. [Google Scholar]
- 22.Wiley RH, Richards DG. Physical constraints on acoustic communication in atmosphere-implications for evolution of animal vocalizations. Behav Ecol Sociobiol. 1978;3:69–94. [Google Scholar]
- 23.Blumenrath SH, Dabelsteen T, Pedersen SB. Being inside nest boxes: Does it complicate the receiving conditions for great tit Parus major females? Bioacoustics. 2004;14:209–223. [Google Scholar]
- 24.Kirschel ANG, Blumstein DT, Smith TB. Character displacement of song and morphology in African tinkerbirds. Proc Natl Acad Sci USA. 2009;106:8256–8261. doi: 10.1073/pnas.0810124106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasselquist D, Bensch S, von Schantz T. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature. 1996;381:229–232. [Google Scholar]
- 26.Mennill DJ, Ratcliffe LM, Boag PT. Female eavesdropping on male song contests in songbirds. Science. 2002;296:873. doi: 10.1126/science.296.5569.873. [DOI] [PubMed] [Google Scholar]
- 27.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr Biol. 2010;20:1735–1739. doi: 10.1016/j.cub.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Mace R. The dawn chorus in the great tit parus-major is directly related to female fertility. Nature. 1987;330:745–746. [Google Scholar]
- 29.Moller AP. Why mated songbirds sing so much-mate guarding and male announcement of mate fertility status. Am Nat. 1991;138:994–1014. [Google Scholar]
- 30.Slabbekoorn H, den Boer-Visser A. Cities change the songs of birds. Curr Biol. 2006;16:2326–2331. doi: 10.1016/j.cub.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Mockford EJ, Marshall RC. Effects of urban noise on song and response behavior in great tits. Proc R Soc Lond B Biol Sci. 2009;276:2979–2985. doi: 10.1098/rspb.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohr B, Wright TF, Dooling RJ. Detection and discrimination of natural calls in masking noise by birds: Estimating the active space of a signal. Anim Behav. 2003;65:763–777. [Google Scholar]
- 33.Pohl NU, Slabbekoorn H, Klump GM, Langemann U. Effects of signal features and environmental noise on signal detection in the great tit, Parus major. Anim Behav. 2009;78:1293–1300. [Google Scholar]
- 34.Swaddle JP, Page LC. High levels of environmental noise erode pair preferences in zebra finches: Implications for noise pollution. Anim Behav. 2007;74:363–368. [Google Scholar]
- 35.Gorissen L, Eens M. Interactive communication between male and female Great Tits (Parus major) during the dawn chorus. Auk. 2004;121:184–191. [Google Scholar]
- 36.Blumenrath SH, Dabelsteen T, Pedersen SB. Vocal neighbour-mate discrimination in female great tits despite high song similarity. Anim Behav. 2007;73:789–796. [Google Scholar]
- 37.Double M, Cockburn A. Pre-dawn infidelity: Females control extra-pair mating in superb fairy-wrens. Proc Biol Sci. 2000;267:465–470. doi: 10.1098/rspb.2000.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Podos J. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature. 2001;409:185–188. doi: 10.1038/35051570. [DOI] [PubMed] [Google Scholar]
- 39.Kroodsma DE. The diversity and plasticity of bird song. In: Marler P, Slabbekoorn H, editors. Nature's Music: The Science of Bird Song. London: Elsevier; 2004. pp. 108–130. [Google Scholar]
- 40.Hamao S, Watanabe M, Mori Y. Urban noise and male density affect songs in the great tit Parus major. Ethol Ecol Evol. 2011;23:111–119. [Google Scholar]
- 41.Brumm H, Todt D. Noise-dependent song amplitude regulation in a territorial songbird. Anim Behav. 2002;63:891–897. [Google Scholar]
- 42.Wollerman L, Wiley RH. Background noise from a natural chorus alters female discrimination of male calls in a Neotropical frog. Anim Behav. 2002;63:15–22. [Google Scholar]
- 43.Falls JB, Krebs JR, McGregor PK. Song matching in the great tit (parus-major)—the effect of similarity and familiarity. Anim Behav. 1982;30:997–1009. [Google Scholar]
- 44.Reijnen R, Foppen R. Effect of road traffic on the breeding site tenacity of male willow warblers (phylloscopus-trochilus) J Ornithol. 1991;132:291–295. [Google Scholar]
- 45.Reijnen R, Foppen R. Impact of road traffic on breeding bird populations. In: Davenport J, Davenport JL, editors. The Ecology of Transportation: Managing Mobility for the Environment. Heidelberg: Springer; 2006. pp. 255–274. [Google Scholar]
- 46.Kociolek AV, Clevenger AP, St Clair CC, Proppe DS. Effects of road networks on bird populations. Conserv Biol. 2011;25:241–249. doi: 10.1111/j.1523-1739.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- 47.Forman RTT. Estimate of the area affected ecologically by the road system in the United States. Conserv Biol. 2000;14:31–35. [Google Scholar]
- 48.Rheindt FE. The impact of roads on birds: Does song frequency play a role in determining susceptibility to noise pollution? J Ornithol. 2003;144:295–306. [Google Scholar]
- 49.Brommer JE, et al. Passerine extrapair mating dynamics: A Bayesian modeling approach comparing four species. Am Nat. 2010;176:178–187. doi: 10.1086/653660. [DOI] [PubMed] [Google Scholar]
- 50.Magrath MJL, Vedder O, van der Velde M, Komdeur J. Maternal effects contribute to the superior performance of extra-pair offspring. Curr Biol. 2009;19:792–797. doi: 10.1016/j.cub.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 51.Kalinowski ST, Taper ML, Marshall TC. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol. 2007;16:1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x. [DOI] [PubMed] [Google Scholar]
- 52.Langemann U, Gauger B, Klump GM. Auditory sensitivity in the great tit: Perception of signals in the presence and absence of noise. Anim Behav. 1998;56:763–769. doi: 10.1006/anbe.1998.0879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.