Abstract
Stability and repair of DNA is of principal importance in cell survival. Heme oxygenase-1 (HO-1; Hmox1) is critical in maintaining cellular homeostasis, in large part through its ability to generate CO, but neither molecule has been studied in the setting of DNA damage. Naïve Hmox1−/− mice exhibit excessive tissue levels of γ-histone H2A, whereas administration of genotoxic stressors or irradiation in HO-1–deficient cells resulted in loss of ataxia-telangiectasia mutated/ataxia telangiectasia and Rad3-related protein and breast cancer 1, early onset induction with dysfunctional γ-H2AX foci and marked elevations in DNA damage. HO-1 induction or exposure to CO induced homologous recombination-mediated DNA repair through ataxia-telangiectasia mutated/ataxia telangiectasia and Rad3-related protein. In vivo, exposure of mice to CO followed by genotoxin (Adriamycin) or radiation-induced injury led to diminished tissue DNA damage and improved survival. We characterize a joint role for HO-1 and the gasotransmitter CO for appropriate DNA repair and provide a mechanism for their potent cytoprotective effects in various pathologies.
Keywords: chemotherapy, heme degradation, gas biology, cytoprotection
Efficient DNA damage repair and checkpoint mechanisms are critical components of normal cellular function to maintain the integrity of genomic DNA (reviewed in refs. 1 and 2). DNA lesions are induced in response to UV or ionizing radiation as well as many chemicals including endogenous metabolites and reactive oxygen species (ROS) (1). DNA repair pathways include repair of damaged bases or single-strand DNA breaks (base excision repair) and repair of double-strand DNA breaks (DSB) including homologous recombination (HR) and nonhomologous end-joining (NHEJ). The cellular response to DNA DSBs occurs via an integrated sensing and signaling network that maintains and restores genomic stability (3). DSBs are detected initially by damage sensors that trigger the activation of transducing kinases. These transducers amplify the damage signal and relay it to effector proteins, which in turn regulate cell-cycle progression, DNA repair, and apoptosis (4). DSBs are recognized by the Mre11–Rad50–Nbs1 (MRN) mediator complex which recruits ataxia telangiectasia-mutated (ATM) to the broken DNA (4). Cells also are faced with detection of replication blocks, which is mediated by an ssDNA–replication protein A complex recruiting the kinases ataxia-telangiectasia and rad3-related (ATR) and Rad17 (5). Both ATR and ATM can phosphorylate multiple target proteins [p53 binding protein 1, breast cancer 1, early onset (Brca1), checkpoint kinase 1, checkpoint kinase 2 (Chk2), and the MRN complex, among others; more than 700 proteins have been identified so far] that mediate the downstream signaling. Further, in response to DNA damage or structural alterations of chromatin, histone H2A is phosphorylated on Ser139 by ATM, ATR, or by DNA-dependent protein kinases. This phosphorylation allows recruitment of additional DNA damage-responsive proteins and the formation of DNA damage foci, which further accommodate the repair process.
The degradation of heme to biliverdin, CO, and iron is catalyzed by one of two isoforms of heme oxygenase (HO). HO-1 is the inducible isoform that is thought to be a homeostatic and protective gene against various stress-related conditions. The diversity of HO-1 inducers supports the speculation that HO-1 also may play a vital function in maintaining cellular homeostasis. Analysis of HO-1–null (Hmox1−/−) mice and the report of a HO-1–deficient human support the paradigm that HO-1 is an important molecule in defense against oxidant stress (6, 7). Given the agents that induce HO-1 and potentially DNA damage, we hypothesized a role for HO-1 and CO, a product of HO-1 activity, as defense molecules involved in limiting the mutation of DNA and also participating in the DNA-repair process. Evidence that HO-1 was protective was demonstrated in a model of UV-induced cell death driven in part by oxidative stress and UV-induced DNA damage, although DNA repair was not evaluated (8). Expression of HO-1 is increased 40-fold in the lung after irradiation (9) and in the liver after doxorubicin treatment (10), suggesting that HO-1 and CO may be induced rapidly and be involved in repair mechanisms. The levels of HO-1, an accepted marker of oxidative stress, also were elevated significantly in ataxia telangiectasia (A-T) fibroblasts, in which the ATM gene is mutated (11), as well as in the brain of ATM-knockout mice (12). However, a functional role for HO-1 in DNA damage signaling and response has not been studied. Here we describe a function for HO-1, via generation of CO, as an important modulator of DNA repair and identify ATM kinase as a downstream target.
Results
Lack of HO-1 Results in Accumulation of γ-H2AX Foci in Vivo.
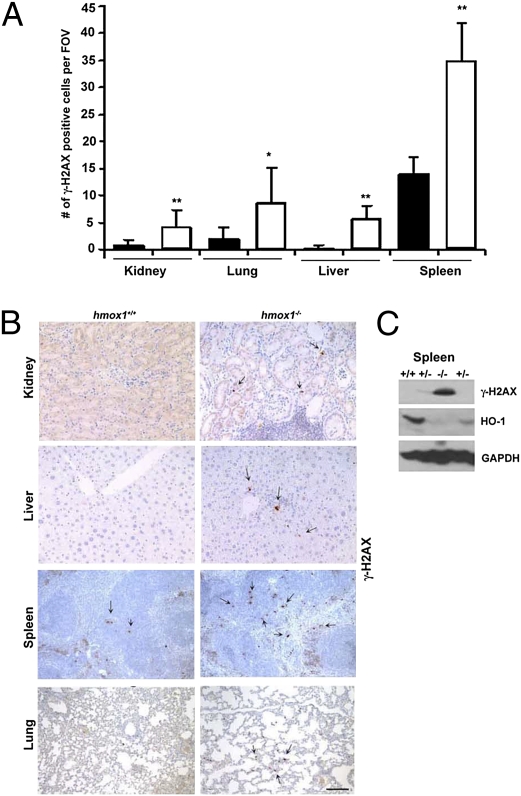
To evaluate the possible role of HO-1 in DNA damage and repair signaling, we performed immunohistochemical analyses of γ-H2AX staining, a marker of ongoing and chronic DNA damage, on various tissues harvested from Hmox1−/− mice. We observed a low degree of γ-H2AX in the spleens and lungs of wild-type animals and nearly undetectable expression in the kidney and liver (Fig. 1 A and B). In contrast, the number of γ-H2AX foci was statistically significantly greater in Hmox1−/− animals than in wild-type controls (Fig. 1 A–C). These data suggested that Hmox1−/− cells are unable to repair broken DNA efficiently or that the extensive oxidative stress in the tissues of Hmox1−/− mice results in accelerated DNA damage.
Fig. 1.
Levels of H2AXγ in tissues from Hmox1−/− mice. (A and B) Immunohistochemical analysis of H2AXγ in kidney, lung, liver, and spleen of wild-type (Hmox1+/+; black bars) and Hmox1−/− mice (white bars). (A) Quantification of the number of H2AXγ foci per field of view. (n = 5–10 fields of view from three or four mice; *P = 0.03; **P < 0.001.) (B) Representative images. (Scale bar: 100 μm.) (C) Immunoblotting with antibodies against H2AXγ and HO-1 in the spleen lysates of wild-type (+/+), Hmox1+/− (+/−), and Hmox1−/− (−/−) mice. Western blot shown is representative of two experiments.
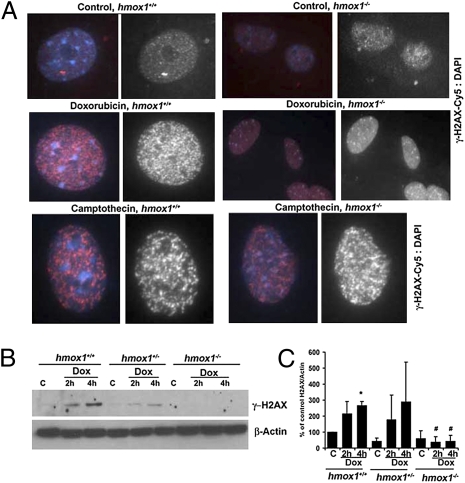
To elucidate whether absence of HO-1 results in accelerated accumulation of DNA damage, we treated cells with doxorubicin or camptothecin, which induce DSBs or single-strand DNA breaks, respectively, as measured by H2AX phosphorylation (Fig. 2). Treatment of Hmox1+/+ fibroblasts with doxorubicin or camptothecin resulted in a strong increase in phosphorylation of histone H2AX and formation of multiple γ-H2AX foci (Fig. 2 A and B). Fibroblasts from Hmox1−/− mice also showed increased foci in response to camptothecin, similar to the response in wild-type cells (Fig. 2A), but unlike wild-type cells, Hmox1−/− fibroblasts treated with doxorubicin showed no γ-H2AX foci formation or phosphorylation of γ-H2AX at any time point tested (Fig. 2 A–C). These data suggested that HO-1 may mediate DNA repair responses specifically to DSBs that are induced by doxorubicin but not those induced by camptothecin, which targets DNA topoisomerase I.
Fig. 2.
Lack of HO-1 results in altered H2AXγ activation in response to DNA damage. (A) Fibroblasts from Hmox1+/+ and Hmox1−/− mice were isolated and treated with camptothecin (1 μg/mL) or doxorubicin (10 μg/mL) for 1 h and stained with antibodies against H2AXγ. Images are shown representative of at least two independent experiments conducted in duplicate. (Scale bar: 25 μm.) (B and C) Immunoblotting analysis of H2AXγ in the lysates of Hmox1+/+, Hmox1−/+, and Hmox1−/− fibroblasts treated with doxorubicin (10 μg/mL) for 2–4 h. (B) Representative immunoblot. (C) Quantitation. Data are representative of at least two independent experiments. *P < 0.05, Hmox1+/+ 4-h treatment versus control; #P < 0.05, Hmox1−/− versus Hmox1+/+.
Absence of HO-1 Results in Decreased DNA Repair Signaling.
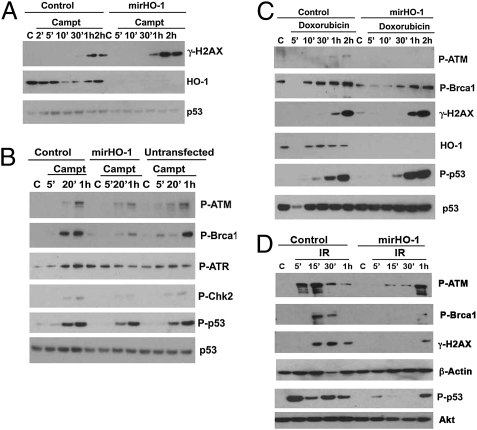
Next we tested the direct effects of HO-1 on signaling pathways in response to the DNA-damaging agents doxorubicin or camptothecin or irradiation in loss- and gain-of-function studies with HO-1. HEK293T (HEK) cells express high basal levels of HO-1 because of the presence of large T antigen. HEK cells were transduced with microRNA-adapted shRNA against HO-1 (mirHO-1) to deplete HO-1 stably and then were exposed to camptothecin, doxorubicin, or irradiation. As expected, camptothecin induced phosphorylation of H2AX in wild-type HEK cells, and this phosphorylation was enhanced slightly in cells without HO-1 (Fig. 3A). These findings correlated with phosphorylation of H2AX observed in the tissues from Hmox1−/− mice (Fig. 1). We next evaluated the status of the major DNA repair kinases, ATR and ATM, and their downstream targets in HEK cells treated with camptothecin. Phosphorylation of ATR, ATM, p53, Chk2, and Brca1 was relatively unchanged in stable latent membrane protein (LMP)-infected HEK control cells compared with nontransfected naïve HEK cells (Fig. 3B). However, knockdown of HO-1 resulted in a significant decrease in phosphorylation of ATM, Brca1, ATR, and Chk2 (Fig. 3B). Importantly, there were no significant changes in phosphorylation of p53, suggesting that HO-1 may be specific and important in DNA repair rather than regulating apoptosis or cell-cycle progression in the presence of a genotoxic stressor.
Fig. 3.
DNA repair signaling in the absence of HO-1. (A and B) HEK293 cells with stable knockdown of HO-1 (mirHO-1) and control cells were treated with camptothecin (1 μg/mL) for 2 min to 2 h. The activation levels of HO-1 and DNA repair signaling proteins were measured by Western blot. (C) Immunoblot analysis in HEK mirHO-1 and control cells treated with doxorubicin (10 μg/mL) for 5 min to 2 h. Data are representative of two experiments. (D) Immunoblot analysis with antibodies against P-ATM, P-Brca1, P-H2AX, and P-p53 in the lysates of HEK293 mirHO-1 and control cells treated with 10 Gy γ-irradiation and harvested 5 min to 1 h after irradiation. Akt is a loading control for the immunoblot for P-p53. Data are representative of two independent experiments conducted in triplicate.
HEK cells treated with doxorubicin responded similarly, with an increase in phosphorylation of ATM, p53, and H2AX (Fig. 3C). Knockdown of HO-1 under these conditions resulted in inhibition in phosphorylated ATM and a delay in Brca1 activation. We did not observe any significant changes in phosphorylated p53 (P-p53) or γ-H2AX in control or mirHO-1–transfected HEK cells in the absence of doxorubicin. Formation of γ-H2AX foci or DNA repair signaling in response to camptothecin or doxorubicin in HEK mirHO-1 cells versus control cells was not ROS dependent (Fig. S1A). Doxorubicin treatment, unlike camptothecin treatment was associated with superoxide production (Fig. S1A), but we did not observe any major differences in ROS generation between mirHO-1 and control HEK cells in response to the two agents (Fig. S1A). There was no significant difference in cell-cycle progression between mirHO-1 HEK and control HEK cells (Fig. S1B) or in the total levels of major DNA repair complexes involved in HR-mediated DNA repair (Fig. S2A).
Further, we investigated the effects of irradiation on DNA in HEK cells in the presence and absence of HO-1 after irradiation (10 Gy). The phosphorylation levels of ATM, Brca1, H2AX, and p53 were significantly decreased or delayed in mirHO-1 HEK cells compared with controls (Fig. 3D).
HO-1/CO Facilitates HR Repair of DSBs.
Because Hmox1−/− cells showed altered H2AXγ foci formation in response to doxorubicin, and mirHO-1 HEK cells showed diminished activation of major signaling cascades leading to DNA repair in response to camptothecin or irradiation, we next assessed the role of HO-1 in HR-mediated DNA repair, which is critical in repair of DSBs induced by doxorubicin, irradiation, or camptothecin (during replication). We used the U20S cell line containing the homologous recombination/sister chromatid recombination (HR/SCR) reporter as previously described (13, 14). I-SceI endonuclease was used to introduce a DSB within the reporter in U2OS cells. The efficiency of HR induced by I-SceI correlated with generation of wild-type GFP by gene conversion (15).
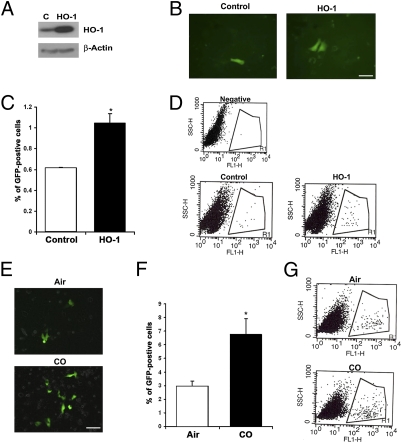
To test the role of HO-1 in DSB, we transiently overexpressed HO-1 together with I-SceI plasmid in U2OS cells and tested the number of cells positive for GFP 24 h posttransfection (Fig. 4 A–C). We observed a significant, twofold increase in GFP levels in cells overexpressing HO-1 as assessed by fluorescence microscopy and flow cytometry (Fig. 4 B–D),whereas nontransfected control cells and vector-transfected control cells showed a low frequency of GFP positivity, indicating poor repair (Fig. 4 B–D). These data support a role for HO-1 in the induction of HR in response to DSBs.
Fig. 4.
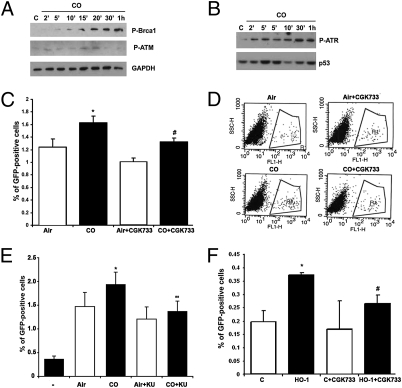
HO-1/CO induces HR-mediated DNA repair. (A) U20S-SCR reporter cells were cotransfected transiently with SceI and HO-1 or control plasmid. HO-1 levels were measured by Western blot. (B–D) The number of GFP+ U20S-SCR reporter cells was measured by fluorescence microscopy (B) and flow cytometry (C and D). Cells were transfected with HO-1 construct, and the number of GFP+ cells was measured 48 h after transfection. Data shown are ± SD and are representative of at least five experiments conducted in duplicate. *P < 0.05, CO versus air. (Scale bar in B: 25 μm.) (E–G) U2OS-SCR reporter cells after transfection with SceI for 24 h were treated with CO (250 ppm) for 24 h. The level of GFP+ cells was measured by fluorescence microscopy (E) or flow cytometry (F and G). Data shown are averages ± SD of three independent experiments. (Scale bar: 25 μm.)
The majority, if not all, of the effects observed with HO-1 are mediated by one or more of its enzymatic products, biliverdin or CO. Therefore, we tested the role of biliverdin and CO separately in HR during DSB and DNA repair. Biliverdin treatment had no effect on the number of GFP+ cells, but CO (250 ppm) significantly increased the number of GFP+ cells (Fig. 4 E–G), suggesting that HO-1 acts in part via CO to regulate DNA repair pathways and facilitate HR in response to DNA damage. Importantly, we did not observed any effects of CO on NHEJ-mediated DNA repair as measured by the number of XHATM-resistant colonies (Fig. S2B).
DNA Repair Responses Modulated by CO/HO-1 Are Dependent on ATM/ATR Activity.
CO induced rapid phosphorylation of Brca1 as well as the upstream kinases ATR and ATM (Fig. 5 A and B), suggesting that CO induces DNA repair pathways in cells that are constantly under risk of oxidative DNA damage. Therefore, we next asked if the two major DNA repair kinases, ATM and ATR, are implicated in accelerated HR in the presence of CO or HO-1. We used the selective inhibitor of ATM and ATR kinases, CGK733, and the more selective inhibitor of ATM, KU55933, to test this hypothesis. Blockade of ATM/ATR or ATM alone significantly decreased CO-mediated HR (Fig. 5 C–E). Further, induction of HO-1 did not increase HR in the presence of CGK733, as otherwise observed in vehicle controls (Fig. 5F and Fig. S2C), strongly suggesting that both ATR and ATM are used by CO/HO-1 in part to regulate HR and DNA repair following damage.
Fig. 5.
CO induces activation of DNA repair signaling. (A and B) Immunoblotting with antibody against P-Brca1 and P-ATM (A) and P-ATR (B) in the lysates of PC3 and HEK cells treated with CO (250 ppm) for 2 min to 1 h. Data are representative of three independent experiments. (C and D) U20S-SCR reporter cells were transfected with SceI for 24 h and treated with CGK733 (10 μM) or DMSO for 1 h before treatment with CO (250 ppm) for 24 h. The level of GFP+ cells was measured by flow cytometry. Quantitation and representative dot plot figures are shown in C and D, respectively. *P < 0.05, CO versus air; #P < 0.05, CO + CGK733 versus CO. (E) U20S-SCR reporter cells were cotransfected with SceI for 24 h and KU55933 (20 μM) or DMSO for 1 h before treatment with CO (250 ppm) for 24 h. The level of GFP+ cells was measured by flow cytometry. *P < 0.05, CO versus air; **P < 0.01, CO + KU55933 versus CO; (−), untransfected cells. (F) U20S-SCR reporter cells were cotransfected with SceI and HO-1 for 24 h; then CGK733 was applied for 24 h. The level of GFP+ cells was measured by flow cytometry. Data are representative of three independent experiments conducted in duplicate. *P < 0.05, HO-1 versus control (C), #P < 0.05, HO-1 + CGK733 versus HO-1.
CO Protects Against DNA Damage in Vivo in Response to Chemotherapy or Radiation.
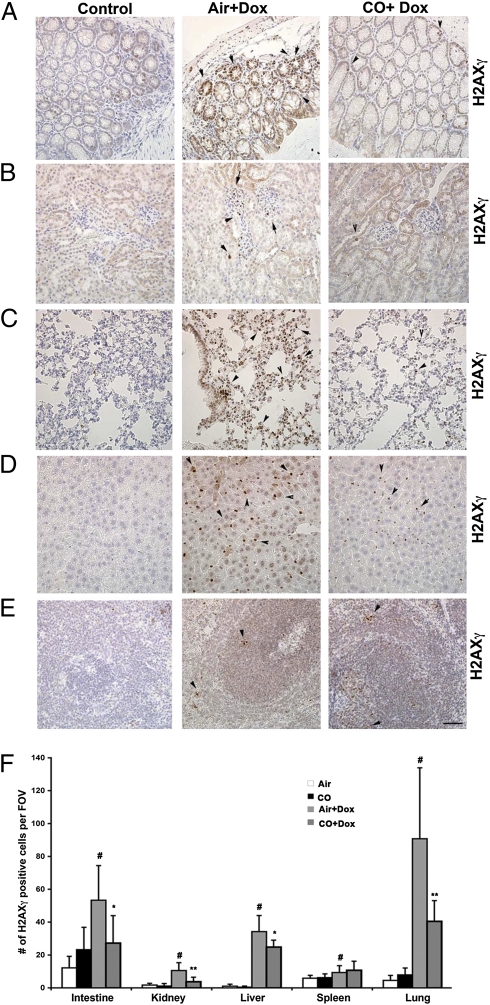
Based on the effects observed in vitro, we next tested the salutary effects of CO in an in vivo model of DNA damage in mice. We used doxorubicin-induced DNA damage in the tissues during chemotherapeutic treatment of tumor xenografts in nude mice. Doxorubicin led to substantial DNA damage in the lung, liver, kidney, and colon and, to a lower extent, in the spleen as determined by accumulation of H2AXγ foci and was associated with increased expression of HO-1 (Fig. 6 A–E and Fig. S3). CO significantly decreased the severity of DNA damage in all organs (Fig. 6 A–F).
Fig. 6.
CO blocks DNA damage in tissues of mice treated with doxorubicin (Dox). (A–E) Immunohistochemistry with antibody against H2AXγ was performed in colon (A), kidney (B), lung (C), liver (D), and spleen (E) tissue. Tissues from nude mice with established PC3 tumors (2 wk) were harvested 14 d after treatment with CO (control) or doxorubicin (8 mg/kg i.v., twice per week) ± CO (250 ppm, 1 h, daily). (Magnification: 40×.) (F) Quantitation of H2AXγ staining in the tissues. Air or CO, n = 3; doxorubicin ± CO, n = 5. Data shown are the number (average ± SD) of H2AXγ+ cells per field of view. (Magnification: 20× for colon and kidney; 40× for spleen, liver, and lung.) #P < 0.05, doxorubicin versus control; *P < 0.05, CO + doxorubicin versus air + doxorubicin; **P < 0.001, CO + doxorubicin versus air + doxorubicin.
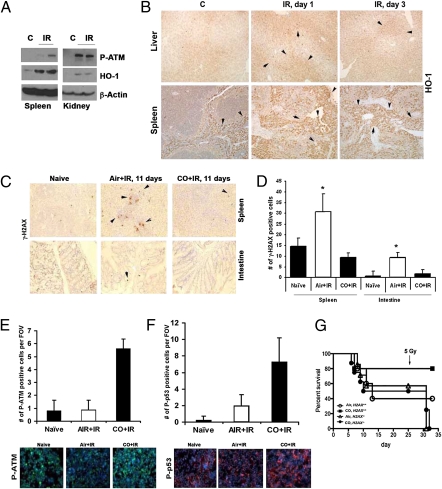
To support the observations with chemical genotoxins, we also used a radiation-induced model of DNA damage. Endogenous HO-1 is induced early in response to irradiation (IR) or doxorubicin, and its expression is sustained for 24–72 h in the spleen, liver, and kidney (Fig. 7 A and B and Fig. S3). To evaluate the role of exogenous CO, mice were pretreated with CO for 1 h before either a lethal or sublethal dose of irradiation and then were treated with CO for l h daily. We observed a chronic elevation of H2AXγ foci in the spleen and intestine of irradiated mice (Fig. 7 C and D). In contrast, treatment of mice with CO led to significant inhibition in sustained H2AXγ foci formation that corresponded to a longer survival rate, with 80% of irradiated, CO-treated mice alive >10 d vs. 50% of air-treated animals (P = 0.031, CO versus air; n = six mice per group). There was a strong induction of P-p53, phosphorylated Brca1 (P-Brca1), and phosphorylated ATM (P-ATM) and early induction of phosphorylated H2AX (P-H2AX), suggesting early resolution of DNA damage, in peripheral blood mononuclear cells and bone marrow (BM) of irradiated animals treated with CO for 1 h compared with air-treated controls (Fig. 7 E and F and Fig. S4 A–F). To evaluate the role of H2AX signaling in CO effects on DNA repair, we used marginal BM transplantation of BM cells from H2ax−/− and H2ax+/+ mice into wild-type irradiated recipients. Lack of H2AX in donor BM cells reversed CO-mediated protection against irradiation-induced death, suggesting a critical role of the H2AX pathway in CO effects (Fig. 7G).
Fig. 7.
CO decreased tissue damage induced by lethal-dose irradiation. (A and B) Immunoblot (A) and immunohistochemistry (B) with antibody against HO-1 in tissues from mice irradiated with 10 Gy. (A) Kidneys and spleen harvested 2 h after irradiation. (B) Liver and spleen harvested 1 and 3 d postirradiation. (C and D) Immunohistochemical analysis of P-H2AX in the spleens and intestines of mice pretreated with air or CO (250 ppm) 1 h before a lethal dose of irradiation (10 Gy) and treated daily with CO for 11 d. Representative images are shown in C, and quantitation of the number of γ-H2AX+ cells is shown in D. n = 3 or 4 views per section from three or four mice per group. *P < 0.05. (E and F) Immunofluorescence staining of P-ATM and P-p53 in mononuclear blood cells of mice treated with CO or air for 1 h before lethal irradiation. Tissues were harvested 2 h after irradiation. Representative images (Lower) and quantitation of the number of positive cells per field of view (Upper) are shown. n = 3 or 4 mice group. (G). Survival of mice after marginal BM transplantation from H2ax−/− and H2ax+/+ mice to wild-type recipients. Mice were treated with CO before a lethal dose of irradiation (10 Gy) and after receiving BM. n = 5–10 mice per group.
Discussion
We demonstrate that HO-1 and CO, both well-characterized salutary molecules, are critically important in the ability of the cell to elicit a repair response after damage to DNA. Indeed, it is possible that the ability of HO-1 and CO to regulate DNA stability underlies their cytoprotective effects in the numerous reports describing their protective capabilities. Here we show that HO-1 and CO are involved directly in the process of DNA repair and describe a mechanism through which the HO-1/CO system acts to limit accumulation of DNA mutations and apoptotic responses in normal cells exposed to oxidative stress or chemotherapeutics. This mechanism is best evidenced in tissues from Hmox1−/− mice, which are characterized by deposition of iron (6) and elevated levels of ROS that probably contribute to DNA damage.
In the original characterization of the Hmox1−/− mice by Poss et al. (6), the mice were described as exhibiting a very low weight and a significant reduction in life span that, to date, remains unexplained. Hmox1−/− mice (8- to 12-mo-old) showed high levels of γ-H2AX foci in all tissues analyzed as compared with aged-matched control mice. The incidence of γ-H2AX foci is significantly elevated in tissues of aged mice as well as in patients who have Werner or Bloom syndrome of premature aging, genomic instability, or who are at an increased risk of cancer. Loss of one copy of H2AX leads to genomic instability (16). Hmox1−/− animals do not show typical premature aging characteristics, but >90% of these animals die in utero. Accelerated ROS generation in Hmox1−/− tissues may be responsible for DNA damage but does not explain why the γ-H2AX foci accumulate and DNA breaks are not repaired.
DNA DSBs are the most cytotoxic lesions and must be repaired for survival. If the damage becomes too extensive, programmed cell death machinery is activated to initiate apoptosis. HO-1–deficient fibroblasts do not form functional γ-H2AX foci in response to the DSB inducer and topoisomerase II poison, doxorubicin, although the presence of H2AX foci in response to camptothecin is not altered. Doxorubicin-treated cells from Hmox1−/− mice are more prone to apoptosis than cells from wild-type mice (17), probably because of abnormalities in the DNA damage response. We determined that HO-1, acting in part through generation of its product CO, directly regulates DNA DSB repair. Lack of HO-1 under conditions of mild to moderate oxidative stress leads to the accumulation of DNA lesions in susceptible tissues such as the lung, liver, and spleen. These organs, in which cellular turnover and metabolic demands are great, are exposed to an increased oxidative burden. Under such conditions, the inability to express HO-1 and produce CO results in increased sensitivity to damage and inappropriate repair, ultimately leading to higher mutation rates.
Lack of HO-1 resulted in pronounced inhibition of ATM phosphorylation and its downstream target Brca1 in response to camptothecin, doxorubicin, or IR treatment. In response to DSB, ATM is activated and phosphorylates a wide number of downstream target proteins to coordinate the appropriate cellular responses, including DNA repair. ATM is missing or inactivated in patients with the genetic disorder A-T, characterized by immunodeficiency, radiation sensitivity, chromosomal instability, and cancer predisposition (18). HO-1 is basally expressed in A-T fibroblasts. A functional MRN complex is required for ATM activation and, consequently, timely activation of ATM-mediated signaling pathways (19). We speculate that HO-1 may be a more proximal signaling molecule acting in part via generation of CO, which induces ATM-dependent DNA repair. CO has been shown to activate signaling pathways that involve hemoproteins such as guanylate cyclase, nitric oxide synthase, and the mitochondrial cytochromes and also has been shown to influence the activation of the nonheme signaling molecules including p38, MAPK, STAT3, peroxisome proliferator-activated receptor-γ, and hypoxia-inducible transcription factor-1α (20, 21). ATM phosphorylation in response to ischemia reperfusion is severely impaired with blockade of the MAPK pathway (22), which is a well-described target for HO-1/CO (23). Importantly, inhibition of the MAPK–ERK pathway reduced the levels of phosphorylated ATM foci, but not γ-H2AX (22), and cells lacking STAT3 showed decreased DNA repair through ATM and ATR (24). We speculate that CO targets the DNA structure either directly or by binding to the metal ions on DNA and thereby influences the function of DNA-associated enzymes. Indeed, one of the nuclear targets of CO may be the heme-binding, RNA-binding protein DiGeorge critical region-8 (25) that has been linked to the DNA damage-associated functions of p53 (26).
Overexpression of HO-1 or treatment with exogenous CO accelerated DNA repair. A GT length-promoter polymorphism for HO-1 has been described that controls the level of HO-1 expression and has been shown to be highly correlative with severity of disease, including cancer (27). Individuals with a long (GT)n repeat, which is associated with low expression levels of HO-1, showed a higher frequency of gastric or lung adenocarcinoma (28) and oral squamous cancer (29) than individuals with short GT repeats and higher HO-1 expression. Additionally, application of CO via a CO-releasing molecule provided a significant inhibition in photocarcinogenesis in mice (30). Early studies by Tyrrell and coworkers (31) elucidated the protective capabilities of HO-1 using a model of UV irradiation. Conclusions made in these reports pioneered the investigation of HO-1 as a cytoprotective gene. Our studies build on this work and provide a potential mechanism of action for HO-1 in addition to its broad anti-inflammatory activity.
In conclusion, we describe a mechanism by which HO-1 and CO promote cell survival and maintain homeostasis. Low or inappropriate HO-1 expression and activity in a prooxidant environment leads to genomic instability, accelerated DNA damage, poor DNA repair, and potentially to the development of disease pathologies. We demonstrate that HO-1 modulates the cellular response to DSBs in genomic DNA via CO and thus probably is involved in preventing cancerogenesis and premature aging.
Materials and Methods
Animals, Irradiation and CO Treatment.
Male C57BL/6 mice 7–9 wk of age were purchased from Jackson Laboratories. H2ax−/− and control littermates were kindly provided by Fred Alt and Shan Zhan (Children's Hospital, Boston, MA). Mice were held under specific pathogen-free conditions, and the experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Beth Israel Deaconess Medical Center (BIDMC). Mice were irradiated with 10 Gy (lethal dose) or 5 Gy (sublethal dose) in a protocol approved by the Radiation Safety Officer at BIDMC and the IACUC. Mice in a Plexiglas chamber were exposed to CO (250 ppm) for 1 h before irradiation and thereafter for 1 h every day as described previously (32).
HR/SCR Reporter Assay, Flow Cytometry, and Fluorescence Microscopy.
HR/SCR reporter U20S cells were used as previously described (14, 15). Briefly, GFP+ cells were analyzed 2 d posttransfection by flow cytometry (FACScan; BD Biosciences) and fluorescence microscopy. Images of GFP+ cells in randomized fields were captured using a Zeiss Apotome fluorescent microscope.
Statistical Analyses.
The significance of differences was determined using ANOVA or student's t test (SPSS Inc.) with significance accepted at P < 0.05. For survival analysis, the log rank Mantel–Cox test was applied.
Supplementary Material
Acknowledgments
We thank Dr. Claudia Stenstrom, the Radiation Safety Officer at Beth Israel Deaconess Medical Center (BIDMC; Boston, MA), for help with irradiation experiments; Drs. Fred Alt (BIDMC) and Shan Zha (Columbia University, New York, NY) for the kind gift of H2ax−/− mice; Drs. Miguel Soares, Rasmus Larsen, and Sofia Rebelo (Instituto Gulbenkian de Ciência, Portugal) for providing tissues and cells from hmox1−/− mice; Drs. Ralph Scully and Anyong Xie (BIDMC, Boston) for providing HR/SCR cells; and Dr. Henning Willers (Massachusetts General Hospital, Boston, MA) for providing NHEJ reporter cells. We thank the Julie Henry Fund at the Transplant Institute of the BIDMC for continued support. This work was supported in part by National Institutes of Health Grant R01GM088666-01 (to L.E.O.) and American Heart Association Grant 10SDG2640091 (to B.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1102295108/-/DCSupplemental.
References
- 1.Lombard DB, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 2.Garinis GA, van der Horst GT, Vijg J, Hoeijmakers JH. DNA damage and ageing: New-age ideas for an age-old problem. Nat Cell Biol. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips ER, McKinnon PJ. DNA double-strand break repair and development. Oncogene. 2007;26:7799–7808. doi: 10.1038/sj.onc.1210877. [DOI] [PubMed] [Google Scholar]
- 4.Nussenzweig A. Causes and consequences of the DNA damage response. Cell Cycle. 2007;6:2339–2340. doi: 10.4161/cc.6.19.4995. [DOI] [PubMed] [Google Scholar]
- 5.Harper JW, Elledge SJ. The DNA damage response: Ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yachie A, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyrrell RM. UV activation of mammalian stress proteins. EXS. 1996;77:255–271. [PubMed] [Google Scholar]
- 9.Risom L, Møller P, Vogel U, Kristjansen PE, Loft S. X-ray-induced oxidative stress: DNA damage and gene expression of HO-1, ERCC1 and OGG1 in mouse lung. Free Radic Res. 2003;37:957–966. doi: 10.1080/1071576031000150788. [DOI] [PubMed] [Google Scholar]
- 10.Ito K, Ozasa H, Sanada K, Horikawa S. Doxorubicin preconditioning: A protection against rat hepatic ischemia-reperfusion injury. Hepatology. 2000;31:416–419. doi: 10.1002/hep.510310222. [DOI] [PubMed] [Google Scholar]
- 11.Lee SA, Dritschilo A, Jung M. Role of ATM in oxidative stress-mediated c-Jun phosphorylation in response to ionizing radiation and CdCl2. J Biol Chem. 2001;276:11783–11790. doi: 10.1074/jbc.M004517200. [DOI] [PubMed] [Google Scholar]
- 12.Barlow C, et al. Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs. Proc Natl Acad Sci USA. 1999;96:9915–9919. doi: 10.1073/pnas.96.17.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie A, et al. Control of sister chromatid recombination by histone H2AX. Mol Cell. 2004;16:1017–1025. doi: 10.1016/j.molcel.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puget N, Knowlton M, Scully R. Molecular analysis of sister chromatid recombination in mammalian cells. DNA Repair (Amst) 2005;4:149–161. doi: 10.1016/j.dnarep.2004.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie A, et al. Distinct roles of chromatin-associated proteins MDC1 and 53BP1 in mammalian double-strand break repair. Mol Cell. 2007;28:1045–1057. doi: 10.1016/j.molcel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celeste A, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371–383. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford TO. Ataxia telangiectasia. Semin Pediatr Neurol. 1998;5:287–294. doi: 10.1016/s1071-9091(98)80007-7. [DOI] [PubMed] [Google Scholar]
- 19.Uziel T, et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin BY, et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci USA. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilban M, et al. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity. 2006;24:601–610. doi: 10.1016/j.immuni.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Golding SE, et al. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046–1053. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 23.Otterbein LE, et al. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury. Am J Pathol. 2003;163:2555–2563. doi: 10.1016/S0002-9440(10)63610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barry SP, et al. STAT3 modulates the DNA damage response pathway. Int J Exp Pathol. 2010;91:506–514. doi: 10.1111/j.1365-2613.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faller M, Matsunaga M, Yin S, Loo JA, Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol. 2007;14:23–29. doi: 10.1038/nsmb1182. [DOI] [PubMed] [Google Scholar]
- 26.Ory B, et al. A microRNA-dependent program controls p53-independent survival and chemosensitivity in human and murine squamous cell carcinoma. J Clin Invest. 2011;121:809–820. doi: 10.1172/JCI43897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kikuchi A, et al. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase-1 gene promoter polymorphism. Hum Genet. 2005;116:354–360. doi: 10.1007/s00439-004-1162-2. [DOI] [PubMed] [Google Scholar]
- 28.Lo SS, et al. Heme oxygenase-1 gene promoter polymorphism is associated with risk of gastric adenocarcinoma and lymphovascular tumor invasion. Ann Surg Oncol. 2007;14:2250–2256. doi: 10.1245/s10434-006-9290-7. [DOI] [PubMed] [Google Scholar]
- 29.Chang KW, et al. Polymorphism in heme oxygenase-1 (HO-1) promoter is related to the risk of oral squamous cell carcinoma occurring on male areca chewers. Br J Cancer. 2004;91:1551–1555. doi: 10.1038/sj.bjc.6602186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allanson M, Reeve VE. Carbon monoxide signalling reduces photocarcinogenesis in the hairless mouse. Cancer Immunol Immunother. 2007;56:1807–1815. doi: 10.1007/s00262-007-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeve VE, Tyrrell RM. Heme oxygenase induction mediates the photoimmunoprotective activity of UVA radiation in the mouse. Proc Natl Acad Sci USA. 1999;96:9317–9321. doi: 10.1073/pnas.96.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegiel B, et al. Nitric oxide-dependent bone marrow progenitor mobilization by carbon monoxide enhances endothelial repair after vascular injury. Circulation. 2010;121:537–548. doi: 10.1161/CIRCULATIONAHA.109.887695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.