Abstract
Body size profoundly affects many aspects of animal biology, including metamorphosis, allometry, size-dependent alternative pathways of gene expression, and the social and ecological roles of individuals. However, regulation of body size is one of the fundamental unsolved problems in developmental biology. The control of body size requires a mechanism that assesses size and stops growth within a characteristic range of sizes. Under normal growth conditions in Manduca sexta, the endocrine cascade that causes the brain to initiate metamorphosis starts when the larva reaches a critical weight. Metamorphosis is initiated by a size-sensing mechanism, but the nature of this mechanism has remained elusive. Here we show that this size-sensing mechanism depends on the limited ability of a fixed tracheal system to sustain the oxygen supply to a growing individual. As body mass increases, the demand for oxygen also increases, but the fixed tracheal system does not allow a corresponding increase in oxygen supply. We show that interinstar molting has the same size-related oxygen-dependent mechanism of regulation as metamorphosis. We show that low oxygen tension induces molting at smaller body size, consistent with the hypothesis that under normal growth conditions, body size is regulated by a mechanism that senses oxygen limitation. We also found that under poor growth conditions, larvae may never attain the critical weight but eventually molt regardless. We show that under these conditions, larvae do not use the critical weight mechanism, but instead use a size-independent mechanism that is independent of the brain.
Most species of animals experience determinate growth and stop growing within a characteristic range of body size (1–4). This raises the question of how body size is sensed such that it is regulated within this range. How body size is sensed remains one of the fundamental unsolved problems in developmental biology.
In insect larvae, the signal to stop growing and initiate a molt is the secretion of the steroid hormone ecdysone. Larval-larval molts are caused by pulses of ecdysone, and in the last larval stage of the tobacco hornworm, Manduca sexta, ecdysone causes the larva to stop feeding and initiate metamorphosis (5). The timing of ecdysone secretion is determined by the critical weight (6–9), a size threshold at which the endocrine events that eventually result in the cessation of feeding, entry into the wandering stage, and metamorphosis are initiated. The discovery of the critical weight demonstrated that initiation of the metamorphic molt depends not on instar duration or growth rate, but rather on size.
The time course of these endocrine events is independent of further nutrition and growth. Therefore, the critical weight can be operationally assessed as the weight at which nutrition is no longer necessary for a normal time course to the cessation of feeding, entry into the wandering stage, and the pupal molt (5, 6, 9). The weight that we measure is a proxy for a physiological variable that is correlated with mass, such as the volume of metabolizing tissues. Studies of genetic strains with different adult body sizes have shown that the critical weight in the last (fifth) larval instar is proportional to the initial weight of that instar (6).
Results and Discussion
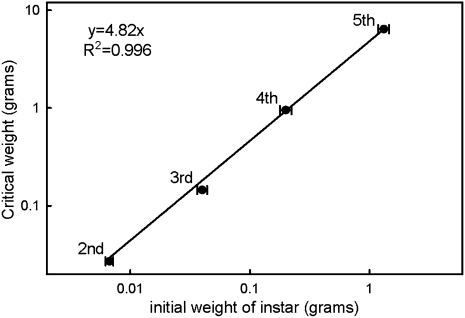
Here we report that under normal rearing conditions, larval-larval molts are also initiated based on a size-sensing mechanism. Regressing the critical weight of the instars on the initial size of the instar gives a regression coefficient of 4.82 (r2 = 0.996), demonstrating that in each instar, the critical weight is 4.82 times the initial size of the instar (Fig. 1). This suggests that there is a common mechanism in every instar that triggers an ecdysone event (a larval-larval molt or cessation of feeding, followed by the pupal molt) once a particular size is reached, and that this critical size is measured relative to some property that is set at the beginning of each instar.
Fig. 1.
Critical weights are proportional to the initial weight of the instar. Critical weights were estimated by means of starvation experiments. Bars are SDs.
The most obvious candidate properties are those associated with the new external skeleton that is deposited at each molt. In caterpillars, the soft exoskeleton of the body wall is not fixed in size but rather grows throughout the instar; thus, it is not a good candidate for limiting growth (10). Although the head capsule does not grow and thus could constrain growth by limiting the food ingestion rate, absorption in the gut, not the ingestion rate, is the rate-limiting step for growth in M. sexta (11). The spiracles and tracheal system deposited at each molt could constrain growth by limiting oxygen delivery. A large body of work shows that oxygen levels influence body size in insects (12–19). Hypoxia not only reduces body size (18), but also constrains the evolution of increased body size by limiting the variation available to selection (16).
In the late stage of each larval instar, the respiration rates of M. sexta caterpillars come close to the maximum oxygen delivery rate allowed by the tracheal system of that instar (20). Similar constraints have been observed in grasshoppers (21). Given that the tracheae could be limiting oxygen supply, and that insects seem to be nearing the maximum delivery rate of oxygen, we hypothesized that critical weight in each instar could be the size at which oxygen delivery becomes compromised.
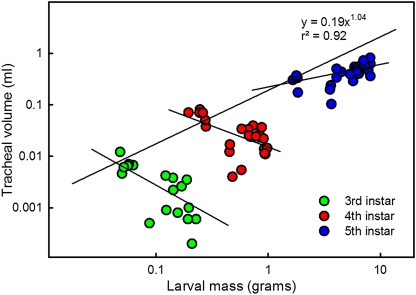
We measured the volume of the tracheal system and found that it does not increase during the course of each instar, but instead increases discretely at each molt (Fig. 2). The methods that we used did not permit assessment of the tracheoles, which might proliferate during the intermolt period; however, the tracheoles do not become functional until the following molt. We found that the tracheal volume at the beginning of each instar scaled with an exponent of 1.04 with respect to the initial mass of the instar (Fig. 2). Thus, the tracheal system scales essentially isometrically with total body volume at the beginning of each successive instar.
Fig. 2.
The tracheal system does not grow within each instar, but increases in size discretely at each molt. There is an apparent slight decrease in tracheal volume during the third and fourth instars. Regression line is for the initial volumes in the instar for data points between 6 and 12 h after ecdysis. Each point represents a single individual.
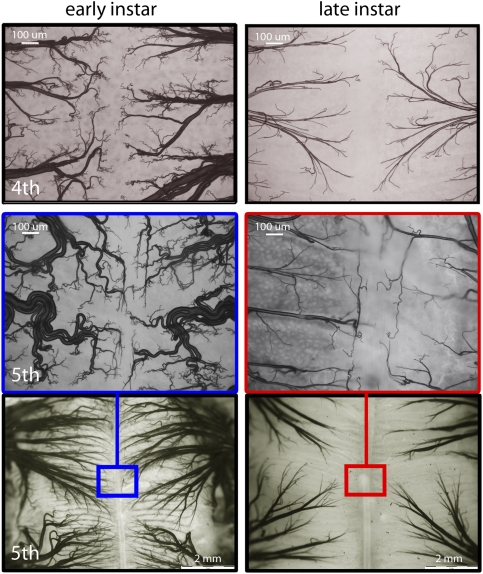
Early in each instar, the tracheae are convoluted and thus have built-in slack to allow extension of the tracheal tubes as the body grows (Fig. 3). As the larval body grows, the tracheal system becomes more sparsely distributed (Fig. 3), because the same tracheal distribution network must supply a much larger volume of tissue. Tracheal size set at the beginning of the instar should limit respiration rates as body mass increases within instars.
Fig. 3.
The tracheal system does not grow within each instar, but the body does. Photos show the distribution of the tracheal network along the midline of the midgut at the beginning and end of the fourth (Top) and fifth (Middle and Bottom) larval instars.
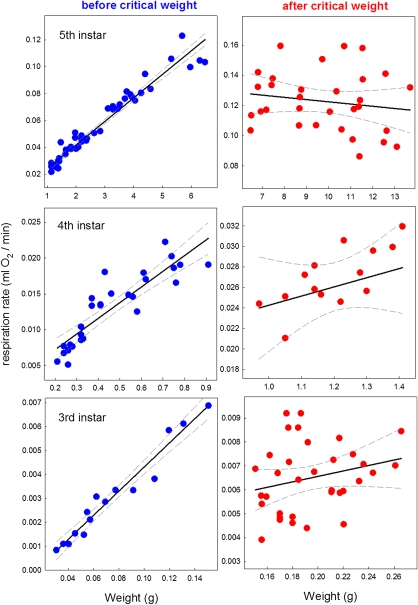
We measured the respiration rate of third, fourth, and fifth instar larvae and found that it initially increased linearly with body mass up to when the larvae reached the critical weight, after which it remained constant (Fig. 4), possibly because it was close to the maximum oxygen delivery rate permitted by the dimensions of the tracheal systems. At the critical weight, oxygen delivery limits growth; indeed, the growth rate of M. sexta larvae was found to follow an sigmoid trajectory with the inflection point at the critical weight, after which the growth rate gradually declines (6). Moreover, Greenlee and Harrison (9) found that late-instar Manduca caterpillars were not able to maintain an adequate oxygen supply at lower oxygen tensions. Thus, late-instar caterpillars consume oxygen at the maximum oxygen delivery rate permitted by the dimensions of their tracheal systems. These findings suggest that in each instar, the larvae have an oxygen delivery ceiling imposed by the constant dimension of the tracheal system, and that the mechanism that senses this ceiling defines the critical weight.
Fig. 4.
Respiration rate levels off after the critical weight. The rate of respiration was determined for larvae throughout the growth period of the third (Top), fourth (Middle), and fifth (Bottom) larval instars. Linear regressions (solid lines) and 95% confidence limits (dashed lines) are shown. The slopes of the regressions after the critical weight are not significantly different from 0 (third instar, P = 0.326; fourth instar, P = 0.319; fifth instar, P = 0.381).
If oxygen supply is limiting, then the constraint should be lifted when larvae are exposed to hyperoxia. To test this hypothesis, we measured the respiration rate of fourth instar larvae, above the critical weight, that were switched from normoxic to hyperoxic conditions and found that their rate of oxygen consumption increased by 36.9 ± 6.2% (SEM, n = 6; P = 0.0019, t test).
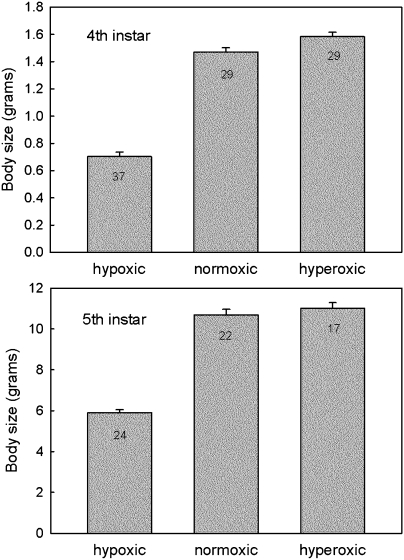
We reasoned that if the critical weight indeed corresponded to the size at which the oxygen supply becomes limiting, then it should be possible to alter the critical weight by changing the oxygen tension in the environment of the larva. We also found that fourth instar larvae kept under 5% hypoxia (5% oxygen and 95% nitrogen) from the beginning of the fourth instar molted to the next instar at a weight well below that of normoxic larvae (Fig. 5). Indeed, they molted at weight below the normoxic critical weight of 1.1 g. Likewise, fifth instar larvae in 5% hypoxia from the beginning of the fifth instar exposed their dorsal vessels at significantly lower weights than normoxic controls (Fig. 5), and close to the normoxic critical weight of ∼7.0 g. Thus larval-larval molts, like the metamorphic molt, are initiated at a critical size, and the two kinds of molts appear to be controlled by the same mechanism.
Fig. 5.
Effect of 5% oxygen (hypoxic) and 40% oxygen (hyperoxic) atmospheres on body size in the fourth (Upper) and fifth (Lower) larval instars. Body size is measured as the weight at which growth stopped in preparation for the molt (fourth instar) or wandering stage (fifth instar). Bars show SEs of measurement (SEM); the numbers inside the bars are sample sizes.
Hyperoxic fourth instar larvae molted at modestly, but nonetheless statistically significantly, higher weights than normoxic larvae (Fig. 5; P = 0.008). In contrast, hyperoxic fifth instar larvae molted at approximately the same weight as normoxic larvae (Fig. 5; P = 0.236). Thus, hyperoxia had less effect on body size than did hypoxia. Our results are consistent with previous work on the effects of oxygen levels on body size showing that hypoxia has a stronger and more consistent effect on body size, whereas the response to hyperoxia is variable, weak, and sometimes nonlinear (16, 18, 19, 22). The inability of hyperoxic conditions to increase the size of the fifth (final) instar suggests that in this instar, a constraint to growth must lie at a different level. We note that if hyperoxia increases the size at the molt in earlier instars, it would have a cumulative effect on body size, so that the initial size of the last larval instar would be larger, as would its critical weight and size at metamorphosis.
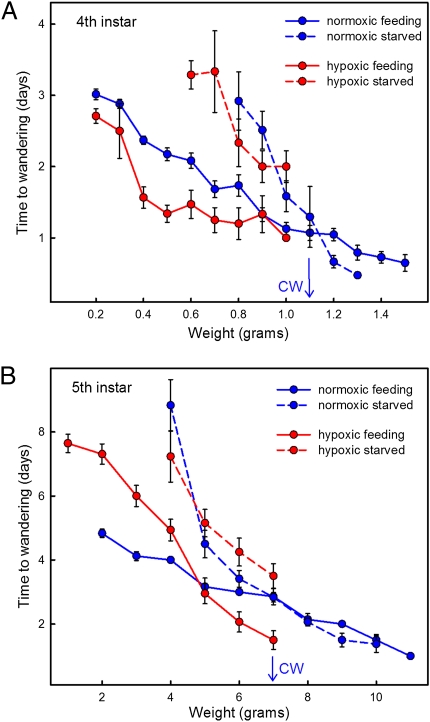
We determined the critical weight of fourth and fifth instar larvae under kept under hypoxia (10% oxygen and 90% nitrogen) from the beginning of the instar, and found that in the fourth instar, the critical weight decreased from 1.1 g to 0.95 g, and in the fifth instar, it decreased from 7.0 g to 5.5 g. We next attempted to determine the critical weight of fourth and fifth instar larvae under 5% hypoxic conditions by placing larvae in hypoxia at the beginning of the instar under study (fourth or fifth instars studied separately), starving hypoxic larvae at various weights, and comparing the time to the next molt (fourth instar) or to the gut purge (fifth instar) with that in larvae that were feeding normally. In both instars, we found that starved hypoxic larvae were always delayed relative to fed hypoxic controls (Fig. 6); there was no size at which starvation did not delay the endocrine cascade.
Fig. 6.
Determination of the critical weight under normoxia in the fourth (A) and fifth (B) larval instars, and the effect of 5% hypoxia. Under normoxia, the critical weight (CW) is operationally assessed as the weight at which there is no difference (by the t test) in the times of feeding (solid lines) and starved (dashed lines) larvae. Under 5% hypoxia, feeding larvae molt at significantly lower weights, and the timing of hypoxic starved larvae does not differ from that of normoxic starved larvae. Bars show SEM.
Starved larvae that failed to reach the critical size eventually molted by some mechanism that evidently did not involve size-dependent oxygen limitation. Hypoxic and normoxic larvae were delayed by the same amount of time (Fig. 6).
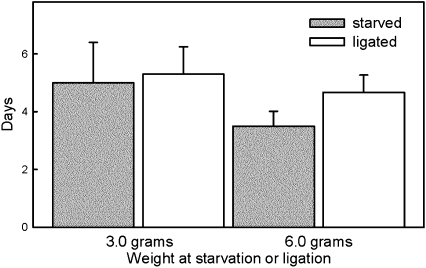
We suspected that the timing of the molt in larvae that were kept below the critical weight was not centrally controlled by the brain via secretion of prothoracicotropic hormone. To test this, we neck-ligated precritical weight larvae, isolating the prothoracic glands from the brain and subesophageal ganglion. We found that larvae ligated at 3 g exposed their dorsal vessel (a sign of entry into the wandering stage) at the same time as unligated but starved larvae (Fig. 7), and that larvae ligated at 6 g exposed their dorsal vessel 1 d after larvae starved at 6 g (Fig. 7). Clearly, these larvae were either ligated or starved before they reached the critical weight and thus did not molt using a size-sensing mechanism. They must have used an alternative mechanism that does not appear to require the brain.
Fig. 7.
Effect of ligation and starvation on timing of entry into the wandering stage of two cohorts of fifth instar larvae that were either starved or neck-ligated at weights below the critical weight (3.0 g and 6.0 g, respectively). Bars are SDs. The differences between neck-ligated and starved larvae at 3.0 g are not significant (P = 0.699, t test); at 6.0 g, there is a 1-d difference (P = 0.411, t test).
A candidate mechanism that is independent of the brain and could account for these observations is provided by the “leaky prothoracic glands” hypothesis (23, 24). It is a well-established fact in insect endocrinology that molting and ecdysone secretion can occur in brainless animals, although usually with significant delay (23–27). The leaky prothoracic glands hypothesis proposes that the prothoracic glands secrete ecdysone at a basal rate, and accumulation of the effects of this low level of ecdysone above a threshold level, triggers the molt (23). This hypothesis emerged from the finding that very small doses of ecdysone infused over a long period have the same effect as large doses infused over a brief period (23). Indeed, we have found gradually rising ecdysone titers in starved and neck-ligated larvae at 6 g (data not shown). Additional support for this hypothesis comes from studies on the control of growth and size in Drosophila showing that larvae whose prothoracicotropic hormone neurons were ablated were still able to pupariate, albeit with a delay (28–32). In addition, insulin signaling in the prothoracic gland increases ecdysone production and causes precocious pupariation. In contrast, suppression of insulin signaling reduces ecdysone secretion and delays the molt. Thus, altering the rate of ecdysone secretion can modify the duration of the larval growth period.
We propose that during normal growth, the brain triggers a molt when a critical size is attained, but under stressful conditions that inhibit growth, such as hypoxia or starvation, the critical size is never reached, and the brain is not in control. Instead, either autonomous activity of the prothoracic glands or stimulation of the glands by extracerebral factors, possibly insulin, induces the molt via the gradual accumulation of ecdysone. In contrast, during normal growth, the size-sensing signal that initiates molting is a reduced availability of oxygen.
Very large and very active insects have dealt with the properties of a tracheal system by evolving large internal air sacs and active mechanisms for ventilating their tracheal system, thereby reducing the length of the path over which oxygen needs to diffuse to reach internal tissues (33, 34). It has been suggested that the evolution of gigantic insects in the Paleozoic was associated with a high level of atmospheric oxygen (13, 17, 18).
The molting cycle in insects is generally assumed to have evolved as a mechanism to deal with a nongrowing external skeleton. Our results show that the nongrowing tracheal system also limits growth, by setting a ceiling on the rate at which oxygen can be supplied to growing tissues (Fig. 4).
Materials and Methods
Animals.
Larvae of M. sexta were reared on an artificial diet in individual containers at 27 °C under a long-day (16-h light/8-h dark) photoperiod.
Tracheal Morphology.
The gut tracheae were chosen because they are easily visible and consistently identifiable (thereby allowing comparisons between individuals). Tracheae were examined using two imaging methods. First, tracheae were stained, and then the larvae were dissected. The tracheae were filled under vacuum with a cobalt solution and then precipitated as black cobalt sulfide (35). Low-magnification images were obtained with an Olympus DP17 camera mounted on an Olympus SZX16 stereo microscope, using Olympus Microsuite Biological Suite software for Imaging Applications. Second, unstained tissue from the dorsal midline of the midgut mounted on a microscope slide was examined at high magnification. These preparations were photographed with a Hamamatsu Orca-ER C4742-95 digital camera mounted on a Leica DMRBE compound microscope, using Simple PCI imaging software (Hamamatsu). In all images, the brightness and contrast were adjusted using the “auto-levels” tool in Adobe Photoshop CS5.
Tracheal Volume Measurement.
Tracheal measurements were done using the water displacement technique of Wigglesworth (35). Larvae were weighed, immersed in water containing 0.1% detergent (Tween), and then placed in a vacuum desiccator attached to a MaximaDry vacuum pump (Fisher Scientific). A vacuum was maintained for 15 min, after which the vessel was allowed to return to atmospheric pressure. As the pressure in the vessel increased, water entered the evacuated tracheal system. The larvae were then dried with a paper towel and reweighed. The difference from the initial weight was taken as the volume of the tracheal system. This method gives an accurate measure of the tracheal volume and gives the same results as the inert gas displacement method of Lease et al. (22); however, this method likely is not able to measure the volume of the finest tracheoles.
Respiration.
Respiration rates for third, fourth, and fifth instar larvae were measured using a constant-pressure respirometer. Measurements were done in a temperature-controlled room at 25 °C. The respirometer was constructed from a 15-mL test tube with a 1-mL pipette inserted in a one-hole stopper. A drop of colored water served as the volumetric marker. CO2 was absorbed by a wad of paper towel soaked in a 20% solution of potassium hydroxide in the test tube. A control tube, identical in all respects except for the presence of an experimental animal, served as a monitor for effects of variation in atmospheric pressure and vessel temperature. After inserting a larva in an experimental tube and sealing the control tube, we waited for 10 min until the volume of the control tube had stabilized, then measured the rate of oxygen consumption in the experimental tubes at 1 min intervals to the closest 0.01 mL The respiration rate was measured for 1–2 h for the third instars, for 0.5–1 h for the fourth instars, and for 15–20 min for the fifth instars. In all cases, there was no change in the volume of the control tubes during the measurement period.
Critical Weight Determination.
Larvae of the fourth and fifth instars were weighed at body masses 0.4–1.4 g (fourth instar) and 4.0–9.0 g (fifth instar). Approximately half of the larvae were allowed to continue feeding, and the rest were starved by removing their food and replacing it with a block of 3% agar. Larvae were checked daily for slippage of the head capsule (fourth instars) or gut purge (fifth instars). Head capsule slippage occurs at 13–16 h after the secretion of ecdysone (24) in the fourth instar, and gut purge occurs at ∼12 h after secretion of ecdysone in the fifth instar (36, 37). The mass at starvation, date of starvation, and date of head capsule slippage or gut purge were recorded for each larva.
Larvae were grouped by 0.1-g weight increments in the fourth instar and by 1.0-g weight increments in the fifth instar, and the average time to molt (or gut purge) was calculated. SEs were calculated as well. For each weight class, a t test was performed to determine whether the time to molt (or gut purge) was statistically different between starved and fed larvae. The critical weight was estimated as the smallest weight at which there was no statistical difference in timing between feeding and starved larvae. Approximately 100 animals in the fourth instar and 140 animals in the fifth instar were used for the critical weight experiments in normoxia (Fig. 6). In the earlier instars (Fig. 1), the critical weight experiment was done as described above, except that the larvae were examined for head capsule slippage (second instar, n = 72; third instar, n = 92; fourth instar, n = 146).
Hypoxia and Hyperoxia.
Larvae were introduced into a Plexiglas glove box (Scienceware) when they were head capsule–slipped from the third to fourth instar (hypoxic fourth instar larvae) or head capsule–slipped from the fourth to fifth instar (hypoxic fifth instar larvae). After larvae were introduced, the glove box was sealed and flushed for 60 min with a mixture of either 10% oxygen:90% nitrogen, 5% oxygen:95% nitrogen, or 40% oxygen:60% nitrogen (National Welders). After the appropriate level of oxygen was achieved the flow of gas was reduced to 250 mL/min for the duration of the experiment. A ToxiRaeII oxygen analyzer (RAE Systems) was used to continuously monitor oxygen levels in the glove box. A ScoutPro electronic balance (Ohaus) within the glove box was used to weigh larvae daily to the nearest 0.01 g.
Critical Weight in Hypoxia.
Critical weight experiments in hypoxia were conducted in the same manner as in normoxia, except that larvae were in the glove box under various oxygen tensions. All larvae were introduced into the relevant atmosphere during head capsule slippage of the molt that led to the instar for which the critical weight was measured. For each graph, there were 55 larvae in fourth instar and 74 larvae in fifth instar under 10% oxygen and 70 larvae in fourth instar and 60 larvae in fifth instar under 5% oxygen.
Neck Ligations.
Larvae to be neck ligated were deeply anesthetized with CO2, and a blood-tight ligature was placed behind the head capsule using waxed dental floss. This ligature isolated both the brain and subesophageal ganglion from the rest of the body. Because the head contains no spiracles, it dies soon after this operation.
Acknowledgments
We thank A. Moczek, K. Preuss, W. Smith, and Y. Suzuki for helpful suggestions and comments on the manuscript; R. Dilling and I. Jalli for discussions; and C. Shreve and L. Grunert for assistance throughout the project. This research was funded by National Science Foundation Grants IOS-0641144 and IOS-0744952 (to H.F.N.) and by Sigma Xi Grants in Aid of Research G2009150531 and G20101015155261 (to V.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Schmidt-Nielsen K. Scaling: Why Is Animal Size So Important? Cambridge, UK: Cambridge Univ Press; 1984. [Google Scholar]
- 2.Roff DA. On being the right size. Am Nat. 1981;118:405–422. [Google Scholar]
- 3.Thompson DAW. On Growth and Form. Cambridge, UK: Cambridge University Press; 1961. [Google Scholar]
- 4.Edgar BA. How flies get their size: Genetics meets physiology. Nat Rev Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- 5.Nijhout HF. Insect Hormones. Princeton, NJ: Princeton Univ Press; 1998. [Google Scholar]
- 6.Nijhout HF, Davidowitz G, Roff DA. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J Biol. 2006;5:16. doi: 10.1186/jbiol43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidowitz G, D'Amico LJ, Nijhout HF. Critical weight in the development of insect body size. Evol Dev. 2003;5:188–197. doi: 10.1046/j.1525-142x.2003.03026.x. [DOI] [PubMed] [Google Scholar]
- 8.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): Cessation of juvenile hormone secretion as a trigger for pupation. J Exp Biol. 1974;61:493–501. doi: 10.1242/jeb.61.2.493. [DOI] [PubMed] [Google Scholar]
- 9.Nijhout HF, Williams CM. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): Growth of the last-instar larva and the decision to pupate. J Exp Biol. 1974;61:481–491. doi: 10.1242/jeb.61.2.481. [DOI] [PubMed] [Google Scholar]
- 10.Wolfgang WJ, Riddiford LM. Larval cuticular morphogenesis in the tobacco hornworm, Manduca sexta, and its hormonal regulation. Dev Biol. 1986;113:305–316. doi: 10.1016/0012-1606(86)90166-1. [DOI] [PubMed] [Google Scholar]
- 11.Woods HA, Kingsolver JG. Feeding rate and the structure of protein digestion and absorption in lepidopteran midguts. Arch Insect Biochem Physiol. 1999;42:74–87. doi: 10.1002/(SICI)1520-6327(199909)42:1<74::AID-ARCH8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Loudon C. Development of Tenebrio molitor in low oxygen levels. J Insect Physiol. 1988;34:97–103. [Google Scholar]
- 13.Dudley R. Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J Exp Biol. 1998;201:1043–1050. doi: 10.1242/jeb.201.8.1043. [DOI] [PubMed] [Google Scholar]
- 14.Harrison JF, Haddad GG. Effects of oxygen on growth and size: Synthesis of molecular, organismal, and evolutionary studies with Drosophila melanogaster. Annu Rev Physiol. 2011;73:95–113. doi: 10.1146/annurev-physiol-012110-142155. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich EC, Farzin M, Klok CJ, Harrison JF. The effect of developmental stage on the sensitivity of cell and body size to hypoxia in Drosophila melanogaster. J Exp Biol. 2011;214:1419–1427. doi: 10.1242/jeb.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klok CJ, Harrison JF. Atmospheric hypoxia limits selection for large body size in insects. PLoS ONE. 2009;4:e3876. doi: 10.1371/journal.pone.0003876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser A, et al. Increase in tracheal investment with beetle size supports hypothesis of oxygen limitation on insect gigantism. Proc Natl Acad Sci USA. 2007;104:13198–13203. doi: 10.1073/pnas.0611544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison JF, et al. Responses of terrestrial insects to hypoxia or hyperoxia. Respir Physiol Neurobiol. 2006;154:4–17. doi: 10.1016/j.resp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Frazier MR, Woods HA, Harrison JF. Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol Biochem Zool. 2001;74:641–650. doi: 10.1086/322172. [DOI] [PubMed] [Google Scholar]
- 20.Greenlee KJ, Harrison JF. Respiratory changes throughout ontogeny in the tobacco hornworm caterpillar, Manduca sexta. J Exp Biol. 2005;208:1385–1392. doi: 10.1242/jeb.01521. [DOI] [PubMed] [Google Scholar]
- 21.Greenlee KJ, Harrison JF. Development of respiratory function in the American locust Schistocerca americana, II: Within-instar effects. J Exp Biol. 2004;207:509–517. doi: 10.1242/jeb.00766. [DOI] [PubMed] [Google Scholar]
- 22.Lease HM, Wolf BO, Harrison JF. Intraspecific variation in tracheal volume in the American locust, Schistocerca americana, measured by a new inert gas method. J Exp Biol. 2006;209:3476–3483. doi: 10.1242/jeb.02343. [DOI] [PubMed] [Google Scholar]
- 23.Nijhout HF. The role of ecdysone in pupation of Manduca sexta. J Insect Physiol. 1976;22:453–463. [Google Scholar]
- 24.Truman JW. Physiology of insect rhythms, I: Circadian organization of the endocrine events underlying the moulting cycle of larval tobacco hornworms. J Exp Biol. 1972;57:805–820. doi: 10.1242/jeb.60.2.371. [DOI] [PubMed] [Google Scholar]
- 25.McBrayer Z, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda S. The hormonal mechanism of larval molting and metamorphosis in the silkworm. J. Fac. Sci. Tokyo Imp. Univ. Sec. 1944;4:477–532. [Google Scholar]
- 27.Judy KJ. Diapause termination and metamorphosis in brainless tobacco hornworms (Lepidoptera) Life Sci. 1972;11:605–611. [Google Scholar]
- 28.Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol. 2005;15:1785–1795. doi: 10.1016/j.cub.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Walkiewicz MA, Stern M. Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS ONE. 2009;4:e5072. doi: 10.1371/journal.pone.0005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Layalle S, Arquier N, Léopold P. The TOR pathway couples nutrition and developmental timing in Drosophila. Dev Cell. 2008;15:568–577. doi: 10.1016/j.devcel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Colombani J, et al. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 33.Chapman RF. The Insects: Structure and Function. Cambridge, UK: Cambridge Univ Press; 1998. [Google Scholar]
- 34.Wigglesworth VB. The respiration of insects. Biol Rev Camb Philos Soc. 1930;6:181–220. [Google Scholar]
- 35.Wigglesworth VB. A new method for injecting the tracheae and tracheoles of insects. Q J Microsc Sci. 1950;91:217–224. [PubMed] [Google Scholar]
- 36.Sakurai S. Temporal organization of endocrine events underlying larval-pupal metamorphosis in the silkworm, Bombyx mori. J Insect Physiol. 1984;30:644–657. [Google Scholar]
- 37.Truman JW, Riddiford LM. Physiology of insect rhythms, 3: The temporal organization of the endocrine events underlying pupation of the tobacco hornworm. J Exp Biol. 1974;60:371–382. doi: 10.1242/jeb.60.2.371. [DOI] [PubMed] [Google Scholar]