Abstract
In humans and other mammals, the hippocampus is critical for episodic memory, the autobiographical record of events, including where and when they happen. When one records from hippocampal pyramidal neurons in awake, behaving rodents, their most obvious firing correlate is the animal's position within a particular environment, earning them the name “place cells.” When an animal explores a novel environment, its pyramidal neurons form their spatial receptive fields over a matter of minutes and are generally stable thereafter. This experience-dependent stabilization of place fields is therefore an attractive candidate neural correlate of the formation of hippocampal memory. However, precisely how the animal's experience of a context translates into stable place fields remains largely unclear. For instance, we still do not know whether observation of a space is sufficient to generate a stable hippocampal representation of that space because the animal must physically visit a spot to demonstrate which cells fire there. We circumvented this problem by comparing the relative stability of place fields of directly experienced space from merely observed space following blockade of NMDA receptors, which preferentially destabilizes newly generated place fields. This allowed us to determine whether place cells stably represent parts of the environment the animal sees, but does not actually occupy. We found that the formation of stable place fields clearly requires direct experience with a space. This suggests that place cells are part of an autobiographical record of events and their spatial context, consistent with providing the “where” information in episodic memory.
Keywords: learning, plasticity, cognitive map, remapping, consolidation
The hippocampus is clearly central to mammalian learning and memory (1), and it is just as clear that an animal's position in space is by far the most obvious firing correlate of hippocampal neurons in awake, behaving rodents (2). However, the precise relationship between memory and place cells is less clear, making the study of the processes governing the formation of place fields particularly interesting. Consistent with the central role for the hippocampus in memory, the balance of experimental evidence supports the idea that place fields are constructed and stabilized by the animal's experience of that space. For example, place cells “remap” (i.e., change to unpredictable locations and/or change their firing rate) in response to even small changes in the sensory environment (3) or even changes in the animal's ongoing behavior (4, 5). Moreover, place fields are quite labile in novel environments until the animal has experienced the environment for several minutes (6, 7). Finally, inhibitors of synaptic plasticity prevent the long-term maintenance (i.e., destabilize) of place fields in novel but not familiar environments (8, 9).
However, recent studies have forced a reexamination of the debate. First, place cells in weanling rats show some adult-like spatial tuning during the animals' first spatial experience away from their nests, suggesting that spatial firing is an innate property of the network (10). Second, ensembles of place cells representing unvisited paths (11) or spaces (12) can become active during periods of rest or sleep before direct experience, a phenomenon called “preplay.” Preplay of unexplored spaces suggests that animals can mentally explore a space before physical exploration, but it remains unclear whether such mental exploration is by itself capable of forming a lasting representation. We therefore sought to determine whether the presumed mental exploration following extensive observation of a space can generate a consolidated representation of that space.
The reason we do not currently know the answer to this fundamental question is that one cannot tell whether an animal has a place field in a particular place until after the animal visits it. This study circumvents this problem by combining a customized environment containing both directly experienced and purely observed areas with pharmacological blockade of NMDA receptor-dependent plasticity, which preferentially destabilizes newly formed place fields (8). Because the pharmacological blockade occurs during the rat's first visit to the previously observed but unexplored space, it enables us to determine whether stable place fields were formed by observation alone before exploration. We find that the rat must physically occupy a space to form a stable hippocampal representation of that space, suggesting that active exploration enables the consolidation of hippocampal place fields. Thus, rather than being a comprehensive map of explored and unexplored spaces, the hippocampal representation of space remains plastic until the animal has explored all parts of the environment. In this respect, place fields are more a representation of the animal's experience within a space rather than the geometry of the space itself.
Results
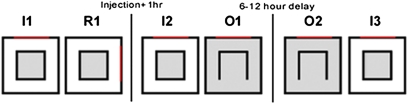
Recordings were performed in a customized behavioral apparatus (Fig. 1) consisting of two concentric boxes: an optically clear Plexiglas inner box inside an opaque outer box with geometric shapes painted on it providing the only asymmetric spatial cues available to the animal. The animal was restricted to the inner box throughout at least a week of once-daily familiarization and screening sessions that lasted 10 min each. The sequence in Fig. 1 was not initiated until well-isolated place cells were found; thus the number of familiarization sessions varied from rat to rat but exceeded more than 70 min for every rat tested (median number of sessions was 15 with a range of 7–29). Thus, the inner box was directly experienced, whereas the perimeter of the outer box could only be observed. Upon finding place cells, the outer box was rotated 90°. The place fields followed the rotation, demonstrating that the animal could see the outer box and used it to orient its place cells (Fig. S1, R1). Following an injection of the NMDA receptor antagonist CPP [(±)-3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid; 10 mg/kg i.p.; Sigma] or saline and another recording session in the inner box (I2), one wall was removed from the inner box (O1), allowing the animal to enter the previously unexplored outer box. After a 6- to 12-h delay, the place cells were recorded again in this modified environment (O2), after which a final session (I3) was run in the initial configuration.
Fig. 1.
Experimental design. The recording environment consisted of two concentric boxes: a clear inner box with no asymmetric cues and an opaque outer box with geometric shapes on its sides to provide spatial cues. The rats were extensively familiarized to the inner box of the environment (I1), after which the outer box was rotated (R1). Following injection and another inner box session (I2), an inner wall was removed, allowing exploration of the entire environment (O1). After a delay the animal was reintroduced to the open configuration (O2) and then back into the closed inner box (I3). Gray portions indicate regions explored by the rat; the red bar indicates cue orientation.
Because NMDA receptor blockade specifically destabilizes newly formed place fields while sparing previously formed place fields (8), CPP injection before session O1 reveals when the outer box place fields were formed. If a hippocampal representation of a space forms by observation alone, then all place fields should be stable, but if it forms only after direct experience, then CPP should specifically destabilize the outer box fields, but not the inner box ones.
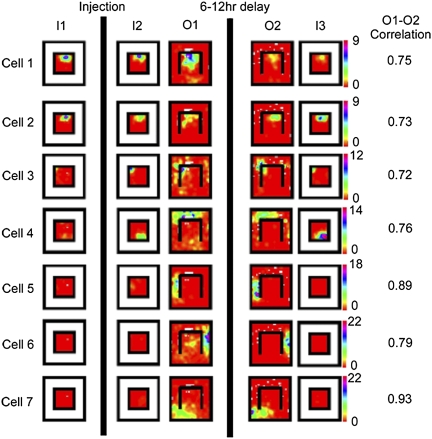
Fig. 2 shows rate maps of place cells recorded from a saline-injected animal throughout the experiments described above. Comparing I2 to O1, the majority of place fields in the inner box did not remap following wall removal. However, consistent with prior work involving manipulations of barriers and walls (13), some neurons (particularly those with place fields near the removed wall) did remap following wall removal (e.g., Fig. 2, cell 4 and Fig. S2). Place fields were evident in the outer box as well, with ∼20% of the cells with fields in the inner box also having fields in the outer box (Fig. 2, cell 3). Removal of the barrier also revealed a previously inactive population of pyramidal cells (e.g., Fig. 2, cells 5–7) that had place fields only in the outer box. To compare the stability of place fields of directly experienced versus observed portions of the environment, we computed separate Pearson's correlation coefficients (stability scores) for the inner and outer box areas (Materials and Methods). Place fields in both compartments were nearly always stable in the saline animals, which would be expected whether the outer box fields were formed during observation or exploration (6). Thus, the saline animals give us three main results: (i) removal of one of the clear, inner walls did not cause a global remapping of place fields in the inner box, although some cells near the removed wall did remap; (ii) “new” place cells turned on only in the outer box of the environment; and (iii) place cells often had fields in both the inner and outer boxes.
Fig. 2.
Place fields of seven simultaneously recorded hippocampal pyramidal neurons across the entire behavioral sequence from a saline-injected rat (with the rotation session omitted for clarity). Rows are cells and columns are sessions. Color bar shows the cell's firing rate values for the entire set of sessions; unvisited pixels are white. Note that all place fields are stable throughout the experiment, with the exception of those inner box cells that remapped in response to wall removal (e.g., cell 4). Whole-environment correlation scores for the O1–O2 comparison are shown to the Right of the ratemaps.
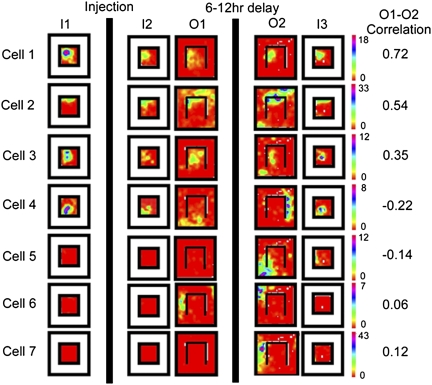
The initial response of the place fields of the CPP-injected animals to wall removal (I2 versus O1) was indistinguishable from that of the saline animals. Most of the inner box fields remained stable, although a minority (particularly those near the removed wall) of inner box fields remapped (Fig. 3, cell 4 and Fig. S2), and new fields appeared in the outer box. The differences between the drug and saline groups arise when one compares the O1 and O2 sessions. Like the place fields of the saline-injected animals, the vast majority of inner box place fields remained stable following CPP (Fig. 3, cells 1–3), except for those that had remapped in response to wall removal, which remapped yet again in the CPP-injected animals (Fig. 3, cell 4). Moreover, every outer box place field (Fig. 3, cells 4–7) also remapped again in the CPP animals, in sharp contrast to both the fields in the saline animals and simultaneously recorded inner box place fields in the CPP group. We quantified this difference by dividing place fields into inner and outer groups for analysis of variance (ANOVA). Post hoc comparisons showed no differences between the stability of the inner and outer box place fields in saline-injected animals or between these two groups and the inner box place fields of the CPP-injected animals. In fact, the only significant difference was between the outer box place cells in CPP-injected animals and all other groups (Fig. 4; F(4,109) = 36.4373, P < 0.001). Outer box place fields were also significantly less active during sharp wave ripples than inner box place fields before experiencing the outer box area (sessions I1 and I2; Fig. S3).
Fig. 3.
Place fields from seven identified pyramidal neurons across the entire behavioral sequence from a CPP-injected rat. Format is the same as in Fig. 2. Note that most neurons with fields in the inner box (e.g., cells 1–3) maintained firing position throughout the experiment, whereas cell 4, which remapped in response to barrier removal in O1, remapped again in O2, consistent with the known effects of CPP on remapping. All outer box firing fields remapped between sessions O1 and O2, even those of cells (2 and 3) with stable inner box fields, which resulted in midrange stability scores for the entire environment.
Fig. 4.
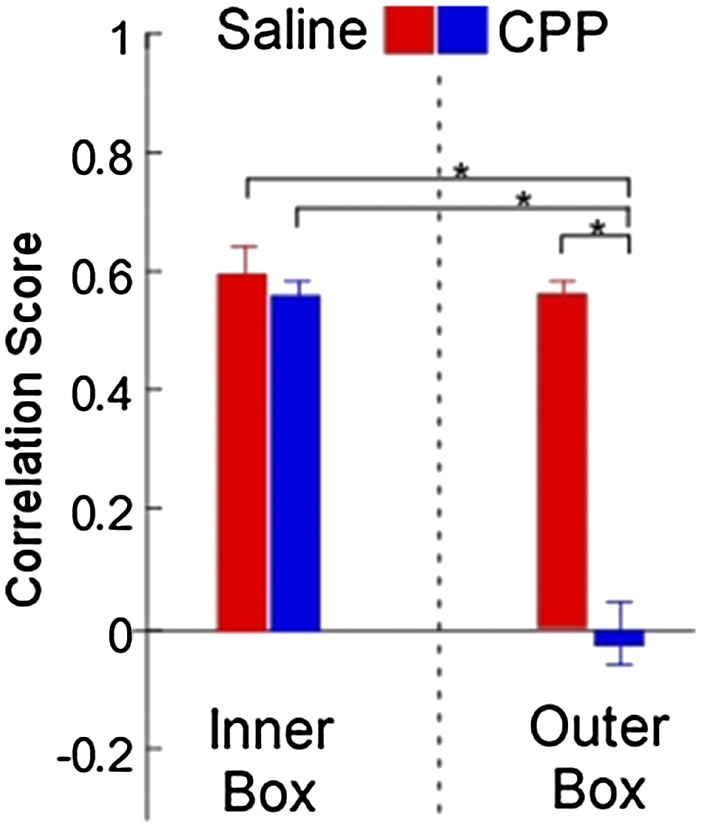
CPP preferentially destabilizes outer box place fields. Mean stability scores for the O1–O2 comparison when broken out into inner and outer box areas (Materials and Methods), error bars are SEM. An ANOVA revealed a significant difference between the four groups (F = 36.4373, P < 0.001). Post hoc comparisons showed that this difference came entirely from the CPP outer box group, which significantly differed from all three other groups (CPP inner, and saline inner and outer; *P < 0.01).
Given recent results (14) showing differential stability of CA1 place fields depending upon their position on the proximodistal axis, we also analyzed whether the instability we observed could be due to uneven anatomical sampling among groups (Figs. S4 and S5). Although we did see the same effect of position on this anatomical axis on place field stability, there was no bias toward one direction or the other in any of the groups. Moreover, the instability effect was far less than what we saw with CPP.
The data in Fig. 4 strongly suggest that the place fields representing the newly explored outer box were made only as the animal first directly experienced it in session O1. Extensive observation of a space is therefore clearly not sufficient for making a stable place cell representation of that space. Indeed, the initial exploration of the outer area has all of the hallmarks of the “complete” remapping that occurs when an animal enters a truly novel environment (15): new place cells started firing in the outer box, and a large fraction (approximately one in five; 16% CPP, 24% saline) of cells had place fields in both the inner and outer box, which are both very rare in a single environment. This proportion (∼20%) is the same as what one would get if the inner and outer boxes were totally distinct, given that the estimated proportion of CA1 neurons that are on in any environment is ∼0.4 (16, 17). Moreover, fast-spiking putative interneurons (i.e., “theta” cells) significantly decreased their firing rate (Fig. S6) as the animal explored the outer box for the first time (n = 12; paired t test, P = 0.0262), consistent with previous studies in novel environments (6, 7). These data suggest that in terms of hippocampal processing, the observed space of the outer box is largely equivalent to an entirely novel space until it is directly experienced.
Discussion
Our primary finding is that NMDA receptor blockade destabilizes the hippocampal representation of areas that are extensively observed but not directly experienced. Because newly made place fields are preferentially destabilized by NMDA receptor blockade (18), this finding strongly suggests that place fields are constructed rather than preconfigured and reflect the animal's experience within that space more than its spatial layout per se. Previous work has already established that hippocampal neurons respond to much more than the animal's current position in the environment (18) and therefore do not simply provide a spatial representation of the environment. The data presented here deal more with the nature of the hippocampal representation of space. The question becomes whether it is better to think of place cells as part of a “third-person” representation of environmental geometry, akin to the “cognitive maps” proposed by Tolman (19) to explain the ability of animals to take novel shortcuts, or a “first-person” representation of the animal's experience within a space more consistent with episodic memory (20). Our results clearly indicate the latter: even though the rotation experiment (Fig. S1) demonstrates that the animal is aware of the outer box area before exploring it and uses its cues to orient its place cells, its place cells appear to treat the outer box as if it were a completely novel environment, making a lasting representation of it only when it was first directly experienced.
Our data also suggest that a place cell “map” of a space is quite plastic, forming only as the animal directly experiences it, and changing to reflect the animal's changing experience within it. As with earlier studies, NMDA receptor blockade did not affect already established fields, arguing against state-dependent effects of CPP, and only mildly affected the firing properties of the newly made place fields (Table S1); so the creation of new firing fields does not necessarily require NMDA receptor-dependent plasticity. However, the newly made fields simply do not appear to get “burned in” without NMDA receptor-dependent plasticity: the outer box cells had either entirely new firing fields in the second session, or none at all. This brings to mind a recent study showing that CPP specifically destabilizes only the place fields that remap in response to a novel behavioral task, sparing the previously learned representation of the task space (5). Taken together, these data suggest that the hippocampal representation of a space is a continuously updated record of the animal's “first-person” experience of its environment, which is recalled as the animal reexperiences that space, and that NMDA-dependent plasticity is required more for the stabilization of place fields rather than for their initial formation.
Of course, our NMDA receptor blockade was systemic, so we cannot make strong claims as to precisely where in the brain this plasticity occurs: it is also possible that the key changes are occurring in upstream brain regions. This raises questions about how observed space might be represented in other brain regions, most notably by the “grid cells” (21) of the medial entorhinal cortex, the major input structure to the hippocampus. In many ways, grid cells appear to be more “hard-wired” than place cells. Their repeating organization and relationship to each other is largely maintained in novel environments (22), suggesting each cell represents distance in space rather than part of a particular environment. Moreover, a grid cell has a similar orientation as its neighbors, unlike place cells that show little or no neighbor relationships, and their firing patterns are immediately present in novel environments (21, 23). The grid cell representation of space therefore seems regular and preconfigured, in contrast to place cells, fields of which are uniquely constructed for every space.
Our data measure the stabilization of place fields rather than their formation per se, so are therefore not inconsistent with recent reports of preplay of novel paths (11) or even novel spaces (12) during sharp wave ripples. However, they do raise questions about the significance of such preplay events in creating lasting hippocampal representations. The preplay phenomenon could be explained by at least three different models. First, a single, hard-wired map could exist for the entire environment regardless of experience, in which case preplay and “replay” (24) just activate different parts of the same map. Second, as suggested by Gupta and colleagues (11), the hippocampus may be able to “mentally explore” unvisited parts of the environment, leading to their consolidation and the generation of a comprehensive cognitive map. Third, place fields may exist stably only in experienced space, and preplay generates a preliminary, unconsolidated map of unexplored space, a “first draft” that stabilizes with experience. Our data show that place fields in the observed space are initially unstable and only become stable upon consolidation by NMDA receptor-dependent plasticity driven by direct experience, and therefore support the third model. Furthermore, the complete remapping of the outer box area on the second day in the CPP animals (Figs. 3 and 4) means that the outer box area is not only independent of the inner environment, but a new outer box map is generated each time the animal experiences the environment by NMDA receptor-independent processes.
These data raise questions about the functional significance of preplay to long-term memory. Although we did not have enough simultaneously recorded cells to look for sequences of activated place cells during sharp wave ripple events before exploration, we did have enough cells to analyze which cells were active during ripple events, which has often been taken as indirect evidence of such sequences (25). When we performed this kind of analysis on the inner box session just before the initial exploration of the outer box, we found that cells that would end up being active in the outer box did in fact fire during ripples, consistent with preplay events (Fig. S3). However, they were much less frequent than those of cells with directly experienced firing fields (i.e., inner box cells), consistent with the findings of both preplay papers. Taken together, these data suggest a relatively simple model whereby the generation of place fields for novel space is not strictly speaking experience-dependent (i.e., the network is able to automatically calculate new fields). However, stably tying them to the available sensory cues very much depends upon their experience and the presumed NMDA receptor-dependent plasticity that this engenders. This would resemble the “two-stage” model of memory encoding (24, 26) if one assumes that the NMDA receptor-dependent stabilization of place fields occurs during sharp waves.
In such a model, direct and “virtual” experience of environmental space need not necessarily be fundamentally different; they could reflect a quantitative rather than qualitative distinction. The relatively rare occurrence of preplay events (11, 12) may simply not provide enough (virtual) experience to consolidate place fields. If so, the opposite result may happen if the unexplored space were somehow made more attentionally salient to the animal. This also brings up the equally interesting question of whether one would expect the same result in other animals that explore environmental space in a less egocentric manner. For instance, bats explore an environment with sonar calls, whereas humans (and presumably nonhuman primates) are capable of mental imagery (i.e., mentally exploring environmental space). Although place cells have been reported in all of these species, ranging from bats (27) to nonhuman primates (28) to humans (29), nonhuman primates and bats both also have “spatial view cells” (30), which do not require the animal to occupy the space. Whereas it is entirely possible that one might therefore see different results if one did the analogous experiment in these species, we would still maintain that such disparities would reflect quantitative rather than qualitative differences in hippocampal processing. There is increasing evidence that the moment-to-moment firing patterns of hippocampal neurons (25, 31) reflect space that the animal has been given reason to pay attention to, even when it is not physically occupying the space. Such attentional processes have also been suggested to underlie the stabilization of place fields over the long term (32, 33). Conversely, whereas humans are certainly capable of mental imagery, actual physical exploration is a far more effective way to learn a spatial layout, as anyone who has tried to learn a route by looking at a map can attest to.
Materials and Methods
Animals, Surgery, and Behavior.
We included data from 18 (nine saline-injected and nine CPP-injected) male Long Evans rats (Charles River; 3–9 mo old; 300–450 g at time of testing) implanted with tetrode microdrives. Microdrives contained six tetrodes moveable as a bundle. Tetrodes were implanted over the CA1 region and slowly lowered into position during screening and familiarization. When well-isolated place cells were present, we initiated the experiment described in Fig. 1. All procedures were carried out in accordance with the Oregon Institutional Animal Care and Use Committee (IACUC) and National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publications no. 80-23).
Data Analysis.
The spiking activity of single units was associated with the rat's position in space at the time of the spike. The position of the rat and the spikes were then placed into 4 × 4 cm bins. The binned spikes were then divided by the binned occupancy to create an unsmoothed rate map. Smoothing of the rate maps was performed with a 3 × 3 Gaussian kernel. Correlation scores based on smoothed rate maps were generated for session pairs by correlating the two maps. A Pearson's correlation score was calculated between equivalent bins, with unvisited and common-zero bins ignored. A cell was eligible for the measure only if it was judged to be the same between the two sessions and showed a place field in either of the two sessions being compared. In addition, the rat must have occupied >85% of the bins in the rate maps and the majority of fields needed to follow the rotation of the cues. These three requirements (recording stability, rotation, and coverage) reduced the number of rats to six saline (48 cells) and six CPP (51 cells) rats for the critical O1–O2 comparison. Data from the other rats and cells that were either unstable between sessions or stable between other session pairs were included in single-session statistics or comparisons, when appropriate (SI Materials and Methods, Table S1, and Fig. S1). For the O1–O2 comparison, we divided the environment into an inner and outer box area and computed a separate stability score for the two regions. We first found the place fields (defined as a contiguous 80-cm2 region where the cell fired above 20% of its peak firing rate for the whole environment) of a cell, and extracted the center of mass for each field. If a place cell had a field centered in the inner box in either session, then a stability score was taken for that cell in the inner box. This procedure was then repeated for the outer box (Fig. 4). Some cells contributed to both the inner and the outer box groups, as approximately one of five cells showed fields in both compartments (e.g., Fig. 2, cell 3). Additional details can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank A. D. Redish for helpful comments on an earlier version of this manuscript. D.C.R was supported in part by National Institutes of Health Training Grant 5 T32 GM007257-33.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105445108/-/DCSupplemental.
References
- 1.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 3.Bostock E, Muller RU, Kubie JL. Experience-dependent modifications of hippocampal place cell firing. Hippocampus. 1991;1:193–205. doi: 10.1002/hipo.450010207. [DOI] [PubMed] [Google Scholar]
- 4.Markus EJ, et al. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci. 2004;24:7681–7689. doi: 10.1523/JNEUROSCI.1958-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 8.Kentros C, et al. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998;280:2121–2126. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- 9.Agnihotri NT, Hawkins RD, Kandel ER, Kentros C. The long-term stability of new hippocampal place fields requires new protein synthesis. Proc Natl Acad Sci USA. 2004;101:3656–3661. doi: 10.1073/pnas.0400385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wills TJ, Cacucci F, Burgess N, O'Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science. 2010;328:1573–1576. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barry C, et al. The boundary vector cell model of place cell firing and spatial memory. Rev Neurosci. 2006;17:71–97. doi: 10.1515/revneuro.2006.17.1-2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henriksen EJ, et al. Spatial representation along the proximodistal axis of CA1. Neuron. 2010;68:127–137. doi: 10.1016/j.neuron.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leutgeb S, et al. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- 16.Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- 17.Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 19.Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 20.Buzsáki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 21.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 22.Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- 23.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 24.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 25.Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64:910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buzsáki G. Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 27.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 28.Hori E, et al. Representation of place by monkey hippocampal neurons in real and virtual translocation. Hippocampus. 2003;13:190–196. doi: 10.1002/hipo.10062. [DOI] [PubMed] [Google Scholar]
- 29.Ekstrom AD, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 30.Rolls ET, Robertson RG, Georges-François P. Spatial view cells in the primate hippocampus. Eur J Neurosci. 1997;9:1789–1794. doi: 10.1111/j.1460-9568.1997.tb01538.x. [DOI] [PubMed] [Google Scholar]
- 31.Johnson A, Fenton AA, Kentros C, Redish AD. Looking for cognition in the structure within the noise. Trends Cogn Sci. 2009;13:55–64. doi: 10.1016/j.tics.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- 33.Muzzio IA, et al. Attention enhances the retrieval and stability of visuospatial and olfactory representations in the dorsal hippocampus. PLoS Biol. 2009;7:e1000140. doi: 10.1371/journal.pbio.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.