Abstract
Despite the great demands for treating craniofacial injuries or abnormalities, effective treatments are currently lacking. One promising approach involves human elastic cartilage reconstruction using autologous stem/progenitor populations. Nevertheless, definitive evidence of the presence of stem cells in human auricular cartilage remains to be established. Here, we demonstrate that human auricular perichondrium, which can be obtained via a minimally invasive approach, harbors a unique cell population, termed as cartilage stem/progenitor cells (CSPCs). The clonogenic progeny of a single CD44+ CD90+ CSPC displays a number of features characteristic of stem cells. Highly chondrogenic CSPCs were shown to reconstruct large (>2 cm) elastic cartilage after extended expansion and differentiation. CSPC-derived cartilage was encapsulated by a perichondrium layer, which contains a CD44+ CD90+ self-renewing stem/progenitor population and was maintained without calcification or tumor formation even after 10 mo. This is a unique report demonstrating the presence of stem cells in auricular cartilage. Utilization of CSPCs will provide a promising reconstructive material for treating craniofacial defects with successful long-term tissue restoration.
Keywords: stem cell identification, flow cytometry, cell transplantation, regenerative medicine, plastic surgery
Craniofacial injuries and abnormalities affect millions of patients worldwide, highlighting the need for novel therapeutic strategies (1). Conventional approaches to treat these defects rely on reconstructive materials, such as autocartilage and bone grafts or synthetic compounds (2–6). Collecting autologous tissue, however, places a significant burden on donor sites, and invariable absorption of the transplanted autografts often leads to treatment failure (7, 8). Moreover, owing to the limited amount of available autografts, severe craniofacial anomalies such as Treacher Collins syndrome and Nager syndrome still remain incurable (9, 10). The implantation of synthetic materials is associated with a number of potential complications, such as inflammation, extrusion, calcification, and abnormal skin (5, 6). To overcome these problems, many researchers have attempted to reconstruct elastic cartilage using cell-based engineering approaches that provide a sufficiently large volume of reconstructive material.
Several cell populations are potential sources for human elastic cartilage reconstruction (11–14). Human auricular chondrocytes, which is the only example already clinically applied, are highly chondrogenic and can produce significant levels of cartilage extracellular matrix (ECM). However, donor site morbidity is commonly observed and, more importantly, poor tissue maintenance remains a significant issue. It is expected that the use of stem cells will address these issues, because self-renewing stem cells can lead to permanent restoration of tissues characterized by high and continuous self-renewal (15). Although bone marrow mesenchymal stem cells (MSCs) were thought to be a promising resource, their use was associated with many serious problems, including low chondrogenic potential, vascularization, and mineralization (16, 17). Various tissue-derived MSCs, such as adipose-derived stem cells, also do not produce enough cartilage ECM to support 3D structures (18–20). The identification of an alternative source of stem cells with high chondrogenic potential is crucial to realize elastic cartilage regenerative therapy.
Previous papers have suggested that some of the cells derived from rabbit auricular (ear) perichondrium appear to be a stem or progenitor population, on the basis of in vitro multidifferentiation assays or label-retaining cell assays (21–23). However, considerable heterogeneities between these studies have precluded definitive evidence of stem cells in elastic cartilage, and the therapeutic potency of these stem/progenitor cells toward cartilage regenerative therapies remains to be determined. The goal of the present study was to determine whether human auricular perichondrium harbors cells with a stem cell character and to develop cell-manipulation techniques for reconstructing larger elastic cartilage with successful long-term tissue restoration aimed at future clinical use. Here, we show that human auricular perichondrium contains a promising stem/progenitor cell population. By combinations of newly identified stem cell markers, we successfully isolated a clonal population of CD44+ CD90+ stem cells from human auricular perichondrocytes, hereafter referred to as cartilage stem/progenitor cells (CSPCs). CSPCs proliferate robustly, show multiple differentiation capabilities, self-renew, and participate in tissue reconstruction. Our layered culture system enables CSPCs to efficiently differentiate into mature chondrocytes. Subcutaneously (s.c.) injected human CSPC-containing perichondrocytes reconstructed over 2 cm of elastic cartilage, consisting both of perichondrium and chondrium layers, and restored these structures without any ectopic tissue formation even after 10 mo.
Results
Human Perichondrocytes Are Highly Proliferative.
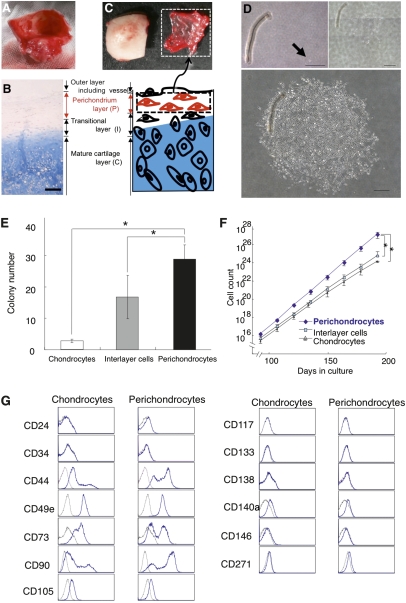
We hypothesized that auricular perichondrium contains stem/progenitor cells that are highly proliferative, are able to differentiate into multiple lineages, can self-renew, and can contribute to tissue reconstruction. To test this hypothesis, we performed experiments using auricular cartilage remnants from microtia patients (Fig. 1 A and B). The patients included 19 males and 11 females, with a mean age of 10.6 ± 1.4 y (mean ± SD). There were no significant differences in the following experiments on the basis of their disease classification or complication. Auricular cartilage remnants were manually separated into the perichondrium layer, the interlayer, and the chondrium layer (Fig. 1C and Fig. S1, Left). After digesting each layer, the cells were cultured to yield perichondrocytes, interlayer cells, and chondrocytes, respectively. To examine their colony-forming capacities, we cultured cells at low density (52 cells/cm2). After 4 wk, single perichondrocytes formed colonies that contained over 50 cells (Fig. 1D). We then counted the semiclonal colonies. Perichondrocytes, interlayer cells, and chondrocytes formed 23.9 ± 4.5, 9.9 ± 6.8, and 2.3 ± 0.4 colonies, respectively (Fig. 1E). These results show that perichondrocytes possessed the highest clonogenicity.
Fig. 1.
Isolation and cultivation of human perichondrocytes. (A and B) Gross morphology and Alcian blue staining of auricular cartilage remnants from microtia patients. (Scale bars, 4 mm and 200 μm.) (C) Separation of the perichondrium layer from auricular cartilage. Separation was confirmed by Alcian blue staining of the separated chondrium layer (C), interlayer (I), and perichondrium layer (P) (also Fig. S1). (Scale bars, 200 μm.) (D) Clonal colony formation using cells derived from each layer after 4 wk (Bottom) of culture. Arrows indicate a single cell on day 1. (Scale bars, 500 μm.) (The figure is a composite of multiple panels.) (E) The colony-forming efficiency of cells derived from each layer. Each colony had to include more than 50 cells. Data are shown as the mean ± SD (n = 9). *P < 0.001. (F) Growth curves for cells derived from each layer after 196 d of culture. Data are shown as the mean ± SD (n = 5). *P < 0.01. (G) Flow cytometry analysis of the expression of cell-surface markers related to various stem cells on chondrocytes (Left) and perichondrocytes (Right). Isotype control antibodies were used for control samples (black dotted line). Perichondrocytes showed higher expression levels of CD44 and CD90 than was observed for chondrocytes.
Next, we analyzed the long-term proliferating capability to determine the proliferative rates of perichondrocytes. Perichondrocytes morphologically resembled fibroblasts and maintained this appearance after long-term culture (Fig. S1, Right). The proliferation rate of perichondrocytes and chondrocytes was determined from growth curves after 196 d of culture (Fig. 1F). On the basis of the growth curves, the doubling time of perichondrocytes was calculated to be 2.6 d compared with 3.0 d for chondrocytes. Thus, perichondrocytes contain highly expandable clones and proliferate 13% faster than chondrocytes.
Cell-Surface Marker Characterization of Human Perichondrocytes.
To characterize the cell-surface marker profile of human CSPC populations, we performed flow cytometry. The hematopoietic stem cell markers CD34 and c-kit (CD117), and the MSC markers CD44, CD73, CD90, CD105, CD133, CD140a, CD146, and CD271 (24–28) were analyzed (Fig. 1G). Most marker expressions of perichondrocytes were similar to that of chondrocytes. However, CD44 and CD90 expressions showed significant differences between them. Higher expressions of CD44 and CD90 were observed in perichondrocytes compared with those of chondrocytes (CD44, 58.8 ± 8.6% and 35.4 ± 7.3%; CD90, 63.0 ± 1.7% and 46.5 ± 3.9%), indicating a potential candidate for clonal isolation of stem cells.
In Vitro Elastic Cartilage Differentiation of Human Perichondrocytes.
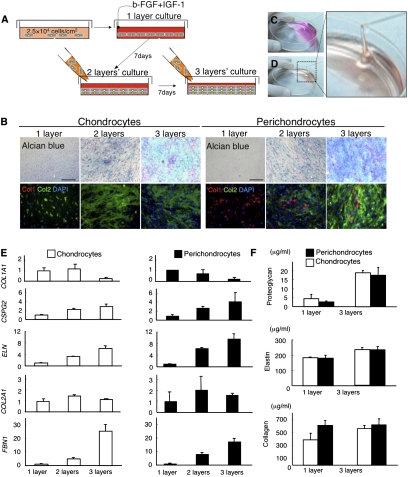
It is crucial to obtain a highly chondrogenic population with plenty of cartilage ECMs following in vitro cultivation before cell transplantation into a lesion. To develop suitable culture conditions for cell transplantation, we focused on the use of a layered culture system combined with several cytokines, which have been shown to enhance hyaline cartilage regeneration in vitro (29). Cultured human perichondrocytes were layered to differentiate them into mature chondrocytes in the presence of bFGF (basic fibroblast growth factor) and IGF1 (insulin-like growth factor 1) (Fig. 2A). The perichondrocytes gradually differentiated into chondrocytes on the basis of the layering and began to produce proteoglycan and type II collagen (Fig. 2B). Several mucopolysaccharides were secreted from the perichondrocytes, making the culture media viscous and matrix-like (Fig. 2 C and D and Movie S1). This is important because high viscosity encourages cells to stay near the site where transplanted.
Fig. 2.
Human perichondrium-derived cells show a highly chondrogenic profile. (A) Schematic diagram of the layered culture system used for chondrogenic induction. (B) Cytochemistry staining of chondrocytes (Left) and perichondrocytes (Right) after the cells were cultured in the layered system. Perichondrocytes differentiated into chondrocytes that produced proteoglycans (Alcian blue) and type II collagen (Col2, Green). (Scale bars, 200 μm.) (C and D) Aspiration of untreated (C) and treated (D) culture media. The secretion of several mucopolysaccharides from the perichondrocytes after layered induction made the media viscous and matrix-like (Movie S1). (E) Real-time PCR analyses of gene expression profiles related to elastic cartilage in chondrocytes (Left) and perichondrocytes (Right) after the cells were cultured in the layered system. Data are shown as the mean ± SD (n = 3). (F) ELISAs specific for proteoglycans, elastic fibers (elastin), and collagen secretion from chondrocytes (Left) and perichondrocytes (Right) after the cells were cultured in monolayers or a trilayered culture. Data are shown as the mean ± SD (n = 3).
Then, to quantify and compare the chondrogenic potential of the perichondrocytes with that of chondrocytes, we performed real-time PCR analysis of genes related to elastic cartilage differentiation. Under differentiation conditions, we observed increased expression of a number of chondrium markers, including versican (CSPG2, 4.2-fold), elastin (ELN, 9.6-fold), alpha 1 type II collagen (COL2A1, 2.1-fold), and fibrillin 1 (FBN1, 17.2-fold), whereas expression of the perichondrium marker alpha 1 type I collagen (COL1A1) decreased to 0.18-fold of the original level (Fig. 2E).
We also performed ELISAs to evaluate the secretion of elastic cartilage ECM proteins. The concentrations of secreted ECM proteins in perichondrocyte cultures under differentiation conditions were 17.5 ± 4.3 μg/mL proteoglycan, 235.6 ± 19.9 μg/mL elastin, and 61.8 ± 7.5 μg/mL collagen. Surprisingly, similar concentrations of secreted proteins were observed for chondrocytes (19.0 ± 1.3, 234.0 ± 16.3, and 55.8 ± 4.9 μg/mL, respectively), highlighting the high chondrogenic potential of the perichondrocytes (Fig. 2F).
Human Perichondrocytes Reconstructed Elastic Cartilage Containing Both Perichondrium and Chondrium Layers.
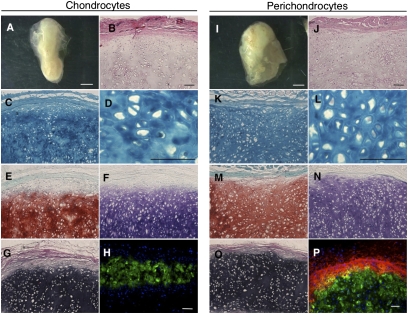
Human perichondrocytes were expanded, subjected to cartilage differentiation conditions, and s.c. injected into nonobese diabetic [(NOD)/SCID] mice. We performed the same experiments with human chondrocytes (Fig. 3 A–H). Histochemical analyses demonstrated that perichondrocytes differentiated into mature chondrocytes and formed an elastic cartilage rich with proteoglycans and elastic fibers (Fig. 3 I–O). Immunohistochemistry revealed that regenerated cartilage contained a Col I+ capsule enveloping a Col II+ chondrium layer (Fig. 3P). These results indicated that perichondrocytes contain a putative stem/progenitor population with regenerative capacity.
Fig. 3.
Tissue reconstitution capability of human perichondrocytes. (A and I) Chondrocytes (Left) and perichondrocytes (Right) formed cartilage-like tissues 3 mo after s.c. transplantation. (Scale bars, 1 mm.) (B and J) Hematoxylin/eosin, (C, D, K, and L) Alcian blue, (E and M) Toluidine blue, and (F and N) Safranin O staining showed that the reconstructed tissue contained mature chondrocytes in lacunae that produced high levels of proteoglycan. (G and O) Elastica Van Gieson staining showed that the reconstructed cartilage contained elastic fibers. (H and P) Immunohistochemistry revealed that perichondrocyte-derived cartilage (Right) contained type I collagen (Col1)+ capsules that enveloped a type II collagen (Col2)+ chondrium layer. (Scale bars, 200 μm.)
Elastic cartilage composed of two layers was maintained even 6 and 10 mo after perichondrocyte transplantation (Fig. S2 A–L). During the growth and maturation period, it is generally accepted that the cell density of the chondrium layer decreases (30). Consistent with this, we found that the cell density decreased from 1.6 to 0.4 × 106 cells/mm2 presumably due to cell maturation (Fig. S2M). The dry weight of perichondrocyte-derived cartilage is heavier than that of chondrocytes with statistical significance (Fig. S2N). Fibrous tissue, blood vessels, bone, or tumors did not develop during the 10-mo observation period. In practical applications for congenital anomalies, larger elastic cartilage should be reconstructed. To achieve this goal, we transplanted larger volumes of perichondrocytes (injection volume = 3 mL; ∼2 × 107 cells) after chondrogenic induction. After 2 mo, over 2 cm of elastic cartilage was generated (Fig. S3A and Movie S2). Reconstructed tissue consisted of homogenously distributed mature chondrocytes with plenty of cartilage ECMs (Fig. S3B). These results are particularly striking, as we were able to control the size of the reconstructed cartilage by adjusting the injection volumes.
Transient Amplification and Transition to a Dormant State of CD44+ CD90+ Cells.
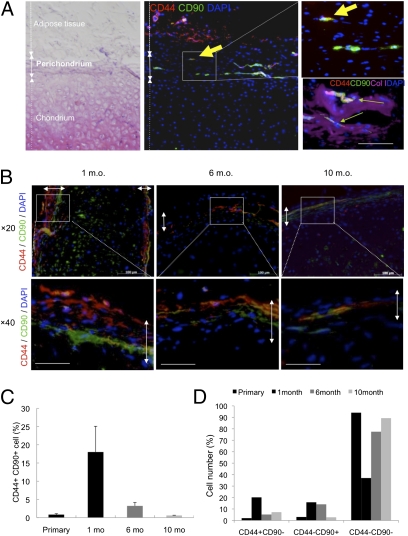
On the basis of FACS analyses, we investigated the distribution of CD44+ CD90+ cells in primary or regenerated tissues to characterize the putative stem cells. Immunohistochemistry revealed that in primary auricular cartilage, a rare population of CD44+ CD90+ cells specifically resided in the perichondrium layer, but not in the chondrium layer (Fig. 4A). The ratio of CD44/CD90 double positive cells was 0.84 ± 0.25% in the perichondrium layer. For regenerated cartilage, CD44+ CD90+ cells were retained in the perichondrium layer throughout the 10 mo (Fig. 4B). Interestingly, the ratio of CD44+ CD90+ cells in the perichondrium was initially amplified up to 18.02 ± 7.06% at 1 mo and then diminished to 0.62 ± 0.05% at 10 mo, the same as observed in primary auricular cartilage remnants (Fig. 4C). CD44 or CD90 single positive cells also experienced the same distributional change (Fig. 4D). These distributional changes seemed to depict the transient amplification of stem/progenitor cells at an initial state of tissue regeneration and transition to a dormant state at a later phase.
Fig. 4.
Presence and retention of CD44+ CD90+ cells in primary or regenerated perichondrium. (A) Rare population of cells in human primary auricular perichondrium (Col I+) specifically express CD44/CD90. Arrows indicate rare double positive cells existing in the outer perichondrium. (Scale bar, 50 μm.) (B) Immunohistochemical analyses of CD44 and CD90 showed apparently distinct distributions at multiple time points (1, 6, and 10 mo). Double-headed arrows show the perichondrium layers. (Scale bars, 100 μm.) (C and D) Quantification of CD44+ CD90+ cells (C) or CD44+ CD90−, CD44− CD90+, and CD44− CD90− cells (D) in primary or regenerated perichondrium.
Prospective Isolation of Stem Cells from Human Auricular Perichondrocytes.
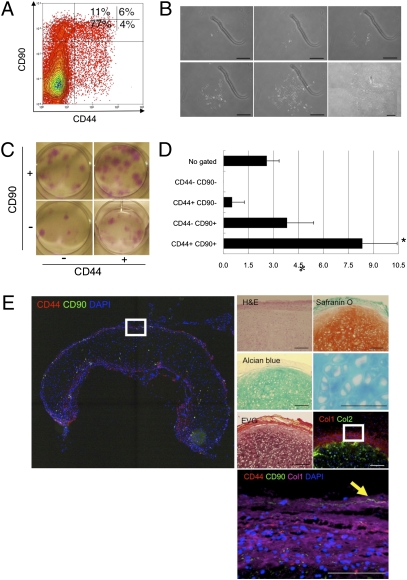
To provide definitive evidence of stem cells in the auricular perichondrium, flow cytometry was used to fractionate the perichondrocytes into four subpopulations on the basis of the expression of CD44 and CD90 (Fig. 5A). We then cultured the cells from each fraction at low density (52 cells/cm2) to identify the fraction that contained the cells that efficiently formed clonal colonies. After 21 d of culture, the CD44+ CD90+ cells clearly formed the most colonies (Fig. 5 B and C). These data suggested that both CD44 and CD90 were good cell-surface markers to enrich the stem/progenitor cells.
Fig. 5.
Reconstruction of human elastic cartilage by a single CD44+ CD90+ stem cell in the human auricular perichondrium. (A) Perichondrocytes were fractionated via flow cytometry on the basis of the expression of CD44 and CD90. The percentages of each subpopulation are shown. (B) Clonal colony formation of CD44+ CD90+ cells. (The figure is a composite of multiple panels.) (C) Macroscopic observation of clonal colonies derived from multicolor sorted cells visualized using Giemsa staining. (D) Ability of sorted perichondrocytes to form large colonies (LCs). The x axis shows the percentages of cells that formed colonies. Data are shown as the mean ± SD (n = 3). *P < 0.05. (E) Reconstruction of human elastic cartilage by clonally cultured CD44+ CD90+ cells. Reconstructed cartilage consisted of mature chondrocytes with plenty of cartilage ECMs, as evaluated by histochemical staining. The arrowhead shows the retention of a CD44+ CD90+ cell in the reconstructed perichondrium layer. (Scale bars, 100 μm.)
Then, we attempted to culture single cells in each well of 96-well plates, following clone sorting of cells on the basis of CD44/CD90 expression. After 21 d, we counted the large colonies (LCs > 100 cells), which potentially reflected a rapid rate of cell proliferation. The results showed that CD44+ CD90+ cells displayed a significantly greater capacity to extensively expand than was observed for CD44+ CD90−, CD44− CD90+, or CD44− CD90− cells (Fig. 5D). Clones isolated from the CD44+ CD90+ fraction most efficiently formed LCs (8.3 ± 2.1%) comprising several hundred cells. The CD44− CD90+ fraction-derived clones were also capable of forming LCs (3.8 ± 1.6%), but the efficiency of LC formation was statistically lower (P < 0.05, n = 3). Far fewer LCs were grown from clones derived from the CD44+ CD90− subpopulation, whereas cells from the CD44− CD90− fraction formed no LCs.
The isolation and cultivation of definitive single-cell–derived CD44+ CD90+ cells enabled us to evaluate several criteria of stem cells. We first examined multipotency using several sorted clones. Compared with unipotential chondrocytes (Fig. 2B and Fig. S4A), all of the six isolated clones possessed chondrogenic, adipogenic, and oseteogenic potential (Fig. S4B).
The identification of multipotent CD44+ CD90+ stem cells with high proliferative potential suggested that these cells could provide a basis for the reconstruction of continuously self-renewing elastic cartilage. To test this approach, we transplanted clonally propagated CD44+ CD90+ cells. Three months after transplantation, elastic cartilage containing both ColI+ perichondrium and Col II+ chondrium were generated. Cells in the chondrium layer consisted of mature chondrocytes packed in lacnae. Surprisingly, CD44+ CD90+ cells were retained in the outer perichondrium layer, suggesting the presence of self-renewing stem cells in the regenerated elastic cartilage.
Discussion
Despite the implications of stem cells in auricular perichondrium from rabbit studies, no studies to date have identified a definitive single-cell–derived stem cell population in elastic cartilage (21). This is attributable to the low frequencies of highly clonogenic stem cells, a common challenge in the field of mesenchymal stem cell-related research (31, 32). Here, we have successfully identified and isolated a human stem/progenitor cell population (CSPCs) existing in the auricular perichondrium by combinations of CD44 and CD90 markers. Clonally propagated CD44+ CD90+ cells from auricular cartilage, on the basis of a number of criteria, appear to be a population of stem cells. The utilization of CSPCs from human auricular cartilage will not only improve our understanding of basic cartilage biology, but will lead to novel therapeutic strategies, including long-term tissue restoration, for patients with craniofacial defects.
Various tissue-derived MSCs have been investigated as possible cellular resources for elastic cartilage reconstruction. These cells, however, do not efficiently differentiate into chondrocytes and fail to produce elastic cartilage-specific ECM components, such as proteoglycans and elastic fibers (16, 18). Furthermore, bone marrow MSC-derived cartilage is associated with hypertrophy, vascular invasion, and ectopic mineralization (17). In contrast to MSCs, CSPCs isolated from the auricular perichondrium displayed a highly chondrogenic profile that was similar to that observed for chondrocytes. However, similar to MSCs, these cells were able to differentiate into adipocytes and osteocytes, suggesting that they are a higher cell lineage than cartilage-committed progenitor cells, which are predetermined to form chondrocytes of elastic cartilage. In line with these expectations, CSPCs expressed CD44 and CD90, similar to MSCs, whereas they did not express the MSC markers CD133, CD140a, CD146, or CD271 (24, 33, 34). Thus, our isolated stem/progenitor cells are closely related to MSCs, yet are a distinct cartilage stem/progenitor cell population. The hierarchy of the mesenchymal cell lineage has not yet been clearly elucidated. A better understanding of this hierarchy may allow CSPCs to be used as a therapeutic resource for applications other than elastic cartilage reconstruction. Moreover, it may lessen the risk of certain adverse effects, such as ectopic ossification, which is a serious problem associated with MSCs.
Continuously self-renewing tissues are successively restored by stem cells. Hematopoiesis provides a well-known paradigm of a stem cell-dependent, steadily self-renewing system. Using postnatal stem cells may significantly alter approaches to cell-based engineering. We found that extensively expanded perichondrocytes that had been subjected to a layered culture system were able to form elastic cartilage after s.c. transplantation. Similar to normal auricular cartilage, the reconstructed cartilage was composed of two layers: perichondrium and chondrium. Transplants successfully restored their original structure even after 10 mo without ectopic tissue formation, indicating that perichondrocytes may be a better resource than MSCs. Our study suggests that the grafting of perichondrocytes should allow long-term restoration of reconstructed tissue owning to self-renewing CSPCs in the perichondrium layer.
Further evaluations of the extracellular matrix components or mechanical evaluations of the regenerated cartilage will be necessary to show the clinical relevance of perichondrocytes (35, 36). However, the use of autologous auricular perichondrocytes represents several important advantages compared with the harvesting of chondrocytes so far. First, harvesting perichondrocytes requires a minimally invasive procedure, whereas collecting chondrocytes from the auricle places a significant burden on donor sites. Perichondrocytes can be obtained from a thin fibrous layer of the auricle even from microtia patients. Second, their high proliferative potential enables us to shorten the culture period. Finally, it is expected that perichondrocytes will enhance morphological preservation through continuous self-renewal because they contain a definitive stem/progenitor population (CSPCs). This is important especially in children, as permanent tissue restoration through many decades is an essential requirement for treating craniofacial anomalies.
There are currently no published reports describing the successful clinical use of a stem cell population for human elastic cartilage reconstruction. Taken together, our innovative discoveries provide three different modes of treatment strategies using an autologous stem/progenitor cell population, as shown in Fig. S5: (i) Direct cell injection and (ii) Two-stage transplantation without a scaffold. First, direct cell injection, which consists of simple procedures, is ready for clinical application. The application is limited to small or simple-shaped lesions, but this method makes it possible to reconstruct self-renewing elastic cartilage after extended expansion of patients’ own cells. For larger and more complex deformities like microtia, we propose two-stage transplantation without a scaffold that is also clinically applicable. In this procedure, a sufficient volume of elastic cartilage is obtained from primary cell transplantation into a non–weight-bearing area like the lower abdomen. Then, a generated cartilage block is used for the framework designed for a given patient's deformities and is then transplanted into a lesion. The optimization of technical aspects (e.g., culture duration, injection volumes, and techniques for sculpting the framework) and further studies concerning safety should lead to the establishment of promising treatment options for currently incurable craniofacial anomalies.
Methods
Isolation and Cultivation of Human Perichondrocytes.
We obtained elastic cartilage samples from microtia patients following the approved guidelines set by the ethical committee at Yokohama City University (approval no. 03–074). We stripped off the adipose tissue and microscopically separated the cartilage into three layers: the chondrium layer, interlayer, and perichondrium layer. Dissected tissues were cut into small pieces and digested for 2 h at 37 °C in PBS containing 0.2% collagenase type II (Worthington) with shaking. After passing through a 100-μm nylon mesh (BD Falcon), the cells were washed three times with PBS. Cell suspensions were cultured in Dulbecco's modified Eagle medium and Ham's F-12 medium (DMEM/F-12; Nissui Pharmaceutical) supplemented with 10% FBS (Moregate) and 1% antibiotic antimycotic solution (AMS; Sigma) in 5% CO2 at 37 °C.
FACS Analysis and Cell Sorting.
We immunolabeled cells with 1 mg of fluorescence-conjugated mouse antihuman monoclonal antibodies or isotype-matched IgGs (Table S1) for 30 min at 4 °C. The fluorescence-labeled cells were analyzed and separated with a MoFlo cell sorter (DakoCytomation).
Cell Multipotency in Vitro.
For chondrogenic induction, each sample was seeded at a density of 2.5 × 104 cells/cm2. For the 48 h after seeding, cells were cultured under our standard culture medium. Thereafter, cells were cultured for 5 d using DMEM/F-12 containing 10% FBS, 1% AMS, L-ascorbic acid 2-phosphate, dexamethasone, insulin-like growth factor-1, and fibroblast growth factor-2. After 7 d of monolayer induction, detached cells from the same donor-derived subculture were adjusted to a density of 2.5 × 104 cells/cm2 and seeded on the monolayer culture. These steps were repeated twice with an interval of 1 wk. We also tested the adipogenic and osteogenic potential of perichondrocytes as previously described (37).
Gene Expression Analysis.
For reverse transcription-polymerase chain reaction (RT-PCR) experiments, we designed primers (SI Methods) using Primer3 software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi). The following quantitative PCR (qPCR) primers were used in TaqMan gene expression assays: COL1A1, Hs00266273_ml; COL2A1, Hs00164099_ml; CSPG2, Hs01007933_m1; ELN, Hs00355783_ml; and FBN1, Hs00171191_m1 (Applied Biosystems).
ELISA.
We quantitatively determined the chondrogenic potential of perichondrocytes by measuring proteoglycan, elastin, and collagen production using ELISAs. We subjected supernatants from the cell culture dish to Blyscan, Fastin, and Sircol assays (Biocolor) (38).
In Vivo Transplantation.
Cells that had been subjected to chondrogenic differentiation were scraped with a cell lifter. The scraped cells were collected into a 2.5-mL syringe (Terumo) equipped with a 23-gauge injection needle (Terumo). An appropriate volume of the culture was s.c. injected into NOD/SCID mice (Sankyo Laboratory). The mice were bred and maintained in accordance with our institutional guidelines for the use of laboratory animals.
Histochemical and Immunohistochemical Analysis.
We stained the sections and/or cultured cells with HE, AB, Toluidine blue, Safranin O, EVG, Alizarin Red S (Muto Pure Chemicals), or Oil Red O (Sigma). For immunohistochemical analysis, the tissue sections and cultured cells were immunolabeled with primary antibodies, rabbit antihuman type I collagen monoclonal antibodies (Monosan), and mouse antihuman type II collagen polyclonal antibodies (Chemicon), at 4 °C overnight. After washing, the sections and/or cells were incubated with Alexa Fluor 488- and/or Cy3-conjugated secondary antibodies (1:800; Molecular Probes) specific for the appropriate species for 1 h at room temperature. The samples were counterstained with 4,6-diamidino-2-phenylindole (DAPI) and analyzed with a LSM510 laser-scanning microscope (Zeiss).
Statistical Analysis.
Data are expressed as the mean ± SD from at least three independent experiments. Differences between three or four groups were analyzed using the Kruskal–Wallis test by ranks, and post hoc comparisons were made with Mann–Whitney U test with Bonferroni correction. Two-tailed P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank N. Sasaki and R. Hirochika for technical support, A. Tanaka and H. Sato for FACS operation, and Y. Yabuki and Y. Yamamoto for providing clinical samples. We are especially grateful to K. Sekine, T. Ogawa, Y. Nagashima, U. Yokoyama, and T. Hirose for critical reading of the manuscript. This work was supported by Grant 20592101 from the Grant-in-Aid for Developmental Scientific Research, the Ministry of Education, Science and Culture. This work was also supported by Grant 19-15 from the Research and Development Project of Yokohama City University, Yokohama, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109767108/-/DCSupplemental.
References
- 1.Chang SC, Tobias G, Roy AK, Vacanti CA, Bonassar LJ. Tissue engineering of autologous cartilage for craniofacial reconstruction by injection molding. Plast Reconstr Surg. 2003;112(3):793–799. doi: 10.1097/01.PRS.0000069711.31021.94. discussion 800–801. [DOI] [PubMed] [Google Scholar]
- 2.Matton G, Anseeuw A, De Keyser F. The history of injectable biomaterials and the biology of collagen. Aesthetic Plast Surg. 1985;9:133–140. doi: 10.1007/BF01570345. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S. Modification of the stages in total reconstruction of the auricle: Part I. Grafting the three-dimensional costal cartilage framework for lobule-type microtia. Plast Reconstr Surg. 1994;93(2):221–230. discussion 267–268. [PubMed] [Google Scholar]
- 4.Maas CS, Monhian N, Shah SB. Implants in rhinoplasty. Facial Plast Surg. 1997;13:279–290. doi: 10.1055/s-0028-1082427. [DOI] [PubMed] [Google Scholar]
- 5.Matarasso A, Elias AC, Elias RL. Labial incompetence: A marker for progressive bone resorption in silastic chin augmentation. Plast Reconstr Surg. 1996;98:1007–1014, discussion 1015. doi: 10.1097/00006534-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Y, Wu W, Yu H, Yang J, Chen G. Silicone implants in augmentation rhinoplasty. Aesthetic Plast Surg. 2002;26:85–88. doi: 10.1007/s00266-002-1493-0. [DOI] [PubMed] [Google Scholar]
- 7.Laurie SW, Kaban LB, Mulliken JB, Murray JE. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933–938. doi: 10.1097/00006534-198406000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Skouteris CA, Sotereanos GC. Donor site morbidity following harvesting of autogenous rib grafts. J Oral Maxillofac Surg. 1989;47:808–812. doi: 10.1016/s0278-2391(89)80038-2. [DOI] [PubMed] [Google Scholar]
- 9.Kline RM, Jr, Wolfe SA. Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 1995;95:5–13, discussion 14–20. [PubMed] [Google Scholar]
- 10.Whitaker LA, et al. Combined report of problems and complications in 793 craniofacial operations. Plast Reconstr Surg. 1979;64:198–203. doi: 10.1097/00006534-197908000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Berry L, Grant ME, McClure J, Rooney P. Bone-marrow-derived chondrogenesis in vitro. J Cell Sci. 1992;101:333–342. doi: 10.1242/jcs.101.2.333. [DOI] [PubMed] [Google Scholar]
- 12.Ma HL, Hung SC, Lin SY, Chen YL, Lo WH. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res A. 2003;64:273–281. doi: 10.1002/jbm.a.10370. [DOI] [PubMed] [Google Scholar]
- 13.Terada S, Fuchs JR, Yoshimoto H, Fauza DO, Vacanti JP. In vitro cartilage regeneration from proliferated adult elastic chondrocytes. Ann Plast Surg. 2005;55:196–201. doi: 10.1097/01.sap.0000164388.33965.4e. [DOI] [PubMed] [Google Scholar]
- 14.Yanaga H, et al. Clinical application of cultured autologous human auricular chondrocytes with autologous serum for craniofacial or nasal augmentation and repair. Plast Reconstr Surg. 2006;117(6):2019–2030. doi: 10.1097/01.prs.0000210662.12267.de. discussion 2031–2032. [DOI] [PubMed] [Google Scholar]
- 15.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 16.Shieh SJ, Terada S, Vacanti JP. Tissue engineering auricular reconstruction: In vitro and in vivo studies. Biomaterials. 2004;25:1545–1557. doi: 10.1016/s0142-9612(03)00501-5. [DOI] [PubMed] [Google Scholar]
- 17.Dickhut A, et al. Calcification or dedifferentiation: Requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–226. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 18.Afizah H, Yang Z, Hui JH, Ouyang HW, Lee EH. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSCs) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659–666. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 19.Koga H, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 21.Togo T, et al. Identification of cartilage progenitor cells in the adult ear perichondrium: Utilization for cartilage reconstruction. Lab Invest. 2006;86:445–457. doi: 10.1038/labinvest.3700409. [DOI] [PubMed] [Google Scholar]
- 22.Duynstee ML, Verwoerd-Verhoef HL, Verwoerd CD, Van Osch GJ. The dual role of perichondrium in cartilage wound healing. Plast Reconstr Surg. 2002;110:1073–1079. doi: 10.1097/01.PRS.0000020991.10201.6C. [DOI] [PubMed] [Google Scholar]
- 23.Haberal Can I, Atilla P, Cakar AN, Onerci M. An animal study on cartilage healing using auricular cartilage as a model. Eur Arch Otorhinolaryngol. 2008;265:307–311. doi: 10.1007/s00405-007-0455-1. [DOI] [PubMed] [Google Scholar]
- 24.Quirici N, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 25.Boiret N, et al. Characterization of nonexpanded mesenchymal progenitor cells from normal adult human bone marrow. Exp Hematol. 2005;33:219–225. doi: 10.1016/j.exphem.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Aslan H, et al. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 2006;24:1728–1737. doi: 10.1634/stemcells.2005-0546. [DOI] [PubMed] [Google Scholar]
- 27.Nimura A, et al. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: Comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58:501–510. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 28.Covas DT, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev. 2010;16:445–453. doi: 10.1089/ten.TEB.2009.0825. [DOI] [PubMed] [Google Scholar]
- 30.Vignon E, Arlot M, Patricot LM, Vignon G. The cell density of human femoral head cartilage. Clin Orthop Relat Res. 1976;(121):303–308. [PubMed] [Google Scholar]
- 31.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 32.Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE. 2009;4:e6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gojo S, et al. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res. 2003;288:51–59. doi: 10.1016/s0014-4827(03)00132-0. [DOI] [PubMed] [Google Scholar]
- 34.Tondreau T, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: Proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 35.Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode frictional properties of engineered cartilaginous tissues. Eur Cell Mater. 2007;14:20–28, discussion 28–29. doi: 10.22203/ecm.v014a02. [DOI] [PubMed] [Google Scholar]
- 36.Roy R, et al. Analysis of bending behavior of native and engineered auricular and costal cartilage. J Biomed Mater Res A. 2004;68:597–602. doi: 10.1002/jbm.a.10068. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi K, et al. Transcripts of unknown function in multiple-signaling pathways involved in human stem cell differentiation. Nucleic Acids Res. 2009;37:4987–5000. doi: 10.1093/nar/gkp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stock UA, et al. Dynamics of extracellular matrix production and turnover in tissue engineered cardiovascular structures. J Cell Biochem. 2001;81:220–228. doi: 10.1002/1097-4644(20010501)81:2<220::aid-jcb1037>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.