Abstract
It is becoming increasingly clear that global warming is taking place; however, its long-term effects on biological populations are largely unknown due to lack of long-term data. Here, we reconstructed a 1,910-y-long time series of outbreaks of Oriental migratory locusts (Locusta migratoria manilensis) in China, on the basis of information extracted from >8,000 historical documents. First by analyzing the most recent period with the best data quality using generalized additive models, we found statistically significant associations between the reconstructed locust abundance and indexes of precipitation and temperature at both annual (A.D. 1512–1911) and decadal (A.D. 1000–1900) scales: There were more locusts under dry and cold conditions and when locust abundance was high in the preceding year or decade. Second, by exploring locust–environment correlations using a 200-y moving window, we tested whether these associations also hold further back in time. The locust–precipitation correlation was found to hold at least as far back as to A.D. 500, supporting the robustness of this link as well as the quality of both reconstructions. The locust–temperature correlation was weaker and less consistent, which may reflect this link being indirect and thus more easily moderated by other factors. We anticipate that further analysis of this unique time series now available to the scientific community will continue to provide insights into biological consequences of climate change in the years to come.
Outbreak of Oriental migratory locusts (Locusta migratoria manilensis) was, together with drought and flood, considered one of the three most severe natural disasters causing damage to crop production in ancient China and has long been noted and consistently recorded in historical documents (1). The earliest known written record of locusts was found inscribed on an ox bone in Oracle Script (Jiaguwen, the earliest Chinese script) 3,500 y ago, asking: “Will locusts appear in the field; will it not rain?” (2). Starting from the History of the Former Han Dynasty (Hanshu) (B.C. 206–A.D. 25), locust records frequently appeared in standard histories (official dynastic histories) (3), and during the Ming-Qing period (A.D. 1368–1911), with the flourishing of gazetteers (local historical chronicles), thousands of detailed locust records were preserved (4).
Using locust records collected from historical documents by Chen (4) and Chen (5), Ma constructed a 1,000-y-long locust series (6). The locust index was derived by summing the reported intensity (ranked 0–5) and spatial extent (ranked 0–5) of locust outbreaks. On the basis of the reconstructed locust series, Ma reported that locust outbreaks in China show no obvious periodicity and that outbreaks generally occurred in drought years or years after floods. For the Hongze Lake region during the period 1913–1962, Ma et al. found that the largest locust outbreaks often occurred in warm and dry years (7). It was supposed that warm temperatures may benefit locusts by increasing winter survival of nymphs, whereas dryness can supply more suitable habitats along riverbanks and lakesides. Using Ma's 1,000-y locust series (6), Stige et al. (8) found that decadal mean locust abundance was negatively associated with temperature and positively associated with the frequencies of droughts and floods. Using wavelet analysis to explore Ma's locust series further, Zhang et al. (9) suggested that 160- to 170-y periodic cooling promotes locust outbreaks by enhancing temperature-associated drought/flood events. In contrast to the studies of Stige et al. (8) and Zhang et al. (9), using more local-scale climate data reconstructed by a general circulation model (GCM), Yu et al. (10) found positive associations between Ma's locust index and temperature at both interannual and interdecadal scales.
These contradictory results have incited us to study the relationship of locusts and temperature further, and a crucial step is to make sure the reconstructed locust series is reliable. The reconstruction method used by Ma (6) has shortcomings that can potentially generate an increasing trend in the locust series. For example, there are higher numbers of local historical documents in more recent periods (especially during the Ming and Qing dynasties) and thus, possibly, higher chance for locust outbreaks to be reported. The territory and its administration units (province, prefecture, or county) also changed from dynasty to dynasty, which may also bias the locust estimation. When studying long-term variation in locust occurrences, such biases should be minimized.
Zhang (11) published a meteorological records compendium based on 8,228 Chinese historical documents. This compendium contains almost the entire available historical records of weather-related phenomena from the 23rd century B.C. until A.D. 1911 and includes >8,000 records of locust occurrence [twice the number Ma (6) used]. The compendium provides a unique opportunity for reconstructing a more reliable locust series for the last two millennia. In this paper, we present the reconstruction of a 1,910-y-long (A.D. 2–1911) locust abundance series and analyze its relationship with different climate proxies. As the association between temperature and precipitation in China may be scale dependent in space, we considered temperature indexes ranging from local (Beijing) to eastern China, entire China, and the northern hemisphere. To draw robust inferences, we first analyzed the more recent and reliable data at both annual and decadal scales using generalized additive models (GAMs) and then tested whether the correlations hold in the earlier parts of the period.
Results
Reconstructed Time Series.
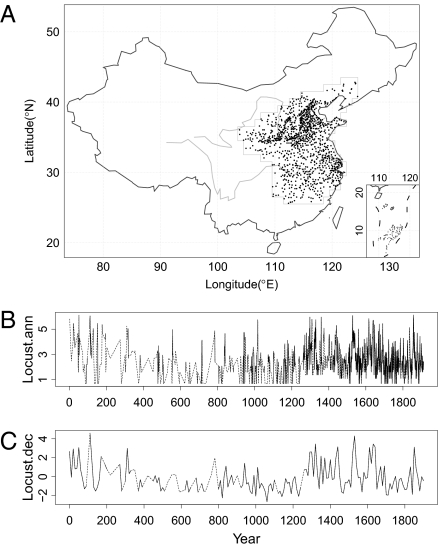
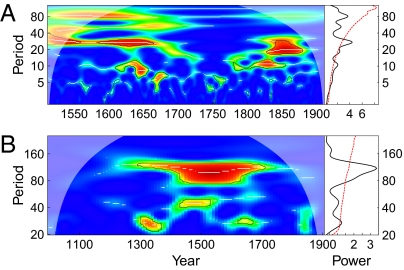
The annual locust index for A.D. 1368–1911 was constructed by multiplying the reported spatial extent (number of counties) and intensity (ranked 1–3) of locust outbreaks and then adjusting for trends in recording effort; locust index values for A.D. 2–1367 were first calculated as the number of prefectures having locust reports and then converted to the same unit as the locust index for A.D. 1368–1911. The annual locust index was natural logarithm transformed for statistical convenience. A decadal index was constructed by averaging the annual (log-transformed) values over each decade and then subtracting its overall mean and dividing by the SE (overall SD/√n, where n is the number of years with locust records in a given decade). The spatial distribution of locust records and the reconstructed locust series are shown in Fig. 1. As seen from the dashed lines in Fig. 1 B and C, the reconstructed locust series contains many no-report years (hereafter referred to as missing values). These missing values are most frequent in the first 1,000 y, probably due to poor recording effort. For drawing robust inferences, we first focused the analysis on periods with few missing values: A.D. 1512–1911 for annual-scale analysis and period A.D. 1000–1910 for decadal-scale analysis. Periodicity analysis for these time periods shows that the annual series displayed a 30-y cycle around A.D. 1512–1660 and 1850 and a 10-y cycle intermittently across the whole period; for the decadal series, a predominant 110-y cycle was found around A.D. 1300–1700, together with shorter-period cycles around A.D. 1350, 1500, and 1650 (Fig. 2).
Fig. 1.
(A) The locations of locust records (solid points) and research region (gray-lined polygon). (B) Annual locust index. (C) Decadal locust index. Dashed lines connect data points across periods with no locust reports.
Fig. 2.
Periodicity analysis for annual and decadal locust series: (Left) wavelet power spectra and (Right) global wavelet power spectra (estimations of the classical Fourier power spectra). (A) Annual data for A.D. 1512–1911; (B) decadal data for A.D. 1000–1910. In the wavelet power spectra, dark contour lines (and the red lines in their global wavelet power spectra) are the 95% confidence interval based on 2,000 Beta-Surrogate series, white lines materialize the maxima of the undulations of the wavelet power spectra, semitransparent cones indicate the regions influenced by edge effects, and the colors code for power values from dark blue (low values) to dark red (high values).
Locusts and Climate.
Correlation analysis.
Correlations between locust and climate indexes (Table 1) were calculated using both original and detrended series (SI Appendix, Fig. S6). Results revealed positive associations between locust abundance and dryness at both annual and decadal scales, although the correlation with the decadal index was statistically significant only after detrending the time series (Table 2, “dryness” and “precipitation” indexes). Further, there was a negative association between locust abundance and annual temperature (Table 2, “temp.T”: Beijing), but it was statistically significant at P < 0.05 only before detrending. The decadal-scale analysis further suggested that the locust–temperature association was stronger when a temperature index representative of entire China (Table 2, “temp.Y”) was used, both compared with a regional-scale index (Table 2, “temp.W”: eastern China) and compared with indexes for northern hemisphere temperature (Table 2, “temp.M” and “temp.L”).
Table 1.
Climate variables used in this study
| Variable | Location | Period (A.D.) | Resolution | Seasonality | Data type | Ref. |
| Dryness | Eastern China (103°–125°E, 25°–43°N) | 1470–2000 | Annual | Spring–autumn | Historical documents, mean of 65 stations | (12) |
| Precipitation | Eastern China (105°–121°E, 25°–40°N) | 501–2000 | Decadal | Spring–autumn | Historical documents, mean of 48 stations | (13) |
| Temp.T | Beijing, China (40°N, 116°E) | 1–1985 | Annual | Summer | Stalagmite layer thickness | (14) |
| Temp.W | Eastern China (106°–127°E, 23°–42°N) | 1000–1999 | Decadal | Annual | Composite of 7 proxies | (15) |
| Temp.Y | China (20°–42°N, 80°–130°E) | 1–1999 | Decadal | Annual | Composite of 9 proxies | (16) |
| Temp.M | Northern Hemisphere (18°–90°N) | 1–1979 | Annual | Annual | Composite of 18 proxies | (17) |
| Temp.L | Northern Hemisphere (30°–90°N) | 1–1999 | Decadal | Annual | Composite of 30 proxies | (18) |
Table 2.
Correlations between reconstructed locust abundance and climate indexes
| Climate index | Period | Original | Detrended |
| Dryness | 1512–1911 | 0.49**** | 0.48**** |
| Precipitation | 1000–1900 | −0.19 | −0.30*** |
| Temp.T | 1512–1911 | −0.23** | −0.09 |
| Temp.W | 1000–1900 | −0.16 | −0.08 |
| Temp.Y | 1000–1900 | −0.32** | −0.20* |
| Temp.M | 1000–1900 | −0.26 | −0.06 |
| Temp.L | 1000–1900 | −0.29 | −0.03 |
Original: correlations between original time series. Detrended: correlations between detrended time series. P values were adjusted for autocorrelation following ref. 30.
*P < 0.10;
**P < 0.05;
***P < 0.01;
****P < 0.001.
Regression analysis.
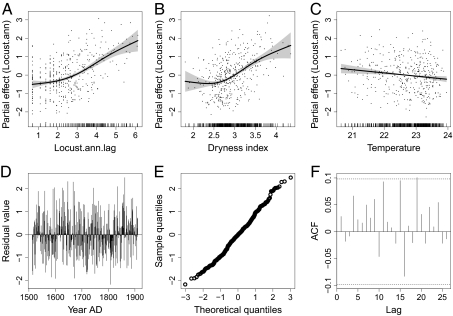
Fig. 3 shows the combined effects of previous year's locust abundance, dryness, and temperature on current year's locust abundance, estimated using the GAM on annual-scale data for the last 400 y. This model (baseline model) explained 47.0% of the variance in the locust series. In comparison, a model with no climate predictor variable (only previous decade's locust abundance) explained 30.8%, a model with temperature as the only climate variable explained 32.7%, and a model with only precipitation explained 45.9% (SI Appendix, Table S1). The model shows that the previous year's locust abundance and dryness have generally positive but somewhat nonlinear effects on the current year's locust abundance, whereas temperature has a weaker, negative effect (Fig. 3 A–C). The nonlinearities in the estimated effects suggest that the previous year's locust abundance has an effect only after reaching a threshold value of ∼1–2 on the locust index scale and dryness after reaching a value of ∼2.5 on the dryness index scale. That is, there appears to be less difference in abundance of locusts between wet and normal years than between normal and dry years. Residual diagnostics (Fig. 3 D–F) show that the residuals are approximately normally distributed and independent. Results were qualitatively similar when all time series were detrended before analysis (SI Appendix, Fig. S7), suggesting that the effects were not artifacts of low-frequency trends in the data.
Fig. 3.
Regression analysis of the effects of climate on annual-scale locust dynamics. (A–C) Estimated effects of previous year locust index, dryness [“dryness”, Table 1; average of regional values ranging from 1 (very wet) to 5 (very dry)], and temperature (“temp.T”, Table 1, unit is °C) on locusts, respectively. Points: partial residuals. Shaded areas: 95% confidence bands. Residual diagnostics reveal no residual trend (D), approximate normal distribution of residuals (E; the quantile plot of residuals forms a nearly straight line), and no significant autocorrelation function (ACF) of residuals (F; autocorrelation marginally surpassed the 5% critical limits indicated by the dotted lines for one out of the 25 time lags considered (lag = 19 y), which is roughly as expected to arise from chance alone).
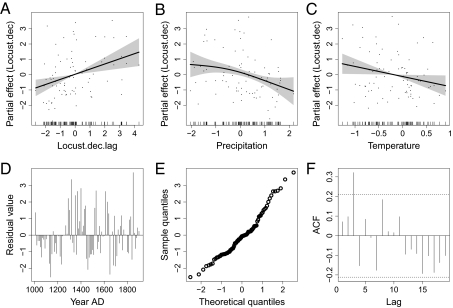
Fig. 4 shows the combined effects of the previous decade's locust abundance, precipitation, and temperature on locust abundance, estimated using the GAM on decadal-scale data for A.D. 1000–1900. This model explained 25.6% of the variance in the decadal locust series. In comparison, a model with no climate predictor variable (only the previous decade's locust abundance) explained 10.8%, a model with temperature as the only climate variable explained 17.1%, and a model with only precipitation explained 21.3% (SI Appendix, Table S1). All variables in the model showed a significant effect: previous decade's locust abundance has a positive linear effect, precipitation has a negative nonlinear effect, and temperature has a linear negative effect on decadal mean locust abundance (Fig. 4 A–C). Residual diagnostics showed that residuals are nearly normally distributed, but not independent (significant autocorrelation at lag 3 decades, Fig. 4F), which means that some of the low-frequency variation in the response was not captured by the model. For example, there were negative anomalies in locust abundance in the two to three first centuries not captured by the predictor variables in the model (Fig. 4D). When detrending the data before analysis, the temperature effect was estimated to be nonlinear, whereas the other results remained qualitatively similar (SI Appendix, Fig. S8). The functional form of the temperature effect is therefore questionable, as the reliability of the linear effect shown in Fig. 4 depends on how well the temperature and locust series preserve the low-frequency variation.
Fig. 4.
Regression analysis of effects of climate on decadal-scale locust dynamics. (A–C) The effects of previous decade's locust abundance, precipitation (Table 1, σ-unit), and temperature (“temp.Y”, Table 1; σ-unit), respectively, on decadal mean locust abundance. (D) Time series of residuals suggest that reconstructed locust abundance tended to be lower than predicted during the first centuries. (E) Residuals were approximately normally distributed. (F) Autocorrelation function of residuals (ACF) reveals significant positive autocorrelation at lag 3 decades.
Correlation back to first 1,000 y.
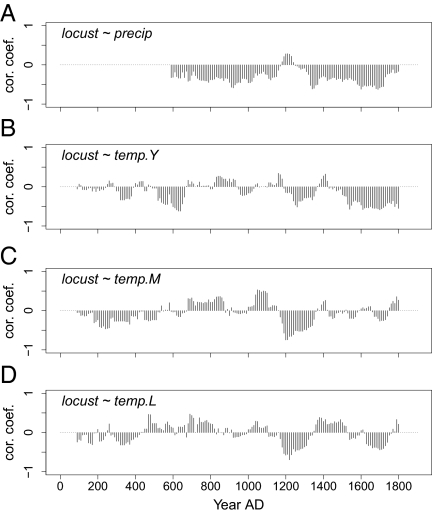
To evaluate the consistency of the locust–climate correlations through the 1910-y period, we computed correlations between decadal locust and climate indexes using a 200-y moving window (Fig. 5). We found that the correlation between locusts and precipitation was consistently negative throughout the period investigated (A.D. 500–1900), except for a short period around AD 1200 (Fig. 5A). The correlation between locusts and temperature in China (“temp.Y”, Fig. 5B) was generally negative, but both weaker and less consistent than the locust–precipitation correlation. For example, around A.D. 0–300, A.D. 700–900, A.D. 1100, and A.D. 1400 the correlations were weak or positive. Also the correlations between locusts and two larger-scale temperature indexes varied through time (Fig. 5 C and D), partly in parallel with those shown in Fig. 5B, suggesting that this variation was not solely an artifact of the temperature reconstruction method.
Fig. 5.
(A–D) Correlations between the reconstructed locust index and decadal climate reconstructions using a 200-y moving window centered at the x-axis values. The precipitation index is available only from A.D. 500 onward.
Discussion
China is the only nation with historical documents systematically recorded and well preserved, covering the past two millennia. These documents contain not only valuable information about history and culture, but also important meteorological, agricultural, and biological information. In this study we reconstructed a nearly 2,000-y locust abundance series, which is, to our knowledge, the longest observation-based time series of biological populations. The source material used in our reconstruction (11) is a long-term, systematic collection of meteorology-related records from thousands of historical documents, which contain twice the number of locust records used in the previous locust dynamic reconstruction by Ma (6). Moreover, our reconstruction has strived to overcome potential shortcomings of Ma's locust series caused by variation in recording effort and administrative division from dynasty to dynasty. Our locust series (natural-logarithm transformed) correlates well with Ma's for A.D. 960–1911 (r = 0.79), suggesting that the reconstructed locust abundance is not very sensitive to the choice of reconstruction method. The uncertainty of the reconstruction varies between periods. During the last 400 y, recording frequency was consistently high, and we believe that both the decadal and the annual locust series have optimal quality. Before this period, recording frequency was lower, especially for the first 1,000 y. Although we have used the most comprehensive material available, many years have no locust reports at all, in some periods for >40 y in a row. Given this limitation, it is noteworthy that the negative correlation between the precipitation index and the locust index held at least back to A.D. 500 (no precipitation data were available before this time), suggesting that there is indeed a biological signal in the locust series also in the earlier periods.
The periodicity of locust outbreaks has attracted ecologists for a long time (8, 9, 19–21). Our reconstructed locust series failed to reveal consistent periodicity, but predominant periods of 10 y, 30 y, and 110 y showed transient significance in the global wavelet power spectrum (Fig. 2). Further, previous decade's locust abundance was found to have a positive effect on decadal mean locust abundance. This finding is consistent with the periodicity analysis, which showed several time periods with cycles of ∼10 y. Also the residual autocorrelation at lag 3 decades is consistent with the periodicity analysis, showing a predominant period around 30 y. As locust abundance may respond rapidly to climate conditions, such transient periodicities seem likely to result from the superimposition of several somewhat irregular climate cycles affecting rainfall and temperature (22). Other examples of possible climate-linked, transient population cycles are oscillations of budmoths in the Alps (23) and lemmings in Scandinavia (24) having disappeared with the recent warming.
The analysis of interannual locust dynamics for the last 400 y showed that locust abundance in a given year depends mainly on the previous year's locust abundance and the current year's precipitation and to a lesser extent on temperature. The effect of the previous year's locust abundance was nonlinear, with a low effect at low values (annual locust index <2), suggesting that it is only intermediate and large outbreaks that increase locust outbreaks in a following year. We hypothesize that this result may be related to the life history of locusts, as they form migratory swarms only after reaching a certain threshold density (6). Further, the dryness index showed a nonlinear effect, consistent with droughts (dryness index >3) leading to more locust outbreaks through effects on the breeding habitat, whereas current year floods (dryness index <3) have no effect on locusts compared with normal years (6).
The results of the analysis of interdecadal locust dynamics largely conformed to the results of the annual-scale analysis, showing a strong, negative effect of precipitation and a weaker, negative effect of temperature. Temperature may affect locusts in an indirect way (8, 9), as cooling decreases summer monsoon rainfall through reduced moisture transport from the surrounding oceans to the Asian continent (25, 26). The fact that it was the temperature index representative of entire China that provided the highest explanatory power, rather than the temperature index for eastern China, from where the locust data derive, is consistent with the locust–temperature association not reflecting a direct, causal link. Across the 1910 y, we found that the correlation between temperature and locusts alternated between positive and negative values (although being negative on average), which may also be more easy to explain if the effect is indirect. Other explanations for the changing temperature–locust correlation could be inaccuracies in either index or the relationship being spurious.
Global climate change has been a focus of both scientists and the public. In general, studies on climate change based on long-term biological data are extremely rare (27, 28), and our newly reconstructed locust series of 1910 y is among the longest time series of biological populations. Global warming is thought to increase the frequencies of several meteorological, agricultural, and biological disasters, for example through temperature-driven increases in insect outbreaks (29). Our study on long-term locust dynamics highlights that other climate factors than temperature per se may be driving biological populations and that predicting global warming effects on precipitation patterns should receive increased attention. As more and better proxies of the historical fluctuations in climate are becoming available to the scientific community, we anticipate that further analysis of this unique locust index (SI Appendix, Datasets S1 and S2) will provide unique insights into the biological consequences of global climate change in the years to come. In particular, we believe the locust series provides a unique opportunity to explore how climate and intrinsic population processes jointly shape population fluctuations at different timescales.
Materials and Methods
Locust Series.
See SI Appendix for more detailed descriptions of the materials and reconstruction of the 1,910-y-long locust index. The annual locust abundance index was constructed on the basis of >8,000 locust records compiled in A Compendium of Chinese Meteorological Records of the Last 3000 Years (11). The total period considered (A.D. 2–1911) can be separated into two periods, A (A.D. 2–1367) and B (A.D. 1368–1911, i.e., the Ming and Qing dynasties). In period A, 598 of 639 locust records derive from standard histories (e.g., Twenty-Four Histories, Comprehensive Mirror to Aid in Government), whereas in period B, 7,519 of 7,677 locust records derive from local gazetteers (provincial, prefectural, and district gazetteers). To ensure consistent spatial coverage throughout the period studied we restricted the study area to eastern China (∼25–43°N, 103–125°E; Fig. 1), which is the most important agricultural region and also coincides with the core distribution area of Oriental migratory locusts in China.
The descriptions of locust outbreaks in period A are relatively simple, and the number of prefectures where locust outbreaks were recorded was chosen as the initial locust index unit for this period. Locust records in period B contain more detailed descriptions on extent and intensity of locust outbreaks, and we used county as a spatial unit and assigned each record an intensity grade ranked 1–3 according to the description of the outbreak. Initial locust index values for period B were calculated by summing the intensity grade values across all counties with recorded locusts in 1 y. The reconstructed locust series for period B displayed a significant increasing trend (P < 0.05), possibly because of bias from increased recording effort. The locust index for period B was therefore adjusted for recording effort, using the total number of historical records in the source archive (11) as recording effort proxy. The initial locust index values for periods A and B cannot be compared directly due to the different units. We therefore converted the initial locust index unit of period A into the recording effort-corrected unit of period B using regressive models, resulting in the 1,910-y-long annual locust time series (Ladj). The reconstructed locust index was highly skewed and for the convenience of statistical analysis, we log-transformed the adjusted locust series: Lann = ln(Ladj + 1).
For comparison with decadal-scale climate proxies, a decadal locust time series was constructed. The annual locust series contains many years with no locust reports (73% of the years in period A and 12% of the years in period B). Because most of these years were in periods with low recording effort (i.e., in period A or early in period B), these years were interpreted as random “missing values” rather than “absence of locusts” in this study. We therefore removed these years before calculating decadal mean locust abundance. As locust abundance in some of these years was probably truly low, removal of these years also removed some of the information in the data, but we considered this step necessary to obtain a series unbiased by sampling effort. A normalized decadal locust index (Ldec), corrected for higher sampling variance in decades with less observations, was defined as
Here, 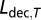 is Ldec for decade T,
is Ldec for decade T, 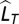 is the mean of Lann for decade T,
is the mean of Lann for decade T, 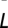 is the long-term mean of the annual locust index Lann, σ is the SD of Lann, and
is the long-term mean of the annual locust index Lann, σ is the SD of Lann, and 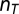 is the number of years with observations in decade T. Note that the denominator is the SE of
is the number of years with observations in decade T. Note that the denominator is the SE of 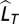 and that the unit of Ldec is SD units. The decadal locust series has 31 decades with missing values, most of which (29) appear in the first 1,000 y.
and that the unit of Ldec is SD units. The decadal locust series has 31 decades with missing values, most of which (29) appear in the first 1,000 y.
The annual locust series for the last 400 y has the highest quality due to the large number of historical documents available for this period. There are still 19 y (5%) with no locust reports, which, considering the high recording effort, we interpreted as the lightest locust outbreaks [Lann = ln(2)] in the analyses. There are two decades with no locust reports in the last 910 y; these decades were left out of most analyses. For wavelet decomposition, which does not allow inclusion of missing values, Ldec for these decades was imputed by linear interpolation.
Climate Data.
Climate data used in this study are listed in Table 1. Precipitation data (dryness and precipitation in Table 1) were extracted from historical documents from China (similar sources to the locust records) and have similar spatial coverage to the locust data. Temp.T is the only available long-term annual temperature proxy inside our study region. The decadal temperature proxy temp.W matches our research region best, temp.Y represents the temperature of entire China, and temp.M and temp.L are two temperature proxies representing the northern hemisphere.
Statistical Analysis.
Detrending.
The detrended versions of the time series were residuals from GAMs of each time series as a smooth function of year (natural cubic spline with the number of knots fixed at 4, that is, with 3 df).
Correlation analysis.
Ecological time series are often autocorrelated, which violates assumptions of independence in correlation tests. To deal with this issue, the effective number of degrees of freedom in significance tests of correlations was adjusted following a method proposed by ref. 30 and modified to allow missing values,
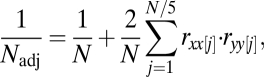 |
where 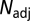 is the adjusted number of degrees of freedom,
is the adjusted number of degrees of freedom, 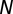 is the sample size with complete observation, and
is the sample size with complete observation, and 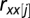 and
and 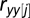 are the autocorrelations of series
are the autocorrelations of series 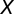 and
and 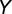 at lag j (j = 1, 2, … , N/5).
at lag j (j = 1, 2, … , N/5). 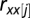 is estimated by
is estimated by
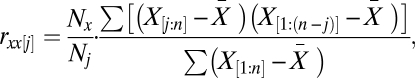 |
Where 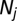 is the length of the vector
is the length of the vector 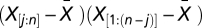 after removing missing values,
after removing missing values, 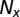 is the length of
is the length of 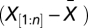 after removing missing values, n is the length of
after removing missing values, n is the length of 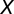 including missing values, and
including missing values, and 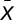 is the average of
is the average of 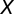 . The significance of the correlation, r, between
. The significance of the correlation, r, between 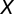 and
and 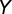 was then tested by a t test with df =
was then tested by a t test with df = 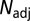 − 2 df.
− 2 df.
Generalized additive models.
GAMs are widely used for conducting nonlinear regression analysis in ecology when the functional forms of the effects of predictor variables are not a priori known (31). We here used the GAM implementation in the package mgcv (version 1.6-2) (31, 32) in the program R (version 2.12.0) (33). The model formula we used for both annual and decadal data was
where LT, PT, and TT are, respectively, reconstructed locust abundance (Lann or Ldec), precipitation index (dryness or precipitation), and temperature index (temp.W or temp.T) for year (or decade) T. The scalar α is the intercept; f, g, and h are smooth functions (natural cubic splines with maximally 4 knots); and εT is an independent and normally distributed error term.
Wavelet.
Decomposition is in many respects an ideal approach for investigating the nonstationary periodicity of ecological time series. Unlike Fourier decomposition, wavelet analysis performs local timescale decomposition of a time series and thus can track the change of its periodicity over time (34, 35). In this study, we adopted the widely used “Morlet” wavelet as the mother wavelet. The significance levels (P = 0.05) were calculated on the basis of 2,000 “Beta-Surrogate” series: surrogates of analyzed time series with similar histogram distributions and autocorrelation features (35–37).
Supplementary Material
Acknowledgments
We thank Dr. Linxi Xu and Dr. Yanping Zhao for their help in locust and meteorological records editing and Prof. Jingyun Zheng for providing precipitation data. This work was supported by the International Cooperation Project of the Chinese Academy of Sciences (GJHZ200810), the Centre for Ecological and Evolutionary Synthesis, and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA05080701).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100189108/-/DCSupplemental.
References
- 1.Guo F, Chen Y, Lu B. The Biology of the Migratory Locusts in China. Jinan, China: Shandong Science and Technology Press; 1991. Chinese. [Google Scholar]
- 2.Fan Y. Locust in yin dynasty. Agric Archaeol. 1983;4:314–317. [Google Scholar]
- 3.Chen M, Jiang T. Complete Collection of Illustrations and Writings from the Earliest to Current Times. Beijing: Ying wu dian; 1728. Chinese. [Google Scholar]
- 4.Chen J. Outbreaks of locust recorded in Chinese literatures. Annu Zhejiang Entomol Bureau. 1936;5:188–241. [Google Scholar]
- 5.Chen G. China Successive Natural and Manmade Disasters Table. Guangzhou, China: Jinan University Book Series; 1939. Chinese. [Google Scholar]
- 6.Ma S. The population dynamics of the oriental migratory locust (Locusta migratoria manilensis) in China. Acta Entomol Sin. 1958;8:1–40. [Google Scholar]
- 7.Ma S, Ding Y, Li D. Study on long-term prediction of locust population fluctuations. Acta Entomol Sin. 1965;14:319–338. [Google Scholar]
- 8.Stige LC, Chan KS, Zhang Z, Frank D, Stenseth NC. Thousand-year-long Chinese time series reveals climatic forcing of decadal locust dynamics. Proc Natl Acad Sci USA. 2007;104:16188–16193. doi: 10.1073/pnas.0706813104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, et al. Periodic temperature-associated drought/flood drives locust plagues in China. Proc R Soc Lond Ser B Biol Sci. 2009;276:823–831. doi: 10.1098/rspb.2008.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu G, Shen H, Liu J. Impacts of climate change on historical locust outbreaks in China. J Geophys Res Atmos. 2009;114(D18):D18104. [Google Scholar]
- 11.Zhang D. A Compendium of Chinese Meteorological Records of the Last 3000 Years. Nanjing, China: Jiangsu Education Publishing House; 2004. Chinese. [Google Scholar]
- 12.Central Meteorological Bureau. Yearly Charts of Dryness-Wetness in China for the Last 500-Year Period. Beijing: Cartographic Publishing House; 1981. Chinese. [Google Scholar]
- 13.Zheng JY, Wang WC, Ge QS, Man ZM, Zhang PY. Precipitation variability and extreme events in eastern China during the past 1500 years. Terr Atmos Ocean Sci. 2006;17:579–592. [Google Scholar]
- 14.Tan M, et al. Cyclic rapid warming on centennial-scale revealed by a 2650-year stalagmite record of warm season temperature. Geophys Res Lett. 2003;30:1617. [Google Scholar]
- 15.Wang S, et al. Reconstruction of temperature series of China for the last 1000 years. Chin Sci Bull. 2007;52:3272–3280. [Google Scholar]
- 16.Yang B, Braeuning A, Johnson K, Shi Y. General characteristics of temperature variation in China during the last two millennia. Geophys Res Lett. 2002;29 10.1029/2001GL014485. [Google Scholar]
- 17.Moberg A, et al. Highly variable Northern Hemisphere temperatures reconstructed from low- and high-resolution proxy data. Nature. 2005;433:613–617. doi: 10.1038/nature03265. [DOI] [PubMed] [Google Scholar]
- 18.Ljungqvist FC. A new reconstruction of temperature variability in the extra-tropical northern hemisphere during the last two millennia. Geogr Ann A. 2010;92A:339–351. [Google Scholar]
- 19.Todd M, Washington R, Cheke R, Kniveton D. Brown locust outbreaks and climate variability in southern Africa. J Appl Ecol. 2002;39:31–42. [Google Scholar]
- 20.Cheke RA, Holt J. Complex dynamics of desert locust plagues. Ecol Entomol. 1993;18:109–115. [Google Scholar]
- 21.Uichanco LB. Secular trends of locust outbreaks in the Philippines and their apparent relation with sunspot cycle. Philipp J Agric. 1936;25:321–354. [Google Scholar]
- 22.Stenseth NC, et al. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 23.Esper J, Büntgen U, Frank DC, Nievergelt D, Liebhold A. 1200 years of regular outbreaks in alpine insects. Proc R Soc Lond Ser B Biol. 2007;274:671–679. doi: 10.1098/rspb.2006.0191. Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kausrud KL, et al. Linking climate change to lemming cycles. Nature. 2008;456:93–97. doi: 10.1038/nature07442. [DOI] [PubMed] [Google Scholar]
- 25.Ueda H, Iwai A, Kuwako K, Hori M. Impact of anthropogenic forcing on the Asian summer monsoon as simulated by eight GCMs. Geophys Res Lett. 2006;33:L06703. [Google Scholar]
- 26.Jiang T, Zhang Q, Blender R, Fraedrich K. Yangtze Delta floods and droughts of the last millennium: Abrupt changes and long term memory. Theor Appl Climatol. 2005;82:131–141. [Google Scholar]
- 27.Cheke RA. Ecology. Thinking long term. Science. 2007;318:577–578. doi: 10.1126/science.1150636. [DOI] [PubMed] [Google Scholar]
- 28.Lima M. Locust plagues, climate variation, and the rhythms of nature. Proc Natl Acad Sci USA. 2007;104:15972–15973. doi: 10.1073/pnas.0708152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Intergovernmental Panel on Climate Change. Fourth Assessment Report on Climate Change 2007: Synthesis Report. Geneva, Switzerland: Intergovernmental Panel on Climate Change; 2007. [Google Scholar]
- 30.Pyper BJ, Peterman RM. Comparison of methods to account for autocorrelation in correlation analyses of fish data. Can J Fish Aquat Sci. 1998;55:2127–2140. [Google Scholar]
- 31.Wood S. Generalized Additive Models: An Introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- 32.Wood S. Fast stable direct fitting and smoothness selection for generalized additive models. J R Stat Soc B. 2008;70:495–518. [Google Scholar]
- 33.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 34.Torrence C, Compo G. A practical guide to wavelet analysis. Bull Am Meteorol Soc. 1998;79:61–78. [Google Scholar]
- 35.Cazelles B, et al. Wavelet analysis of ecological time series. Oecologia. 2008;156:287–304. doi: 10.1007/s00442-008-0993-2. [DOI] [PubMed] [Google Scholar]
- 36.Davison A, Hinkley D. Bootstrap Methods and Their Application. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 37.Rouyer T, Fromentin J, Stenseth N, Cazelles B. Analysing multiple time series and extending significance testing in wavelet analysis. Mar Ecol Prog Ser. 2008;359:11–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.