Abstract
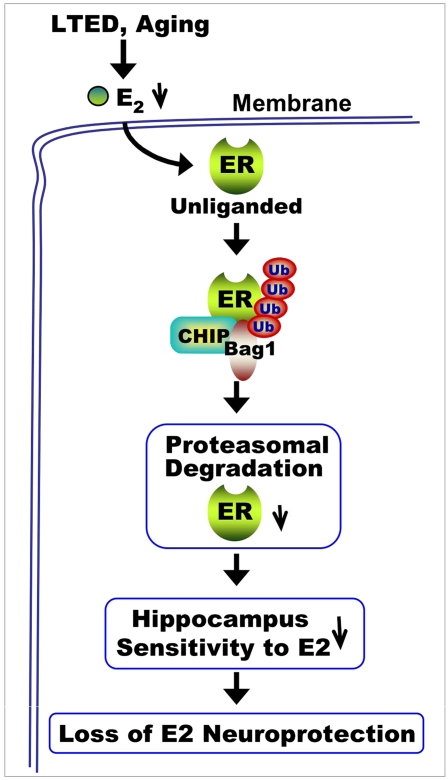
Recent work suggests that timing of 17β-estradiol (E2) therapy may be critical for observing a beneficial neural effect. Along these lines, E2 neuroprotection, but not its uterotropic effect, was shown to be lost following long-term E2 deprivation (LTED), and this effect was associated with a significant decrease of estrogen receptor-α (ERα) in the hippocampus but not the uterus. The purpose of the current study was to determine the mechanism underlying the ERα decrease and to determine whether aging leads to a similar loss of hippocampal ERα and E2 sensitivity. The results of the study show that ERα in the rat hippocampal CA1 region but not the uterus undergoes enhanced interaction with the E3 ubiquitin ligase C terminus of heat shock cognate protein 70 (Hsc70)-interacting protein (CHIP) that leads to its ubiquitination/proteasomal degradation following LTED (10-wk ovariectomy). E2 treatment initiated before but not after LTED prevented the enhanced ERα-CHIP interaction and ERα ubiquitination/degradation and was fully neuroprotective against global cerebral ischemia. Administration of a proteasomal inhibitor or CHIP antisense oligonucleotides to knock down CHIP reversed the LTED-induced down-regulation of ERα. Further work showed that these observations extended to natural aging, because aged rats showed enhanced CHIP interaction; ubiquitination and degradation of both hippocampal ERα and ERβ; and, importantly, a correlated loss of E2 neuroprotection against global cerebral ischemia. In contrast, E2 administration to middle-aged rats was still capable of exerting neuroprotection. As a whole, the study provides support for a “critical period” for E2 neuroprotection of the hippocampus and provides important insight into the mechanism underlying the critical period.
Keywords: estradiol, neuroendocrine, steroid, stroke
Basic science and clinical observation studies have provided evidence of a beneficial effect of 17β-estradiol (E2) on cardiovascular disease, neuroprotection, and neurodegenerative diseases such as stroke and Alzheimer's disease (1–6). However, the Women's Health Initiative (WHI) surprisingly failed to observe a protective effect of hormone therapy on the cardiovascular system and, in fact, reported a small but significant increase in risk for stroke and dementia (7–9). The average age of subjects in the WHI study was 63 y, far past the onset of menopause. It has been suggested that there may be a “critical period” for the beneficial protective effect of E2 on the brain and that estrogen may need to be administered at perimenopause or earlier to observe a beneficial effect on the cardiovascular and neural system (10–12). In support of a critical period hypothesis for E2 beneficial effects in the brain, work by our laboratory and others has shown that long-term E2 deprivation (LTED) leads to a loss of E2 neuroprotection in animal models of focal and global cerebral ischemia (GCI) (13, 14). Intriguingly, recent work by our group showed that the loss of E2 neuroprotection of the hippocampal CA1 region, an area critical for learning and memory, was correlated with a significant decrease in estrogen receptor-α (ERα) but not ERβ following LTED (13). This decrease in ERα and E2 sensitivity was tissue-specific, because ERα did not decrease in the uterus following LTED and the uterus, unlike the hippocampal CA1 region, remained sensitive to the uterotropic action of E2. The mechanisms underlying the decrease of ERα in the hippocampal CA1 region following LTED are unknown. Previous work in breast cancer cells demonstrated that an ubiquitin E3 ligase, C terminus of heat shock cognate protein 70 (Hsc70)-interacting protein (CHIP), binds and promotes degradation of the unliganded ERα via the ubiquitin-proteasome degradation pathway (15, 16). Because LTED results in very low serum E2 levels (e.g., reduced ligand for binding to ER), we hypothesized that the reduced ERα in the hippocampal CA1 following LTED may be attributable to CHIP-mediated proteasomal degradation of ERα. In addition, because LTED is only a model of aging, it is important to determine whether ERα levels and E2 sensitivity of the hippocampal CA1 region are similarly attenuated with natural aging. To address these issues, the goals of the current study were to (i) determine the mechanism underlying LTED-induced attenuation of ERα and E2 sensitivity in the hippocampus, (ii) determine whether a critical period exists for E2 neuroprotection of the aging hippocampal CA1 region, and (iii) determine the mechanism that underlies existence of the critical period.
Results
Ubiquitination and Degradation of Hippocampal CA1 ERα.
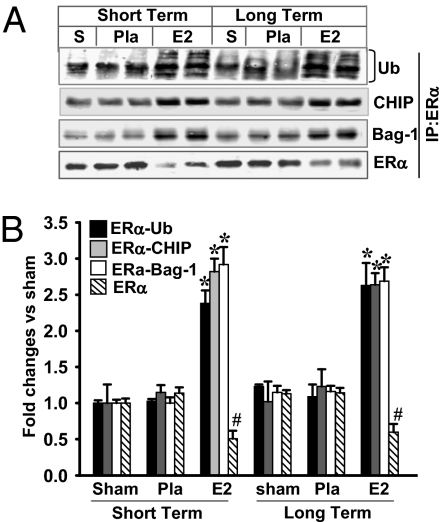
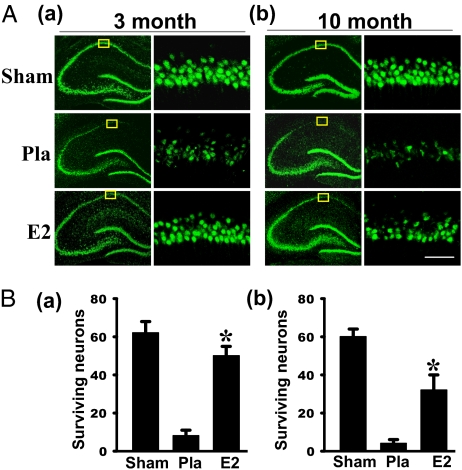
In previous studies, we showed that LTED (10-wk ovariectomy) resulted in a significant decrease of ERα in the hippocampal CA1 and a loss of E2 neuroprotection against GCI (13). To elucidate the mechanisms underlying the decrease of ERα, we first examined whether LTED affected ubiquitination/degradation of ERα in the hippocampal CA1 region of young adult female rats. As shown in Fig. 1 A and B, ERα immunoprecipitation (IP) studies revealed that ubiquitination of ERα in the hippocampal CA1 was increased over twofold in LTED (10-wk) animals compared with short-term E2-deprived (1-wk) animals. The increase was paralleled by a significant decrease of ERα in LTED animals. The increase in ERα ubiquitination occurred in all groups of LTED rats [sham, placebo (Pla), and E2], and E2 treatment at the end of the 10-wk E2 deprivation period was incapable of preventing the ERα ubiquitination/degradation. Reverse IP with ubiquitin and blotting for ERα further confirmed the enhanced ERα ubiquitination in LTED rats (Fig. 1 C and D). In addition, real-time RT-PCR and in situ hybridization results showed that ERα mRNA levels were not significantly different in LTED rats compared with short-term E2-deprived rats (Fig. S1 A and B), which suggests that the decrease in ERα protein levels in the hippocampal CA1 region of LTED rats is primarily attributable to increased degradation. Further work showed that ubiquitination and degradation of ERα was specific, because the levels and ubiquitination of ERβ, unlike ERα, did not change significantly following LTED (Fig. S1 C and D). Because ovariectomy removes many ovarian-generated steroids and factors, it was important to confirm that the changes in ERα observed following long-term ovariectomy are primarily attributable to the loss of E2 and not to some other factor. We also wanted to show that E2 replacement initiated at the time of LTED and maintained throughout the LTED period would be fully capable of exerting neuroprotection. We thus performed an experiment in which E2 replacement was initiated at the time of ovariectomy and maintained for the entire 10 wk, and we examined the effect on ERα levels and degradation as well as E2 neuroprotection against cerebral ischemia. As shown in Fig. 2A, examination of neuron-specific nuclear protein (NeuN) staining, a neuronal-specific marker (Fig. 2 A, a), and quantification of surviving neurons (Fig. 2 A, b) in the hippocampal CA1 region at 7 d after reperfusion revealed that E2 treatment initiated after 10-wk ovariectomy was unable to exert neuroprotection of the hippocampal CA1 region following GCI. However, in animals in which E2 treatment was initiated immediately at ovariectomy and maintained for the period of ovariectomy, E2 exerted robust neuroprotection of the hippocampal CA1 region, as indicated by NeuN staining (Fig. 2 A, a) and quantification of surviving neurons (Fig. 2 A, b). Furthermore, in animals treated with E2 for the entire ovariectomy period, E2 prevented the decrease in ERα in the hippocampal CA1 region (Fig. 2 B, a and B, c) that occurred following long-term ovariectomy, as well as preventing the increase in ubiquitination of ERα (Fig. 2 B, b and B, c). E2 treatment initiated after 10-wk ovariectomy was incapable of preventing the decrease in ERα or its increased ubiquitination and degradation. These results suggest that it is the loss of E2 following ovariectomy that leads to the increased ubiquitination/degradation of ERα in the CA1 region and loss of sensitivity to the E2 neuroprotective effect. The results also support the critical period hypothesis by showing that E2 replacement at the time of LTED and maintained throughout LTED is fully neuroprotective.
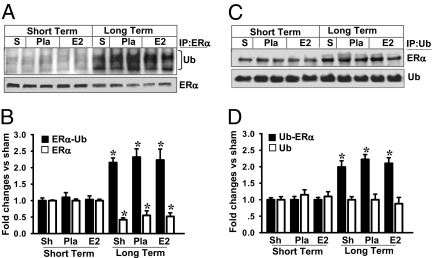
Fig. 1.
LTED (10 wk of ovariectomy) leads to a significant increase of ERα ubiquitination and decrease of ERα protein. (A) Hippocampal CA1 protein samples from sham, short-term (7 d), and 10-wk later (long term) Pla/E2-replaced rats at 3 h of reperfusion were immunoprecipitated with anti-ERα antibody or nonspecific IgG and then separately blotted (Western blot) with antiubiquitin antibody or anti-ERα antibody. S, sham; Ub, ubiquitin. (C) In reciprocal co-IP experiments, samples were subjected to IP with antiubiquitin antibody and the immunocomplexes were probed for the presence of ERα and ubiquitination as indicated. (B and D) Data were shown as mean ± SE from independent animals (n = 4–5) and expressed as fold vs. sham in short-term Pla/E2-treated groups. *P < 0.05 vs. sham, Pla, and E2 in short-term groups.
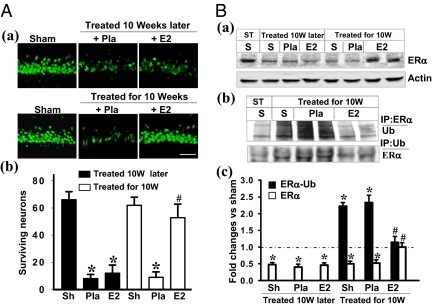
Fig. 2.
Neuroprotection and prevention of ERα degradation/ubiquitination by continuous E2 replacement for 10 wk. (A) NeuN staining of CA1 subregion was performed on the hippocampal sections from sham, animals treated with E2 or Pla following LDED (a, Upper), or animals treated immediately after ovariectomy for the entire 10-wk period (a, Lower). The rats were subjected to sham ischemia or 10 min of ischemia followed by 7 d of reperfusion. (b) Cell-counting study showed that E2 replacement initiated immediately at the time of ovariectomy and maintained for the entire 10-wk period restored its ability to protect CA1 neurons against ischemic damage compared with rats that received E2 replacement only at the end of the long-term deprivation period. (Magnification: 40×; scale bar: 50 μm.) Sh, sham; 10W, 10 wk later. Data were expressed as mean ± SE (n = 6–7 in each group). *P < 0.05 vs. sham; #P < 0.001 vs. Pla treatment in 10-wk later group. (B) At 3 h after ischemic reperfusion, Western blotting analyses of ERα and β-actin were performed with hippocampal CA1 protein from sham in the short-term E2 deprivation group and in the animals treated with E2/Pla 10 wk later after ovariectomy (treated 10 wk later) or treated with E2/Pla immediately for the full 10 wk. ST, short term. (a and c) Note that ERα protein attenuation was blocked in the rats that received replaced E2 for the 10 wk. (b) Coprecipitation between ERα and ubiquitinated proteins was performed with the 3-h reperfusion CA1 protein samples. (b and c) Note that the high level of ERα ubiquitination following LTED was totally blocked by E2 replacement for the 10 wk. Data were shown as mean ± SE from four to six independent animals and were expressed as fold vs. sham in the short-term E2 deprivation group. *P < 0.05 and #P < 0.01 compared with short-term sham and Pla-treated animals, respectively, for the 10-wk later group.
Proteasome Inhibition Attenuates the Decrease of ERα in LTED Rats.
We next examined whether inhibition of proteasomal activity would reinstate ERα levels in the hippocampal CA1 region in LTED rats. Fig. 3A shows that 20s proteasomal activity in the CA1 region is not significantly different between short-term E2-deprived and LTED rats. Administration of the proteasomal inhibitor MG132 significantly decreased hippocampal CA1 region 20s proteasomal activity in sham and Pla-treated (ischemic) LTED rats (Fig. 3B). The decrease of proteasomal activity by MG132 treatment was associated with a significant increase of ERα protein levels in the hippocampal CA1 region of sham and Pla-treated LTED rats (Fig. 3C). This suggests that the increased ubiquitination of ERα following long-term ovariectomy targets it to the proteasome for degradation and that ERα levels can be reinstated by inhibiting the proteasome.
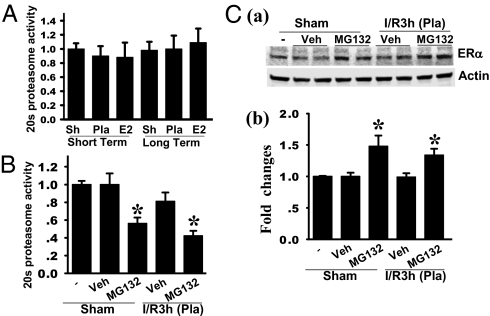
Fig. 3.
Proteasome inhibition by MG132 attenuates the decrease of ERα protein in LTED animals. (A) There were no changes in 20s proteasome activity in the hippocampal CA1 region in the rats following LTED compared with short-term E2 deprivation at 3 h after ischemic reperfusion. (B) Activity of 20s proteasome was significantly attenuated in the hippocampal CA1 region at day 4 following i.c.v. infusion of the 26S proteasome inhibitor MG132, either in the sham-operated rats or in the LTED rats subjected to 3 h of ischemic reperfusion. I/R3h, 3 h of ischemic reperfusion; Veh, vehicle. (C, a) Hippocampal CA1 protein samples from sham, vehicle-treated, and MG132-treated rats following LTED were subjected to Western blotting with anti-ERα or anti–β-actin antibody. (C, b) Band densities for ERα were normalized to β-actin. All the data were expressed as mean ± SE from independent animals (4–6) and expressed as fold changes vs. sham. *P < 0.05 vs. sham and vehicle-treated groups.
Increased Interaction of Hippocampal ERα with the Ubiquitin E3 Ligase CHIP in LTED Rats.
Previous studies in Michigan Cancer Foundation-7 (MCF-7) breast cancer cells demonstrated that the carboxyl terminus of CHIP is an ubiquitin E3 ligase that binds and promotes degradation of unliganded ERα via the ubiquitin-proteasome pathway (15).This finding raises the possibility that CHIP may bind to and ubiquitinate ERα and target it for proteasomal degradation in LTED rats. To address this issue, we examined CHIP interaction with ERα in short-term E2-deprived vs. LTED rats. In Fig. 4 A and B, IP of hippocampal CA1 lysates with ERα antibody and blotting for CHIP revealed that ERα interaction with CHIP is low in short-term E2-deprived rats and that ischemia and E2 have no significant effect on the interaction. In contrast, ERα interaction with CHIP is markedly and significantly increased (2- to 3-fold increase) in all groups in LTED rats compared with short-term E2-deprived rats (Fig. 4 A and B). Reverse IP using CHIP antibody and blotting for ERα yielded similar results. It should be noted that actin and total CHIP levels in the hippocampal CA1 region were not significantly different between short-term E2-deprived and LTED rats. Bcl-2‐associated athanogene 1 (Bag-1) is a proteasome-associated cochaperone that has been shown to promote delivery of the ubiquitinated ER protein to the proteasome for degradation (15–17). We thus examined whether Bag-1 interaction with ERα was increased in LTED rats. As shown in Fig. 4 A and B, ERα interaction with Bag-1 was significantly increased (2- to 3-fold increase) in all groups in LTED rats compared with short-term E2-deprived rats. The results suggest that there is a robust increase in interaction of ERα and the E3 ubiquitin ligase CHIP in the hippocampal CA1 region of LTED rats, which may explain the increased ubiquitination/degradation of ERα observed in the animals. The increased interaction of hippocampal ERα with CHIP and Bag-1 was prevented if E2 was initiated at the time of ovariectomy and maintained for the entire 10 wk of ovariectomy (Fig. 4 C and D) and suggests that the loss of E2 is the critical factor leading to enhanced interaction of ERα with CHIP and Bag-1 as well as the subsequent degradation of ERα in the hippocampal CA1 region in long-term ovariectomized rats. Furthermore, the increased interaction of ERα with CHIP in LTED rats was specific for the hippocampal CA1 region, because additional studies showed that interaction of ERα with CHIP and Bag-1 was not elevated in the uterus of LTED rats compared with short-term E2-deprived rats (Fig. 5A). In addition, there was no significant increase in ubiquitination of uterine ERα in the LTED rats compared with short-term E2-deprived rats and no significant difference in uterine ERα levels (Fig. 5 B, a and B, b). Note that E2 did increase CHIP and Bag-1 interaction and ubiquitination of uterine ERα in both short-term E2-deprived and LTED rats but that overall levels of CHIP–ERα–Bag-1 interaction and ERα ubiquitination between short-term E2-deprived and LTED rats are not significantly different. We previously reported that the uterus of LTED rats remained sensitive to E2 uterotropic effects, whereas E2 neuroprotective effects in the hippocampal CA1 region were lost (13). Our finding that CHIP interaction and ubiquitination of ERα do not increase in the uterus of LTED rats thus provides a potential explanation for these tissue-dependent differences in E2 sensitivity and ERα regulation following LTED.
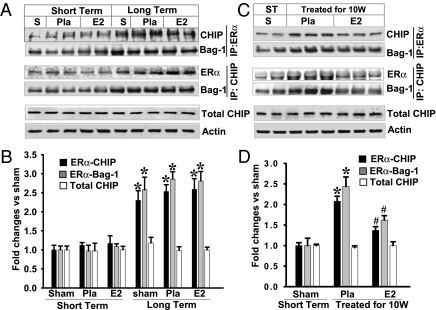
Fig. 4.
LTED leads to increased interaction between ERα, CHIP, and Bag-1 protein, which was blocked by E2 replacement for 10 wk. (A and C) Hippocampal CA1 protein samples at 3 h of ischemic reperfusion from the short-term and 10-wk later Pla/E2-replaced rats as well as from rats treated with E2/Pla for 10 wk were immunoprecipitated with anti-ERα antibody and probed with anti-CHIP or anti–Bag-1 antibody. Samples were also subjected to IP with anti-CHIP, and the immunocomplexes were probed for the presence of ERα or Bag-1. The expression of total CHIP and actin from hippocampal CA1 of the rats was determined by Western blot analyses; no significant changes were observed between groups. These data suggest that ERα degradation involves CHIP and Bag-1 following LTED. S, sham. (B and D) Data were shown as mean ± SE from independent animals (A, n = 4–5; B, n = 5–6) and expressed as fold vs. sham of the short-term E2 deprivation group. *P < 0.05 vs. short-term sham group; #P < 0.05 vs. group treated with Pla 10 wk later.
Fig. 5.
CHIP- and Bag-1–mediated ERα ubiquitination and degradation in the uterus in E2-treated animals. (A) Uterine protein samples from sham of the short-term (7 d) and 10-wk later rats that received replacement of Pla/E2 at 3 h of ischemic reperfusion were immunoprecipitated with anti-ERα antibody and then separately blotted (Western blot) with antiubiquitin, anti-CHIP, anti–Bag-1, or anti-ERα antibody. Note that 10-wk E2 deprivation failed to down-regulate ERα protein expression in the uterus, as was observed in the CA1 region. However, ERα protein ubiquitination was increased and ERα protein expression in the uterus was attenuated by E2 compared with Pla control, and this effect was maintained in LTED (10 wk later) animals. S, sham; Ub, ubiquitin. (B) Data were shown as mean ± SE from four to five independent animals and expressed as fold changes vs. short-term E2-deprived sham. *P < 0.05 vs. sham and Pla-treated groups; #P < 0.05 vs. Pla-treated groups.
Natural Aging Is Associated with Increased ERα and ERβ CHIP Binding and Ubiquitination and with Decreased ERα and ERβ Levels in the Hippocampal CA1 Region.
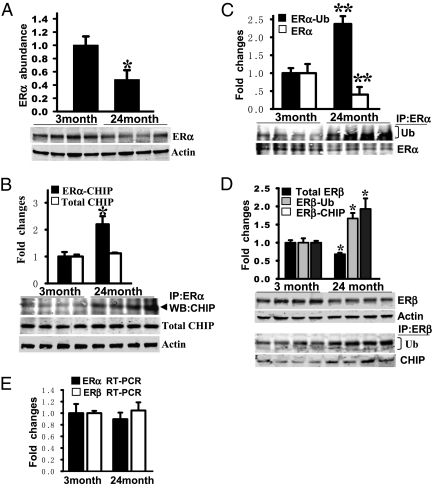
LTED (ovariectomy) is an excellent model of surgical menopause and has also been used as a model of natural menopause. However, the model is generally performed in young animals, and thus may not completely recapitulate or predict changes that occur with natural aging. It is thus important to determine whether the changes in ER degradation and levels we observed in the LTED rat model occur in intact rats with natural aging and to determine whether these changes correlate with changes in sensitivity to the actions of E2. To address with this issue, we used intact young (3-mo-old, diestrus 1) and old (24-mo-old, acyclic) female F344 rats from the National Institute of Aging. As shown in Fig. 6A, Western blot analysis shows that there is an approximate 50–60% decrease in ERα protein levels in the hippocampal CA1 region of old vs. young rats. The decrease in ERα in old rats was correlated with a significant (over 2-fold) increase in ERα interaction with CHIP (Fig. 6B) and robust ubiquitination of ERα (Fig. 6C) in the hippocampal CA1 of old rats compared with young animals. Total CHIP levels did not change with aging (Fig. 6B). Interestingly, in contrast to results observed in the LTED rats, aging was associated with a significant decrease in ERβ in the hippocampal CA1 region, with 24-mo-old rats having a significant decrease of ERβ protein levels compared with 3-mo-old rats (Fig. 6D). The decrease in ERβ in 24-mo-old rats paralleled an increased interaction of ERβ with CHIP and increased ubiquitination of ERβ in 24-mo-old rats compared with 3-mo-old rats. Finally, real-time RT-PCR for ERα and ERβ revealed that mRNA levels for ERα and ERβ in the hippocampal CA1 region are not significantly different between 3-mo-old and 24-mo-old rats (Fig. 6E), suggesting that the decrease in hippocampal ERα and ERβ is predominantly attributable to increased degradation of the protein rather than decreased synthesis.
Fig. 6.
Decreased expression of ER and increased expression of ER ubiquitination and binding with CHIP protein in the hippocampal CA1 in aged (24-mo-old) rats compared with young (3-mo-old) rats. (A) Hippocampal homogenates from young and aged F344 rats were subjected to Western blot analysis to test the expression profile of ERα protein. (B and C) Hippocampal CA1 protein samples were immunoprecipitated with anti-ERα antibody and probed with anti-CHIP, antiubiquitin, or anti-ERα antibody. CHIP protein expression did not change in young vs. aged rats. Ub, ubiquitin; WB, Western blot. (D) ERβ protein expression and its bindings with ubiquitin and CHIP were examined by Western blotting and co-IP analyses, respectively. (E) Quantitative analysis of ERα and ERβ mRNA expression was conducted by RT-PCR analysis in the hippocampal CA1 region of the brain in young and aged rats. ERα and ERβ mRNA expression showed no significant change. All the data were shown as mean ± SE from independent animals (n = 5–7) and expressed as fold vs. group of young animals. *P < 0.05 and **P < 0.001, respectively, compared with young group of animals.
Estrogen Exerts Significant Neuroprotection in Young and Middle-Aged Female Rats but Not in Old Female Rats.
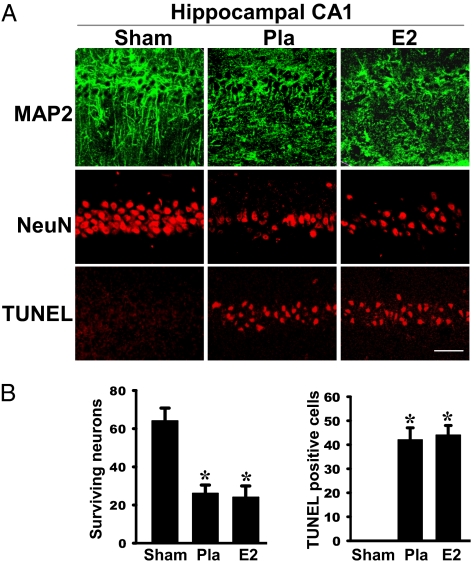
Because there is a significant decrease of ERα and ERβ in the hippocampal CA1 region of old rats, it could suggest there is a critical period for E2 neuroprotection in the hippocampal CA1 region. To address this issue, we examined E2 neuroprotection in young (3-mo-old), middle-aged (10-mo-old), and old (24-mo-old) short-term ovariectomized F344 female rats. The results for the young and middle-aged ovariectomized rats are presented in Fig. 7 A and B, and the results for the old ovariectomized rats are presented in Fig. 8 A and B. As shown in Fig. 7 A and B, NeuN staining and counting of the number of surviving neurons in the hippocampal CA1 region of 3-mo-old and 10-mo-old ovariectomized rats at 7 d after GCI revealed that E2 exerted significant and robust neuroprotection in the 3-mo-old rats. E2 also exerted significant neuroprotection in middle-aged rats, but the effect, although significant, was not as robust as observed in young rats. It should also be noted that the mortality rate for GCI was slightly higher for the middle-aged rats at 7 d of reperfusion (Table 1). When old (24-mo-old rats) were examined, it was found that they were much more susceptible to GCI damage compared with young and middle-aged rats (Table 1). A total of 82 F344 rats were used in the mortality calculation. Over half of the old animals had already died by day 3 after reperfusion. Interestingly, E2 did not decrease mortality in the old rats and, in fact, was slightly higher (66.7% E2 vs. 53.8% Pla). The increased sensitivity and susceptibility of old rats to GCI is consistent with previous reports (18). We next examined neuronal survival in the old rats. Fig. 8A depicts results from examination of neuronal damage/cell death at day 3 after reperfusion in 24-mo-old rats using the neuronal-specific markers MAP2 and NeuN. MAP2 is a sensitive and early indicator of neuropathology associated with ischemia (19). NeuN is a neuron-specific marker used to indicate survival of neurons following ischemia. As shown in Fig. 8A, there is robust widespread MAP2 staining of pyramidal cells in sham nonischemic animals, showing excellent neuronal morphology with long axons and dendrites. In contrast, MAP2 staining in Pla-treated ischemic rats revealed poor morphology of CA1 pyramidal neurons, indicating significant neuronal damage following cerebral ischemia in the old rats. Intriguingly, MAP2 staining in E2-treated animals showed a similar poor neuronal morphology as observed in Pla-treated rats, suggesting that E2 is unable to exert neuroprotection against GCI in old rats. In support of this suggestion, NeuN staining and counting of NeuN-positive surviving neurons revealed that GCI induced a significant loss of NeuN-positive neurons in the hippocampal CA1 in Pla-treated animals compared with sham animals and that E2 treatment was unable to prevent the loss of NeuN-positive cells (Fig. 8 A and B, a). Finally, immunohistochemistry for TUNEL, a marker of apoptosis, and counting of the number of TUNEL-positive cells revealed that Pla-treated ischemic animals had a significant increase in apoptosis in the hippocampal CA1 region compared with sham control animals (Fig. 8 A and B, b). Furthermore, in agreement with the MAP2 and NeuN results, E2 treatment was unable to reduce apoptosis in the old animals (Fig. 8 A and B, a), further confirming that E2 is incapable of exerting significant neuroprotection in old rats, as it does in young and middle-aged rats. The loss of sensitivity to E2 neuroprotective effects in old rats is also consistent with the increased degradation and significant decrease of hippocampal ERα and ERβ observed in old rats.
Fig. 7.
Neuroprotective effects of E2 in hippocampal CA1 region in young (a, 3-mo-old) and middle-aged (b, 10-mo-old) F344 rats following global ischemia. (A) Typical photomicrographs of NeuN staining in brain hippocampus from sham, Pla, and E2-treated female ovariectomized F344 rats following 7 d of ischemic reperfusion. NeuN-positive CA1 pyramidal cells showing intact and round nuclei following ischemia were counted as surviving cells. (B) Quantitative summary of data (mean ± SE, n = 6–8 animals per group) shows the numbers of surviving neurons per 250-μm length of medial CA1. (Magnification: 40×; scale bar: 50 μm.) *P < 0.01 vs. Pla group.
Fig. 8.
No neuroprotective effects of E2 in hippocampal CA1 region in aged rats following global ischemia. (A) Typical photomicrographs of hippocampal CA1 region showing MAP2 staining (Top), NeuN staining (Middle), and TUNEL staining (Bottom) of 24-mo-old F344 rats following 3 d of ischemic reperfusion. Note that in sham animals, pyramidal cells show long axons and many dendrites, whereas the widespread staining of neurons was not present in the Pla- and E2-treated rats following ischemia. (B) Quantitative summary of data (mean ± SE, n = 5–6 animals per group) shows the numbers of surviving neurons and apoptotic neurons per 250-μm length of medial CA1. (Magnification: 40×; scale bar: 50 μm.) *P < 0.01 vs. sham group.
Table 1.
Mortality rate of F344 rats at different ages
| Mortality |
||||
| Age, mo | Sham, % | Pla, % | E2, % | Reperfusion, d |
| 3 | 0 | 10.0 | 0 | 7 |
| 10 | 0 | 18.2 | 18.2 | 7 |
| 24 | 0 | 53.8 | 66.7 | 3 |
Discussion
The current study provides support for the critical period hypothesis that E2 must be given before a period of LTED (e.g., at perimenopause) to observe its beneficial neuroprotective effects in the hippocampal CA1 region, a region critical for cognitive function and commonly affected in dementia. It also provides a potential mechanistic explanation for existence of the critical period by demonstrating that LTED leads to a significant down-regulation of ERα in the hippocampal CA1 region via a CHIP-mediated ubiquitination and proteasomal degradation pathway. Previous work in breast cancer cells has demonstrated that CHIP can ubiquitinate the unliganded ERα and that the proteasome-associated cochaperone Bag-1 promotes delivery of the ubiquitinated ER protein to the proteasome for degradation (15–17). Our study extends the CHIP–Bag-1–ERα proteasomal degradation pathway to the brain and demonstrates that it mediates down-regulation of ERα in the hippocampal CA1 region following LTED. It is currently unknown which lysine sites are polyubiquitinated on ERα and which sites/motifs are critical regulators of ERα stability/degradation. However, previous work has suggested that lysines K302 and K303 play an important role in protecting ERα from degradation. Mutation of lysines K302 and K303 to alanine resulted in increased degradation of ERα (17), possibly by altering receptor-HSP90-cochaperone interactions. ER stability has also been implicated to be regulated by posttranslational modification. For instance, phosphorylation of human ERα at Ser118 and Ser167 has been reported to alter ERα stability (20, 21), with phosphorylated Ser118 (pSer118)-ERα being resistant to proteasomal degradation (20). We examined pSer118-ERα levels in LTED rats and observed a robust decline in hippocampal pSER118-ERα levels following LTED; however, when the phospho-ERα levels were corrected with total ERα protein, there was no significant effect of LTED on pSER118-ERα levels (Fig. S2 A and B). We were unable to find a pSER167-ERα antibody that worked in rats, and thus could not examine for changes in pSER167-ERα. It is possible that in the absence of ligand, ERα interaction with ER coregulators is altered, leading to a conformation change or changes in cochaperone interactions that facilitate interaction with CHIP and resultant ubiquitination and proteasomal degradation. In support of this possibility, the expression and ERα interaction of several ERα coregulator proteins, proline-, glutamic acid-, and leucine-rich protein-1(PELP1), metastasis-associated protein 1 (MTA1), and receptor interacting protein 140 (RIP140). Have been shown to decrease significantly in the aged brain (22, 23). We should add that further work by our group has found that ovariectomy itself does not increase CHIP expression in the hippocampal CA1 region; thus, the increased interaction of CHIP with ERα is not attributable to increased CHIP levels (Fig. S3).
It should be pointed out that degradation of ERα following LTED may explain not only the loss of E2 neuroprotective action observed in our study but the loss of other critical E2 actions reported previously following LTED, such as loss of E2 enhancement of long-term potentiation (LTP) and synaptic density (24) and elevation of choline acetyltransferase (25). Thus, our results may help to explain the loss of other well-known estrogen actions in the hippocampus following prolonged hypoestrogenicity. It is important to note that CHIP is not the only E3 ubiquitin ligase implicated to ubiquitinate/degrade ERα. Studies in breast cancer cells have also implicated neural precursor cell-expressed developmentally down-regulated (NEDD8) as an ERα ubiquitinating enzyme (26). However, when we examined NEDD8 interaction with ERα in our study, we did not find any increased interaction of NEDD8 with hippocampal ERα following LTED (Fig. S4 A and B). Thus, this suggests that the increased interaction is specific for CHIP, possibly attributable to the fact that CHIP ubiquitinates and target for degradation the unliganded ERα, which should represent the major form of the receptor in the hypoestrogenic state of LTED. Further work by our group showed that CHIP knockdown in the hippocampal CA1 region using a CHIP antisense oligonucleotides approach reversed ERα ubiquitination and down-regulation/degradation in LTED rats, strongly suggesting that CHIP plays a critical role in ERα ubiquitination/degradation after LTED (Fig. S5 A–D).
An additional unique aspect of our study was that we found that the same CHIP-mediated degradation of hippocampal CA1 ERα observed in the LTED model occurs in intact nonischemic rats during natural aging. Compared with young (3-mo-old) rats, old (24-mo-old) rats had significantly enhanced ERα ubiquitination and interaction with CHIP as well as marked down-regulation of ERα in the hippocampal CA1 region. Interestingly, unlike in the LTED model, we found that ERβ also showed enhanced ubiquitination, CHIP interaction, and down-regulation in aged rats. This is consistent with previous reports that in addition to ubiquitination of ERα, CHIP can ubiquitinate and target ERβ for proteasomal degradation (27). The additional degradation of ERβ in aged rats could be attributable to the fact that the period of hypoestrogenicity in aged rats is significantly longer than the 10 wk we used in the LTED model (e.g., a longer period of hypoestrogenicity may be needed to down-regulate ERβ). Our finding of a decrease of ERα and ERβ in the hippocampal CA1 region of aged F344 rats is consistent with a previous report in 24-mo-old Wister rats, where there was a 56% and 41% decrease of ERα and ERβ, respectively, compared with young rats (28). A significant decrease of ERα and ERβ has also been reported in hippocampal synapses in old ovariectomized rats (29). Thus, in addition to the increased degradation of ER observed in our study, defects in targeting of ER to the synapse could contribute to decreased sensitivity to beneficial E2 neural effects in aged animals.
The decrease in ERα and ERβ levels in the hippocampal CA1 region of aged rats observed in our study was correlated with a loss of low-dose E2 neuroprotective effects following GCI in old rats. Because our study used a low diestrus level dose of E2, it was of interest to determine if a higher proestrus-type E2 dose (0.050 mg) would be able to exert neuroprotection against GCI in LTED and aged rats. This higher E2 dose has been previously shown to produce serum E2 levels in the proestrus range (64 ± 7 pg/mL) and to be neuroprotective against GCI in young rats (30). As shown in Fig. S6A, high-dose E2 treatment was strongly neuroprotective against GCI in short-term ovariectomized rats, but this neuroprotective effect was lost in LTED rats. Likewise, although high-dose E2 was strongly neuroprotective of the hippocampal CA1 region in young (3-mo-old) ovariectomized rats, it was incapable of exerting neuroprotection against GCI in aged (24-mo-old) rats (Fig. S6B). The loss of E2 beneficial effect in old rats is reminiscent of the results of the WHI study, where estrogen therapy was found not to be beneficial on stroke and dementia risk in old subjects, and actually led to an increased risk (7–9). Similarly, our study also revealed a pattern for worse outcome in the old animals, because low-dose E2-treated aged rats had a 16% increase in mortality compared with young rats. The finding of a pattern for increased mortality in E2-treated aged rats after GCI is consistent with the proposed “healthy cell bias of estrogen action” proposed by Brinton (31) and Brinton and colleagues (32), which holds that once cells are damaged as a result of aging or other factors, E2 may actually be detrimental and unable to protect the cell. Aged rats were also much more susceptible to GCI, which is consistent with previous reports (18). These findings highlight the risk of extrapolating findings in young animals to old animals and the need to perform correlate studies in aged animals before drawing conclusions. Although the findings are in agreement with those of the WHI, our studies also provide support for the critical period hypothesis of estrogen benefit. Along these lines, our study found that low-dose E2 replacement in middle-aged rats was capable of exerting significant neuroprotection against GCI, in contrast to a lack of protection in old rats. Our finding that E2 is still protective in middle-aged rats is consistent with previous reports by others in both focal and GCI models (33–35). Low-dose E2 therapy would be preferred in humans so as to minimize negative side effects. Interestingly, a recent clinical study used a low dose of estrogen therapy and found it did not enhance stroke risk, compared with the increased risk seen in the WHI study, which used a higher estrogen dose (36).
In conclusion, the current study demonstrates that there is a CHIP-mediated degradation of ER in the hippocampal CA1 region following both LTED and aging, which is correlated with a loss of estrogen neuroprotection. The CHIP-mediated down-regulation of ER was tissue-dependent because it was not observed in the uterus, which remains responsive to E2 following both LTED and aging. As a whole, the results provide support and a potential mechanistic explanation for the critical period hypothesis of estrogen beneficial effect in the brain and add to a growing literature suggesting that low-dose E2 therapy begun before menopause onset may be necessary to yield maximal beneficial neural effects of E2.
Methods
Animals, GCI, and Drug Administration.
Adult (3-mo-old) Sprague–Dawley (SD) female rats (Harlan) or adult young (3-mo-old), middle-aged (10-mo-old), and aged (24-mo-old) Fischer 344 rats (National Institute of Aging) were bilaterally ovariectomized. Pla or E2 Alzet minipumps (0.025 mg, 21-d release) were implanted s.c. in the upper midback region under the skin at the time of ovariectomy, and GCI was performed 1 wk later. The dose of E2 used produces serum E2 levels of ∼10–15 pg/mL, which represents physiological low diestrus 1 levels of E2 (37). In some studies, a higher E2 dose was used (0.050 mg, 21-d release), which was administered at the time of ovariectomy (1 wk before ischemia). This dose has been reported to produce proestrus levels of E2 (∼64 pg/mL), as measured in animals killed after ischemia (30). In some SD animals, LTED, in which the animals were ovariectomized, was performed; 10 wk later, Pla or an E2 minipump was implanted, and 1 wk later, GCI was performed. Finally, some LTED animals had E2 replacement initiated immediately and maintained throughout the 10 wk of ovariectomy (10-wk continuous). For GCI, all animals (except sham control) underwent four-vessel occlusion GCI performed as described previously (37). To compare the estrogen receptor levels between intact young and aged Fischer 344 rats, the ovarian cycle was monitored as described previously (13); young cycling rats were used on diestrus 1, and acyclical aged rats showing persistent diestrus were used in the studies. The reversible potent 26S proteasome inhibitor MG132 (no. 474790, EMD Biosciences, Inc.) was dissolved in 10% DMSO at a concentration of 100 μM. Five microliters of the solution was administrated to both of the cerebral ventricles of rats 66 d after ovariectomy through i.c.v. injection; the rats were killed 4 d later. Control rats were infused with 10% DMSO as vehicle control. To knock down CHIP protein, 10 nmol of end-phosphorothioated HPLC-purified antisense oligodeoxynucleotides (5′-CAGCGAGCCTATAGT TTGGC-3′) was mixed with 5 μL of in vivo-jetPEI (Polyplus Transfection) and administrated i.c.v. in both cerebral ventricles of rats 66 d after ovariectomy every 24 h for 4 d. The last infusion was administered 12 h before the rats were killed. The same dose of scrambled missense oligodeoxynucleotides was used as a control.
Tissue Collection, IP, Western Blotting, and Histological Analysis.
For brain tissue preparation, rats were killed under anesthesia at the indicated time points. Whole brains were removed, and the hippocampal CA1 regions were microdissected from both sides of the hippocampal fissure and immediately frozen in dry ice. Protein samples were extracted as described previously (13). IP and Western blotting were performed as described previously (13). Histological examination of the ischemic brain was performed by NeuN and TUNEL staining as described previously by our laboratory (13). For quantitative analyses, the numbers of surviving neurons and TUNEL-positive cells per 250-μm length of medial CA1 pyramidal cell layer were counted. A mean ± SE was calculated from the data in each group, and statistical analysis was performed as described below.
Proteasome Activity Assay, Quantitative RT-PCR, and in Situ Hybridization.
We analyzed 20S protease activity, which is the catalytical core of the 26S proteasome. The activity was measured using a 20S Proteasome Activity Assay Kit (APT280; Chemicon International) following the manufacturer's instructions. Real-time RT-PCR assays were performed on a Cepheid Smart Cycler using the RNA Amplification SYBR Green I kit (Roche), according to the manufacturer's protocol, as previously described by our laboratory (38). For in situ hybridization, locked nucleic acid (LNA)-modified oligonucleotide probes were digoxigenin-labeled by miRCURY LNA technology (Exiqon). The probe sequence was /5DigN/TAAGACGGAAGGAAGGAATGTG/3Dig-N/. A scrambled oligonucleotide was used as a negative control. In situ hybridization was performed according to a protocol described previously (39).
Statistical Analysis.
Statistical analysis was performed using either one-way ANOVA or two-way ANOVA analysis, followed by Student–Newman–Keuls post hoc tests to determine group differences. When groups were compared with a control group (e.g., sham), Dunnett's test was adopted for post hoc analyses after ANOVA. When only two groups were compared, a Student's t test was used. Statistical significance was accepted at the 95% confidence level (P < 0.05). Data were expressed as mean ± SE.
Supplementary Material
Acknowledgments
This study was supported by Research Grant NS050730 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and by American Heart Association Scientist Development Grant 10SDG2560092.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 14375.
See Author Summary on page 14387.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104391108/-/DCSupplemental.
References
- 1.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: Clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpkins JW, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 4.Simpkins JW, Singh M. More than a decade of estrogen neuroprotection. Alzheimers Dement. 2008;4(Suppl 1):S131–S136. doi: 10.1016/j.jalz.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Henderson VW. Estrogen-containing hormone therapy and Alzheimer's disease risk: Understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Henderson VW. Cognitive changes after menopause: Influence of estrogen. Clin Obstet Gynecol. 2008;51:618–626. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espeland MA, et al. Women's Health Initiative Memory Study Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 8.Shumaker SA, et al. WHIMS Investigators Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women's Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 9.Wassertheil-Smoller S, et al. WHI Investigators Effect of estrogen plus progestin on stroke in postmenopausal women: The Women's Health Initiative: A randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin BB. The critical period hypothesis: Can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 11.Craig MC, Murphy DG. Estrogen therapy and Alzheimer's dementia. Ann N Y Acad Sci. 2010;1205:245–253. doi: 10.1111/j.1749-6632.2010.05673.x. [DOI] [PubMed] [Google Scholar]
- 12.Sherwin BB. Estrogen therapy: Is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–627. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- 13.Zhang QG, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki S, et al. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci USA. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan M, Park A, Nephew KP. CHIP (carboxyl terminus of Hsc70-interacting protein) promotes basal and geldanamycin-induced degradation of estrogen receptor-alpha. Mol Endocrinol. 2005;19:2901–2914. doi: 10.1210/me.2005-0111. [DOI] [PubMed] [Google Scholar]
- 16.Tateishi Y, et al. Ligand-dependent switching of ubiquitin-proteasome pathways for estrogen receptor. EMBO J. 2004;23:4813–4823. doi: 10.1038/sj.emboj.7600472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry NB, Fan M, Nephew KP. Estrogen receptor-alpha hinge-region lysines 302 and 303 regulate receptor degradation by the proteasome. Mol Endocrinol. 2008;22:1535–1551. doi: 10.1210/me.2007-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canese R, Fortuna S, Lorenzini P, Podo F, Michalek H. Transient global brain ischemia in young and aged rats: Differences in severity and progression, but not localisation, of lesions evaluated by magnetic resonance imaging. MAGMA. 1998;7:28–34. doi: 10.1007/BF02592254. [DOI] [PubMed] [Google Scholar]
- 19.Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP2 immunostaining: Description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab. 1996;16:170–174. doi: 10.1097/00004647-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Valley CC, Solodin NM, Powers GL, Ellison SJ, Alarid ET. Temporal variation in estrogen receptor-alpha protein turnover in the presence of estrogen. J Mol Endocrinol. 2008;40:23–34. doi: 10.1677/JME-07-0067. [DOI] [PubMed] [Google Scholar]
- 21.Weitsman GE, Weebadda W, Ung K, Murphy LC. Reactive oxygen species induce phosphorylation of serine 118 and 167 on estrogen receptor alpha. Breast Cancer Res Treat. 2009;118:269–279. doi: 10.1007/s10549-008-0221-0. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S, Thakur MK. Interaction of estrogen receptor-alpha ligand binding domain with nuclear proteins of aging mouse brain. J Neurosci Res. 2009;87:2591–2600. doi: 10.1002/jnr.22068. [DOI] [PubMed] [Google Scholar]
- 23.Thakur MK, Ghosh S. Interaction of estrogen receptor alpha transactivation domain with MTA1 decreases in old mouse brain. J Mol Neurosci. 2009;37:269–273. doi: 10.1007/s12031-008-9131-1. [DOI] [PubMed] [Google Scholar]
- 24.Smith CC, Vedder LC, Nelson AR, Bredemann TM, McMahon LL. Duration of estrogen deprivation, not chronological age, prevents estrogen's ability to enhance hippocampal synaptic physiology. Proc Natl Acad Sci USA. 2010;107:19543–19548. doi: 10.1073/pnas.1009307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 26.Fan M, Bigsby RM, Nephew KP. The NEDD8 pathway is required for proteasome-mediated degradation of human estrogen receptor (ER)-alpha and essential for the antiproliferative activity of ICI 182,780 in ERalpha-positive breast cancer cells. Mol Endocrinol. 2003;17:356–365. doi: 10.1210/me.2002-0323. [DOI] [PubMed] [Google Scholar]
- 27.Tateishi Y, et al. Turning off estrogen receptor beta-mediated transcription requires estrogen-dependent receptor proteolysis. Mol Cell Biol. 2006;26:7966–7976. doi: 10.1128/MCB.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: A qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 29.Adams MM, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- 31.Brinton RD. The healthy cell bias of estrogen action: Mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Nilsen J, Brinton RD. Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: Therapeutic implications. Endocrinology. 2006;147:5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- 33.Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 34.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 35.Lebesgue D, et al. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS ONE. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speroff L. Transdermal hormone therapy and the risk of stroke and venous thrombosis. Climacteric. 2010;13:429–432. doi: 10.3109/13697137.2010.507111. [DOI] [PubMed] [Google Scholar]
- 37.Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan MM, et al. Cloning, distribution, and colocalization of MNAR/PELP1 with glucocorticoid receptors in primate and nonprimate brain. Neuroendocrinology. 2006;84:317–329. doi: 10.1159/000097746. [DOI] [PubMed] [Google Scholar]
- 39.Obernosterer G, Martinez J, Alenius M. Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat Protoc. 2007;2:1508–1514. doi: 10.1038/nprot.2007.153. [DOI] [PubMed] [Google Scholar]