Abstract
Optical mapping is a tool used in cardiac electrophysiology to study the heart's normal rhythm and arrhythmias. The optical mapping technique provides a unique opportunity to obtain membrane potential recordings with a higher temporal and spatial resolution than electrical mapping. Additionally, it allows simultaneous recording of membrane potential and calcium transients in the whole heart. This article presents the basic concepts of optical mapping techniques as an introduction for students and investigators in experimental laboratories unfamiliar with it.
Keywords: optical mapping, cardiac electrophysiology, ventricular arrhythmias
Cardiac mapping is a broad term that includes different types of mapping techniques, such as epicardial and endocardial mapping (Gupta, Maheshwari, Thakur, & Lokhandwala, 2002). Epicardial mapping is one of the most frequent mapping techniques and requires an open-chest procedure. Arrays of electrodes are placed on the heart's surface and electrical activities are recorded simultaneously from a limited number of sites (e.g., 100–300). Although the electrical mapping has evolved recording 250 kB of data per second by sampling each of the 128 channels at 1000 Hz (Ideker, Smith, & Wolf, 1989), multisite contact mapping techniques do not provide adequate spatial and temporal resolution to elucidate the mechanism of cardiac arrhythmias. Furthermore, the interpretation of measured activation and repolarization times is uncertain in some conditions (e.g., ischemia; Efimov, Nikolski, & Salama, 2004), and the repolarization times are estimated indirectly (Packer, 2001).
Rapid technical innovations during the last decades of the 20th century have led to the development of sophisticated optical mapping techniques. Optical mapping is performed by using voltage-sensitive dyes and imaging systems with high temporal and spatial resolutions (i.e., acquiring thousands of pixels in a few milliseconds) and can be used in a variety of settings from the subcellular level in vitro to the whole heart in vivo. Both activation and repolarization times can be measured directly from different sites in both normal and diseased heart (e.g., ischemic heart).
The purpose of this article is to provide a basic review of optical mapping techniques in the whole heart as they are used to study ventricular arrhythmias, including both ventricular tachycardia (VT) and ventricular fibrillation (VF). We also describe the basic concepts underlying the technical aspects of optical mapping methods.
Historical Background of Optical Mapping
Membrane potential has generally been studied by intracellular recordings from cells that have been impaled with glass pipette microelectrodes. The limitations of this recording method include the inability to study small cells and subcellular organelles and the difficulty in obtaining simultaneous and stable recordings of membrane potential at multiple sites (Efimov et al., 2004).
The possibility of optical recordings of membrane potential was first demonstrated in 1968. Although the initial recordings had poor signal-to-noise ratio (SNR or S/N), both intrinsic and extrinsic signals were obtained. Intrinsic signals were obtained from recordings of light scattering or birefringence of axons (Cohen, Keynes, & Hille, 1968), whereas extrinsic signals were obtained by staining axons with fluorescent dyes (Tasaki, Watanabe, Sandling, & Carnay, 1968). In search of better signals, more than 1,000 dyes were screened as possible probes of membrane potential (Cohen & Salzberg, 1978). Optical action potentials (APs) were recorded from mammalian hearts by using voltage-sensitive dyes (Salama & Morad, 1976). The first cardiac application of this method was the localization of pacemaker activity in embryonic heart preparations (Kamino, Hirota, & Fujii, 1981). Although optical mapping of the heart was initially restricted to a few laboratories, this technique became more common in the 1990s.
Major Components of Optical Mapping System
The goal of the optical mapping system is to provide optical signals with a high SNR and high temporal and spatial resolution, while minimizing the probability of side effects, including photobleaching. All cardiac optical mapping studies require staining the heart with voltage-sensitive and/or calcium dye and then using imaging systems to record electrical activity and calcium transients from the surface of the heart. Detailed descriptions of each optical mapping component are presented below.
Voltage-Sensitive Dyes
Mechanism of action
Voltage-sensitive dyes contain molecules that bind to cell membranes when they are delivered to the whole heart, cardiac tissue, or myocytes through coronary perfusion or superfusion (Lichtman & Conchello, 2005; Rosenbaum, 2001). Both electrochromic and solvatochromic theories have been used to explain the mechanism of action of voltage-sensitive dyes. According to the electrochromic theory, molecules undergo an electronic redistribution during excitation of membrane potential, and, if the direction of the charge shift is parallel to an external field (membrane potential), the energy of the electronic transition is altered. Fast voltage-sensitive dyes can track voltage changes rapidly during excitation, and there is an instantaneous coupling between the external field (membrane potential) and the electronic states of the chromophore (the chemical group that gives color to a molecule; Fluhler, Burnham, & Loew, 1985). However, the response of certain dyes (e.g., RH421) cannot be explained by the electrochromic theory. The mechanism of such dyes may be explained by the solvatochromic theory, which suggests that the electrical field may reorient the dye molecules (change in polarity) in the lipid environment by the voltage gradient (Efimov, Biermann, & Zipes, 2003).
Characteristics of optical signals
Merocyanine I (or M-540) was the first dye used to optically record APs (Salama, 2001). Optical recordings of APs from frog ventricular tissue stained with M-540 have shown an excellent correlation with microelectrode recordings. Furthermore, optical and intracellular microelectrode recordings of APs have shown similar results with different interventions, including increasing the frequency of stimulation (shortened AP), cooling (prolonged AP), pharmacological interventions (i.e., epinephrine), and ionic intervention (e.g., addition of Ni2+; Salama & Morad,1976). Moreover, in the intact heart, the results of surface electrograms and optical recordings are in agreement (Nygren, Baczko, & Giles, 2006).
A recent study (Himel & Knisley, 2007) investigated the pattern of excitation during VF on ventricular epicardium of three healthy perfused rabbit hearts by simultaneous optical (using di-4-ANEPPS dye and laser scanner) and extracellular potential recordings. The means of optical deflection times occurred earlier than the means of electrical deflection times by approximately 1 ms. The authors ascribed this result to an earlier occurrence in subsurface tissue than in the heart surface.
Optical measurements are reported either as a change in fluorescence (ΔF) or as a fractional fluorescence change (ΔF/F—the change in fluorescence intensity during a given voltage step divided by the background fluorescence; Ebner & Chen, 1995; Rohr, 2002). The ΔF/F corresponds to depolarization during an AP recording (approximately 100 mV). However, ΔF/F does not reflect the absolute resting membrane potential because fluorescence can be affected by tissue autofluorescence, bleaching, or the loss of the dye from the tissue. The SNR can be optimized by choosing dyes that provide the highest possible ΔF/F (Rohr, 2002). The ΔF is small (8% to 10%/100 mV) when using di-4-ANEPPS (Laurita & Rosenbaum, 2003).
Only fast voltage-sensitive dyes are used in cardiac electrophysiology because their voltage-dependent optical response occurs within microseconds; consequently, APs can be measured. Although optical recordings reproduce the temporal course of APs, they do not provide absolute values of membrane potential voltages (Lichtman & Conchello, 2005; Rosenbaum, 2001). The styryl dyes, such as di-4-ANEPPS, are the most popular dyes used in experimental studies of cardiac electrophysiology. They have the following characteristics: large SNR, short excitation wavelengths, and large Stokes shifts that make it possible to exclude the scattered and reflected light and the filtering away of the background autofluorescence (Efimov et al., 2003; Wuskell et al., 2006).
The orientation of styryl dye molecules in the cell membrane is affected by the presence of lipophilic and hydrophilic groups at opposite ends of the molecule. Each styryl molecule is anchored in the aqueous extracellular space by a hydrophilic negatively charged sulphonyl group, whereas the other end of the molecule is held within the bilayer lipid membrane by a lipophilic hydrocarbon chain (Efimov & Cheng, 2002). Most styryl dyes require an additional reagent (i.e., dimethyl sulphoxide [DMSO] or Pluronics) to transport them from the aqueous medium to the tissue (Patrick et al., 2007).
Other than voltage-sensitive dyes, calcium indicators are the only ones available to study the cellular activity of myocardium in the whole heart on a beat-to-beat basis. Calcium ions play crucial roles in the regulation of many cellular processes including the excitation-contraction coupling in the heart. The properties of calcium indicators that are used frequently in the whole heart to study cardiac arrhythmias are described below.
Characteristics and measurements for calcium indicators
Two types of calcium dyes are available to record calcium transients ([Ca2+]i) in the whole heart including dual wavelength ratiometric dyes and single wavelength nonratiometric dyes (Rudolf, Mongillo, Rizzuto, & Pozzan, 2003). Indo-1 is considered to be a dual wavelength ratiometric dye and its emission spectrum shifts on binding calcium. The ratiometric measurement for Indo-1 is obtained by taking the ratio between the fluorescence intensity values corresponding to two emission wavelengths: calcium-bound and calcium-free conditions (Lichtman & Conchello, 2005; Rudolf et al., 2003). The emission wavelength of the fluorescence maximum shifts rather than just increasing in intensity when Indo-1 binds with calcium, whereas the excitation spectra remain unchanged (Rudolf et al., 2003). This measures [Ca2+]i and is independent of dye concentration. Indo-1 requires light in the ultraviolet range of 340–380 nm for excitation (Lee, & Clusin, 1987) and 405/485 nm is the emission ratio for [Ca2+]i measurements (405 = calcium bound and 485 = calcium free). Although ratiometric [Ca2+]i may reflect the relative changes in intracellular calcium, the absolute calcium levels are not certain in some cases (Katra & Laurita, 2005). The following equation (Grynkiewicz, Poenie, & Tsien, 1985) is used for dual wavelength ratiometric dyes to measure calcium and it has been used to measure [Ca2+]i in the whole heart by Katra, Pruvot, & Laurita, 2004:
Kd is the dissociation constant representing the concentration at which 50% of the calcium indicator molecules are combined with calcium (Richter & Hader, 2000). The present ratio is R, Rmax is R at saturating calcium concentration, and Rmin is R at zero calcium concentration. (Sf2) is the value at zero calcium concentration for wavelength two (485 nm) and (Sb2) is the value at saturating calcium concentration for wavelength two (Rudolf et al., 2003).
Indo-1 acetoxymethyl (AM) was first used in both normal and ischemic whole heart (Figure 1; Lee, Smith, Mohabir, & Clusin, 1987). Cell permeant AM esters of calcium dyes (e.g., Indo-1) make the dye neutral, so that it can cross the cell membrane (Richter & Hader, 2000). Specific enzymes (esterases) that are present in the cell hydrolyze the AM esters to release the calcium dye. The heart is loaded with the dye for 30 or 45 min, which is followed by 20 or 30 min of washout to remove unhydrolyzed or partially hydrolyzed dye (Katra et al., 2004; Lee et al., 1987).
Figure 1.
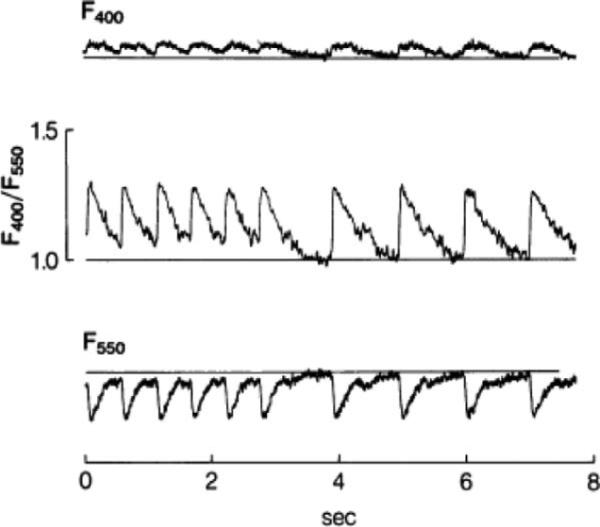
First recording of calcium transients in intact heart loaded with Indo-1. Fluorescence increases at 400 ± 12.5 nm (top trace) and decreases at 550 ± 20 nm (bottom trace). In the middle trace, the first six beats of calcium transients were induced by pacing of right ventricle whereas the last four beats occurred spontaneously. The fluorescence was collected by fiber-optic cables before reaching photomultiplier tubes. The photomultiplier is a photodetector used when the light level is very low (Adapted from Lee et al., 1987). Reprinted with permission.
Rhod-2 is a single wavelength nonratiometric dye and calcium concentration is determined by a relative increase in the fluorescence intensity on binding with calcium (Rudolf et al., 2003). Calibration in the whole heart is performed (Choi & Salama, 2000) using the Grynkiewitz formula:
The F is the fluorescence intensity (Richter & Hader, 2000), Fmin is the fluorescence under calcium-free condition, and Fmax is the dye fluorescence in the calcium-saturated condition, which is generally achieved with a calcium ionophore (Choi & Salama, 2000).
The required excitation light for Rhod-2 is at visible wavelengths of 500–600 nm (Del Nido et al., 1998). Rhod-2 is excited at 520 nm with a peak emission at 580 nm. The heart can be loaded with the dye for more than 10 min and generally washes out 15–30 min of its delivery to the heart tissue (Hwang et al., 2006). A transient decrease in heart rate (300–340 ms cycle length) and perfusion pressure (<6 mmHg) has been noted while staining with Rhod-2; however, hearts can recover fully within 5 min. A high concentration (0.6–0.8 mg Rhod-2 in 0.5 ml DMSO) should be avoided because it results in a 10%–15% decline in pressure. Rhod-2 staining provides stable recordings of calcium and excellent SNR for more than 2 hr (Choi & Salama, 2000).
Although it is assumed that fluorescence indicators are distributed in the cytosol homogeneously, the AM esters of rhodamine-based dyes are cationic, which may potentially result in mitochondrial uptake. However, manganese quenching has determined that Rhod-2 loading in subcellular compartments, including mitochondria, is minimal (<5%; Del Nido et al., 1998).
Optical mapping methods provide an opportunity not only to study [Ca2+]i in the intact heart but also to simultaneously record membrane potential and [Ca2+]i (Figure 2). Basically, there are two methods for simultaneous recordings and they involve different dyes and light sources. One method uses Indo-1 and di-4-ANEPPS with an ultraviolet source and a visible wavelength. The other method uses Rhod-2, which has a long wavelength, and RH-237, which has an even longer wavelength. Both dyes are excited by the same visible wavelength. Both of these methods also produce similar results (Clusin, 2008), and the cross talk between voltage and [Ca2+]i signals (e.g., RH-237 & Rhod-2) is not significant (Omichi et al., 2004).
Figure 2.
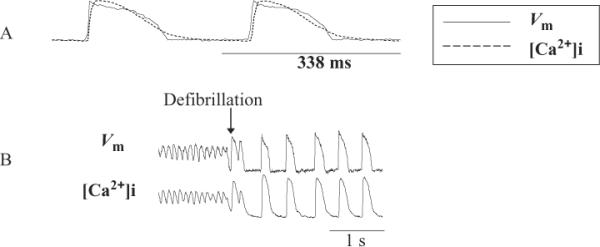
Simultaneous recording of membrane potential and calcium transients during normal sinus rhythm, ventricular fibrillation (VF), and defibrillation. (A) Optical signals from membrane potential and calcium transients during normal sinus rhythm. (B) Optical signals during VF, defibrillation and normal sinus rhythm in Langendorff-perfused rabbit heart (Attin, 2005).
Light Sources
One of the most common conventional lights is tungsten-halogen bulbs, which are nonlaser light sources. The tungsten-halogen lamp, with a power of 100 or 250 W, emits a smooth, continuous spectrum with shorter wavelengths than other lights. The small amount of halogen gas inside the lamp promotes long life and operates at high temperature. The advantages of this light are its low cost and its ability to obtain a high output when operated by a well-regulated direct current (DC) power supply (Parker, 2003). Switching DC supplies should be avoided. These lamps can be turned on from 0.5 to 4 min during the recording and are turned off to avoid excessive heat to the lamp bases and electrodes. They have sufficiently low noise characteristics for measurement of the AP through absorption changes of the dye (Salama, 2001). Moreover, they have a spectrum in the visible range, which makes them flexible for choosing the excitation wavelength. The 250 W bulb filament has dimensions of 7 mm × 3.5 mm, which makes it appropriate for illumination of medium-sized preparations (i.e., rabbit hearts). Usually, the tungsten-halogen lamps should be considered as a first choice in optical mapping (Fast, 2005).
Laser light is monochromatic (one wavelength) and is considered to be the perfect light source from an optical perspective (Parker, 2003). Laser has a high cost, a short lifetime, and high power consumption, but its intense illumination can be easily and rapidly applied to a small spot (Parker, 2003). The variation in beam intensity is within 1%–5%, comparable to the average intensity recorded from most voltage-sensitive dyes. A new version of the classic laser light has a lower noise level, which is comparable to the tungsten-halogen or arc lamp but it costs $55,000 or more (Fast, 2005). Safety precautions should be taken, such as wearing protective eyewear, posting warning signs, restricting access to the room during laser exposure, and using a low optical table so that the laser beam is below eye level even when sitting (Parker, 2003).
Light-emitting diodes (LEDs) are an attractive alternative to the conventional white light sources and laser light because of low power consumption and fewer imaging artifacts (Moe et al., 2005). The wide range of wavelengths with narrow spectral output reduces the need for optical filtering (Lakowicz, 2006).
In general, a larger signal can be produced by a larger emitted fluorescence resulting from more intense excitation. Continuous illumination causes photobleaching, so one common preventive method is to keep the sample in the dark and use only enough light to get the image that is needed (Lichtman & Conchello, 2005).
Detectors
There are two types of photodetectors: photodiode arrays and charged coupled device (CCD) cameras. The photodetectors of these devices consist of two-dimensional arrays of silicon elements that transduce the energy of light photons into the energy of electrical charges (Fast, 2005).
A photodiode is a semiconductor junction that converts light into electrical current (Parker, 2003). The current output from each photodetector must be passed to a voltage converter; the voltage signals are then sent to a data acquisition computer (Rosenbaum, 2001). Photodiodes are best suited to moderate or high intensity light that is focused on a small spot, because the noise decreases and the speed of response increases when the detector area is decreased (Parker, 2003). The advantage of a photodiode array is continuous generation of photocurrent in response to changes in membrane potential. This process leads to digitally sampling the AP at very rapid rates. The photodiode array for optical mapping of cardiac activation has high spatial (150 μm) and temporal (3.4 kHz) resolution (Rothenberg, Watanabe, Eloff, & Rosenbaum, 2005). Usually, two photodiodes are used for simultaneous recordings of Vm and [Ca2+]i, and they should be aligned precisely to record the same areas (Choi & Salama, 2000).
Another detector is the CCD video camera that is a semiconductor device segmented into an array of light-sensitive recording pixels. The high spatial resolution of the CCD video camera is due to the presence of 106 or more pixels (Rosenbaum, 2001). Electrons are released when a photon hits a pixel, leading to an increase in charge contained on that pixel. The pixel size of CCD imagers is in range of micrometers. Each frame of a CCD movie integrates light over a discrete interval in a manner analogous to a photographic process. The pixels generate charge as they collect light over an interval. The accumulated charge is then shifted off the CCD chip pixel by pixel and converted to a video signal comprised of voltages. One interval corresponds to a single frame or picture, and each frame represents light collected simultaneously by all pixels over the same interval (Baxter, Davidenko, Loew, Wuskell, & Jalife, 1997).
Originally, CCD cameras were used to obtain higher spatial resolution; however, they have slow frame rates. The speed can be increased by pixel binning (reading from several pixels at once), but this will decrease the spatial resolution (128 × 128 pixels at 490 frames/s; Efimov et al., 2004). The speed and quality of video recording depend on reducing the number of pixels. Recent studies have used CCD cameras with 420 frames/s (Chou et al., 2005) or 500 frames/s (Gray, Iyer, Berenfeld, Pertsov, & Hyatt, 2006). Cameras with high quantum efficiency or low noise allow for shorter exposure and less bleaching (Lichtman & Conchello, 2005). For simultaneous recordings of Vm and [Ca2+]i, two CCD cameras need to be aligned (e.g., by using a grid) to image the same region (Omichi et al., 2004).
Filters
Optical filters allow only selected wavelengths of light to reach the detector and are essential to reduce background light. The high-quality filters can also decrease the probability of bleaching, while allowing all, or nearly all, of the emitted wavelengths to pass through (Lichtman & Conchello, 2005). An excitation filter is a color filter that transmits only wavelengths of light that excite a specific dye (Yuste & Konnerth, 2005). Emission filters transmit the emission and block light at the excitation wavelength and may be shortpass, longpass, or bandpass filters. The shortpass filter attenuates longer wavelengths and transmits shorter wavelengths over the active range of the target spectrum. In contrast, the longpass filter attenuates shorter wavelengths and transmits longer wavelengths over the active range of the target spectrum. Bandpass filters are denoted by their center wavelength and bandwidth. The center wavelength is the arithmetic mean of the wavelengths at 50% of peak transmission.
Motion Artifact and Excitation-Contraction Uncouplers
The motion artifact produced by contraction of heart muscle distorts signals by changing the relative position of the heart tissue to the photon sensor (Wu, Biermann, Rubart, & Zipes, 1998). There are different ways to prevent the heart's motion, including restraining the isolated heart or tissue mechanically, reducing or abolishing extracellular calcium, and using pharmacological agents, such as excitation-contraction (E-C) uncouplers (Qin et al., 2003).
Uncoupler agents including 2,3-butanedione monoxime (BDM) and cytochalasin D (Cyto-D, a fungal metabolite) are frequently used for optical mapping studies. Although BDM is cost-effective, it reduces calcium current (Gwathmey, Hajjar, & Solaro, 1991) and AP durations (Wu et al., 1998), prevents VF induction, and converts VF to VT at high concentrations (Lee et al., 2001). Although the mechanism of action of BDM is not fully understood due to contradictory findings, it seems that low concentrations primarily affect the calcium transients and high concentrations inhibit myofibrillar proteins (Sellin & McArdle, 1994).
Cyto-D's effects have been reported in different species and these effects may be partly related to the methodological approaches used (Table 1). Cyto-D does not change the repolarization time, and trans-mural propagation is not slowed at a concentration of 25 μmol/L for 15 min with subsequent perfusion of 10 μmol/L in wedge preparation of canine left ventricle (Wu et al., 1998). However, it has caused hyperpolarization in mice (Jalife, Morley, Tallini, & Vaidya, 1998) as well as prolongation of APs in mice (Jalife et al., 1998) and rabbits (Hayashi et al., 2003; data in rabbits were obtained with a microelectrode). Cyto-D has increased both diastolic and systolic measures of [Ca2+]i, with no effect on its duration (Baker et al., 2004).
Table 1.
Effect of Cytochalasin D on Ventricular Arrhythmias
| Reference | Species/Preparation | Concentration | Characteristics of VT/VF |
|---|---|---|---|
| Lee et al. (2001) | Isolated perfused swine right ventricle | 10–40 μmol/L | No effect on the dynamics of VF |
| Banville and Gray (2002) | Retrograde perfusion of rabbit hearts | 10 μmol/L with additional injections of 2.5 μmol/L if needed | Inability to induce sustained ventricular arrhythmias |
| Qin et al. (2003) | Langendorff-perfused pig hearts | 10 μmol/L | Slowing and regularization of VF pattern due to loss of excitability |
| Baker et al. (2004) | Perfused FVB mice heart | Continuous perfusion with various concentrations; minimum concentration 5 μM | Could not induce VT/VF |
| Cheng, Nikolski, Wallick, and Efimov (2004) | Langendorff-perfused rabbit hearts | 20 μM in Tyrode's solution | Shock-induced arrhythmias resembled mostly polymorphic VT |
NOTE: VF = ventricular fibrillation; VT = ventricular tachycardia; FVB = inbred strains of mice.
The exact mechanism of Cyto-D is unknown. Cyto-D may affect electrical properties, such as slowing the rate of inactivation of voltage-dependent sodium channels (Undrovinas, Shander, & Makielski, 1995). The direct interaction of Cyto-D and actin causes depolymerization of F-actin in cytoskeleton, leading to contraction failure (Wu et al., 1998). A more recent study (Rueckschloss & Isenberg, 2001) indicated that Cyto-D mediates dephosphorylation and activates actin-depolymerizing factor (ADF/cofilin), which eventually causes the depolymerization of F-actin, with a concomitant reduction of calcium currents.
A new E-C agent, blebbistatin, has shown no effect on electrophysiological properties or [Ca2+]i morphologies in Langendorff-perfused rabbit hearts, isolated rabbit right ventricle and right atrium, and single rat ventricular myocytes (Fedorov et al., 2007). Blebbistatin inhibits actin-myosin interaction in cardiac muscle (Dou, Arlock, & Arner, 2007) and myosin II (2–10 mM) in cardiac cells (Fedorov et al., 2007).
Clinical Relevance
Depolarization of cardiac muscle generates an AP and the pattern of AP propagation is the basis of cardiac rhythm and arrhythmias. Although AP propagation can be viewed as an electrical wave, reentry as one of the most common underlying causes of VT/VF can be defined as “an excitation wave that repeatedly travels along a closed path” (Wellner & Berenfeld, 2004). Anatomical reentry is a circulating wave around an anatomical obstacle (e.g., scar) and was described in the beginning of the last century. The functional reentry is a circulating wave around an area with an altered electrophysiological property (e.g., slow conduction) that was described almost 60 years later, known as “leading circle” (Allessie, Bonke, & Schopman, 1977). However, the leading circle theory does not consider biophysical properties of cardiac tissue in relation to reentry.
Advances in spiral wave research have been based on two approaches: (a) the experimental study of the Belouzov-Zhabothinsky chemical reaction and (b) numerical/theoretical work on mathematical models of cellular electrical activity, especially the Fitzhugh-Nagumo model. The spiral wave has also been reproduced in models of cardiac tissue (i.e., computer simulation) and has been studied by optical mapping techniques (Comtois, Kneller, & Nattel, 2005; Davidenko, Pertsov, Salomonsz, Baxter, & Jalife, 1992). Using optical mapping system, reentry is currently viewed as spiral wave in two-dimensional tissue and as a scroll wave in three-dimensional tissue. The spiral wave occurs as a result of a wavebreak that is an interaction between a wavefront, corresponding to AP-depolarization during phase zero of the AP, and a waveback, corresponding to repolarization during phase three of the AP (Weiss et al., 2005). Currently, the properties of the spiral wave during VT/VF in both small (i.e., rabbit) and large (i.e., swine) hearts is being investigated intensively by use of optical mapping systems.
Optical mapping techniques have also provided a unique opportunity to visualize the locations of arrhythmias and quantify the propagation of electrical activity, which ultimately helps elucidate the mechanisms of VT/VF. For example, the characteristics of papillary muscle (PM) as an important source of VF (Kim et al., 1999) have been investigated by using optical mapping techniques by Pak et al. (2003). They used propranolol to convert multiple-wavelet VF into a slower focal source of VF, and used ablation to terminate VF in the PM of the Langendorff-perfused rabbit heart. Furthermore, clinical mapping and ablation of PM has eliminated the focal source of VF in a limited number of patients (Haissaguerre et al., 2003; Kohsaka, Razavi, & Massumi, 2007). These clinical studies have validated the utility of optical mapping techniques.
Optical mapping techniques have been used extensively to study the underlying causes of sudden death (SD) including VT/VF. The prevalence of SD is not known despite it being a major public health problem: there are approximately 200,000–450,000 or more sudden cardiac deaths per year in the United States (European Heart Rhythm Association et al., 2006). The mechanism of sudden cardiac death due to VT/VF is not fully understood, despite intense research for more than a century. Defibrillation is the only effective treatment for these lethal arrhythmias, and the mechanism of defibrillation is still not clear despite its discovery decades ago. Simultaneous recordings of Vm and [Ca2+]i by optical mapping techniques have led to new insights into the mechanism of VT/VF and defibrillation. For example, the first post-shock activation after defibrillation has been suggested to be calcium mediated (Attin, 2005; Hwang et al., 2006). Furthermore, involvement of the calcium transient and AP alternans in the genesis of VF has been studied by optical mapping methods and has been comprehensively reviewed by Clusin (2008).
Theories have been proposed for the mechanism of VF based on experimental studies using optical mapping techniques. However, the findings of these studies are based on animals (e.g., rabbits) and may not apply to the dynamics of the human heart. Recently, the first recording of optical mapping using Langendorff-perfused human heart has been published (Nanthakumar et al., 2007). Scientists from different disciplines including nursing may find answers to the genesis of heart rhythm in both normal and diseased human heart by using complex techniques such as optical mapping.
Summary
Optical mapping techniques provide a unique opportunity for studying ventricular arrhythmias in the whole heart. Rapid technological innovations will permit the study of VT/VF in complex settings in normal and diseased human heart. Performing optical mapping experiments requires in-depth knowledge and technical skills. Development of more sophisticated optical mapping systems, such as panoramic optical mapping, may provide even greater insight into arrhythmia mechanisms.
Acknowledgments
Supported by #T32 NR007075 Training in Biobehavioral Nursing Research grant from the National Institute of Nursing Research.
Appendix: Glossary
Calcium Transients
Ion channels determine the membrane potential (Vm) in living cells. Intracellular calcium transients [Ca2+]i also shape AP characteristics by modulating different ionic currents including the L-type calcium channel. Calcium (Ca2+) provides the link between the electrical and mechanical state of the heart. During the plateau phase of the AP, Ca2+ enters the cell through channels called L-type Ca+2 receptors (dihydropyridine receptors; DHP). These channels are located at the sarcolemmal-sarcoplasmic reticulum (SR) junction, where the SR Ca2+-release channels (or ryanodine receptors [RYRs]) are found. The influx of Ca2+ (2–4 Ca2+ ions binding to the RYR) triggers Ca2+ release from the SR. This process is known as calcium-induced calcium release (CICR). The release of Ca2+ from the SR is controlled by at least three regulators: (a) the magnitude of the triggering Ca2+ influx, (b) the state of the RYRs, and (c) the Ca2+ content within the SR (Volders et al., 2000). The total rise and fall of [Ca2+]i due to influx and SR release is called the calcium transients. Spontaneous calcium release from the SR can increase [Ca2+]i and cause depolarization or even an AP (Capogrossi, Houser, Bahinski, & Lakatta, 1987). Furthermore, the role of [Ca2+]i in relation to the mechanism of cardiac arrhythmias has been under intense investigation using calcium-sensitive dyes.
Langendorff Perfusion of an Isolated Heart
In the Langendorff preparation, the aorta of an excised intact heart is cannulated, and then attached to a reservoir containing oxygenated perfused fluid (95% oxygen and 5% carbon dioxide). The perfusion is maintained at 37°C continuously and the fluid is delivered in a retrograde direction down the aorta either at a constant hydrostatic pressure (60–100 mmHg) or at a constant flow rate. The retrograde flow shuts the leaflets of the aortic valve. Consequently, the perfusion solution cannot enter the left ventricle and is displaced via the coronary ostia into coronary arteries. After perfusing the entire ventricular mass, it drains into the right atrium via the coronary sinus (Skrzypiec-Spring, Grotthus, Szelag, & Schulz, 2007; Sutherland & Hearse, 2000). Heart isolation causes VF to become more organized and slower with a possible shift in the mean direction of propagation with respect to the endocardium (Qin et al., 2003).
Light
Light is a small part of the electromagnetic spectrum, extending from wavelengths of about 200 nm to those above 2000 nm (Parker, 2003). The human eye can only see from 400 nm (violet) to 700 nm (deep red). Light has a dual nature, behaving as both a wave and as particles (photons). The background light is any detectable light that is not due to fluorescence such as light leaking through pinholes in filters, electronic noise in cameras, or autofluorescence.
Mapping
Excitation mapping is the measurement of electrical changes at many points either simultaneously or sequentially (Smith, 1999). Currently, “cardiac mapping” refers to measuring electrical activity of the heart in two or three dimensions that can be viewed in two-dimensional representations (Sih & Berbari, 2003).
Noise
Noise is defined as the variation of the signal in repeated observations (Stelzer, 1998). Noise in imaging data has two main sources: biological (i.e., motion due to heart contraction) and technical (i.e., cameras and electronic instrumentation). Detection of minimal signal in any experiment depends on the SNR or S/N. An engineering term, SNR expresses the level of noise in a system in reference to the level of signal (Rubin, 1987). The signal must be greater than the noise inherent in the measurement of the signal to measure the signal accurately. The SNR must be sufficiently large to ensure the validity of the measurement; that usually requires an SNR of 2–5 as a minimum. The SNR can be kept reasonably stable by limiting light exposure over time to minimize bleaching of the dye. The calculation of SNR for a detector allows a measure of the device's sensitivity (Christenson, 2005). The SNR not only depends on the dye itself but also in its mode of delivery (i.e., coronary perfusion provides more uniform dye delivery).
Principles of Fluorescence
The principles of fluorescence can be explained by using a “Perrin-Jablonski diagram.” Most texts use the title “Jablonski diagram” instead of “Perrin-Jablonski diagram;” however, Perrin used energy level diagrams to describe fluorescence phenomena before Jablonski (Jameson, Croney, & Moens, 2003). The energy state of a molecule that is capable of fluorescing is shown in Figure 3. The “ground state” is S0, which represents the energy of a molecule not being excited by light. Light (i.e., at a discrete wavelength) can be used to excite the dye molecule in the ground state. The “excited state” or S1 occurs when there is absorption of the light energy (photon) by a molecule. The transition to the excited state occurs very rapidly (in femtoseconds). Excitation causes alterations in the electronic, vibrational and rotational states of the molecule (i.e., movement of an electron into a different orbital that is farther away from the nucleus). As the dye molecule returns to its “ground state,” it emits light of a different wavelength (generally longer) in nanoseconds (10−9; Lichtman & Conchello, 2005). This light is known as fluorescence. The basic principles of fluorescence involve two opposite processes: absorption and emission (it should be noted that these processes are often called excitation and emission). Absorption occurs when a photon is taken in by a molecule (i.e., a dye molecule), and its energy is converted into an atomic or molecular process (i.e., an electron changes orbit). Fluorescence is the subsequent emission of light that is at a lower energy or longer wavelengths. This cycle can be repeated until the dye molecule is destroyed. This is often referred to as “photobleaching.” Once a molecule is photobleached, it is no longer fluorescent (Johnson & Landers, 2004). Bleaching can also be described as processes that cause the fluorescence signal to fade permanently. Usually, fluorophores cycle ground and excited states approximately between 10,000 and 40,000 cycles before permanent bleaching occurs (Lichtman & Conchello, 2005).
Figure 3.
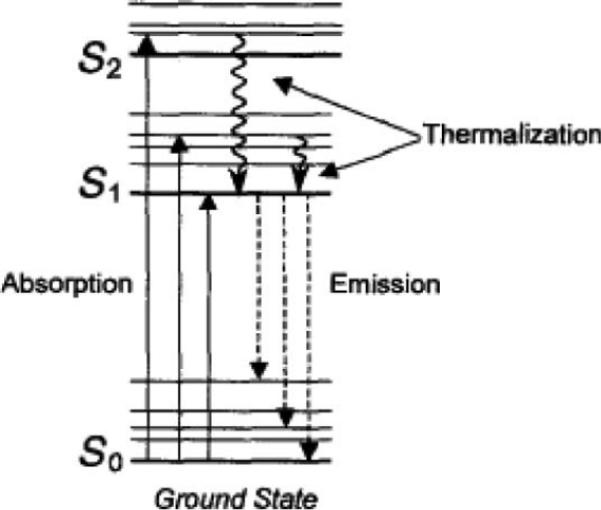
Perrin-Jablonski diagram. S0 = ground state; S1 = excited state (Adapted from Jameson et al., 2003). Reprinted with permission.
As noted above, the energy of the emitted photon (longer wavelength) is usually less than the absorbed photon (shorter wavelength). This difference in energy is called the “Stokes shift.” The separation of exciting and emitting light is easier when there is a large Stokes shift. However, not all fluorescent emissions occur at longer wavelengths than the exciting light, leading to an overlap of emission and excitation spectra. By knowing the fluorescence excitation and emission spectra, it is possible to select filters that minimize cross-talk and prevent inclusion of excitation photons in the background light measured by the detector. Moreover, this can be done with minimum attenuation of emitted fluorescence. The goal of fluorescence detection is to maximize signal while minimizing background.
Resolution
Resolution is determined by different factors, including the wavelength of the light (i.e., shorter wavelength improves resolution), the numerical aperture of the optical system, and the type of specimen (Jonkman, Swoger, Kress, Rohrbach, & Stelzer, 2003). Both spatial (space) and temporal (time) resolution should be determined before recording data during an experiment. There is always a trade-off between the temporal and spatial resolution (Lin & Wikswo, 1999). Spatial resolution refers to the number of pixels displayed by a monitor or image, measured horizontally and vertically (i.e., 128 × 128). Temporal resolution refers to the speed of recording an image or frame. Usually, increasing spatial resolution decreases the temporal resolution and visa versa.
Contrast quantifies the resolution and is considered as an important factor for achieving maximum resolution. Contrast can be defined as the difference between the minimum and maximum intensity of two points in the image. Resolution is different from “image quality,” which is a subjective term depending on the person's view. A high-resolution image can have a lower contrast or be noisy, whereas an image with low resolution can appear as a good and pleasing image (Jonkman et al., 2003).
References
- Allessie MA, Bonke FI, Schopman FJ. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: A new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circulation Research. 1977;41:9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- Attin M. Unpublished doctoral dissertation. University of California; Los Angeles: 2005. Calcium transients: An insight into ventricular defibrillation. [Google Scholar]
- Baker LC, Wolk R, Choi B-R, Watkins S, Plan P, Shah A, et al. Effects of mechanical uncouplers, diacetyl monoxime, and cytochalasin-D on the electro-physiology of perfused mouse hearts. American Journal of Physiology. 2004;287:H1771–H1779. doi: 10.1152/ajpheart.00234.2004. [DOI] [PubMed] [Google Scholar]
- Banville I, Gray R. Effect of action potential duration and conduction velocity restitution and their spatial dispersion on alternans and the stability of arrhythmias. Journal of Cardiovascular Electrophysiology. 2002;13:1141–1149. doi: 10.1046/j.1540-8167.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- Baxter WT, Davidenko JM, Loew LM, Wuskell JP, Jalife J. Technical features of a CCD video camera system to record cardiac fluorescence data. Annals of Biomedical Engineering. 1997;25:713–725. doi: 10.1007/BF02684848. [DOI] [PubMed] [Google Scholar]
- Capogrossi MC, Houser SR, Bahinski A, Lakatta EG. Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Circulation Research. 1987;61:498–503. doi: 10.1161/01.res.61.4.498. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Li L, Nikolski V, Wallick DW, Efimov IG. Shock induced arrhythmogenesis is enhanced by 2,3-butanedione monoxime compared with cytochalasin D. American Journal of Physiology. 2004;286:H310–H318. doi: 10.1152/ajpheart.00092.2003. [DOI] [PubMed] [Google Scholar]
- Choi B-R, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: Mechanisms underlying concordant alternans. Journal of Physiology. 2000;529:171–188. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C-C, Nihei M, Zhou S, Tan A, Kawase A, Macias ES, et al. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- Christenson M. The application of scientific grade CCD cameras to biological imaging. In: Yuste R, Konnerth A, editors. Imaging in neuroscience and development: A Laboratory manual. Cold Spring Harbor Laboratory Press; New York: 2005. pp. 23–33. [Google Scholar]
- Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. American Journal of Physiology. 2008;294:H1–H10. doi: 10.1152/ajpheart.00802.2007. [DOI] [PubMed] [Google Scholar]
- Cohen LB, Keynes RD, Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968;218:438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Cohen LB, Salzberg BM. Optical measurement of membrane potential. Review of Physiology, Biochemistry and Pharmacology. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Comtois P, Kneller J, Nattel S. Of circles and spirals: Bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace. 2005;7:10–20. doi: 10.1016/j.eupc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992;355:349–351. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- Del Nido PJ, Glynn P, Buenaventura P, Salama G, Koretsky AP. Fluorescence measurement of calcium transients in perfused rabbit heart using Rhod-2. The American Journal of Physiology. 1998;274:H728–H741. doi: 10.1152/ajpheart.1998.274.2.H728. [DOI] [PubMed] [Google Scholar]
- Dou Y, Arlock P, Arner A. Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. American Journal of Physiology. 2007;293:C1148–C1153. doi: 10.1152/ajpcell.00551.2006. [DOI] [PubMed] [Google Scholar]
- Ebner TJ, Chen G. Use of voltage-sensitive dyes and optical recordings in the central nervous system. Progress in Neurobiology. 1995;46:463–506. doi: 10.1016/0301-0082(95)00010-s. [DOI] [PubMed] [Google Scholar]
- Efimov IR, Biermann M, Zipes D. Fast fluorescent mapping of electrical activity in the heart: Practical guide to experimental design and applications. In: Shenasa M, Borggrefe M, Breithardt G, editors. Cardiac mapping. Blackwell Publishing Inc./Futura; New York: 2003. pp. 131–156. [Google Scholar]
- Efimov IR, Cheng Y. Optical mapping of cardiac stimulation: Fluorescent imaging with a photodiode array. In: Cabo C, Rosenbaum DS, editors. Quantitative cardiac electrophysiology. Marcel Dekker; New York: 2002. pp. 583–621. [Google Scholar]
- Efimov IR, Nikolski VP, Bub G. Optical mapping. In: Wenk GE, Bowlin GL, editors. Encyclopedia of biomaterials and biomedical engineering. Marcel Dekker; New York: 2004. pp. 1133–1142. [Google Scholar]
- Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circulation Research. 2004;95:21–33. doi: 10.1161/01.RES.0000130529.18016.35. [DOI] [PubMed] [Google Scholar]
- European Heart Rhythm Association. Heart Rhythm Society. Zipes DP, Camm AJ, Borggrefe M, Buxton, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Journal of the American College of Cardiology. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Fast VG. Recording action potentials using voltage sensitive dyes. In: Dhein S, Mohr FW, Delmoar M, editors. Practical methods in cardiovascular research. Springer; New York: 2005. pp. 233–255. [Google Scholar]
- Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke W, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- Fluhler E, Burnham VG, Loew LM. Spectra, membrane binding, and potentiometric responses to new charge shift probes. Biochemistry. 1985;24:5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- Gray RA, Iyer A, Berenfeld O, Pertsov AM, Hyatt CJ. Interdependence of virtual electrode polarization and conduction velocity during premature stimulation. Journal of Electrocardiology. 2006;39:S13–S18. doi: 10.1016/j.jelectrocard.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca+2 indictors with greatly improved fluorescence properties. The Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gupta AK, Maheshwari A, Thakur R, Lokhandwala YY. Cardiac mapping: Utility or futility? Indian Pacing and Electrophysiology Journal. 2002;2:20–32. [PMC free article] [PubMed] [Google Scholar]
- Gwathmey JK, Hajjar RJ, Solaro RJ. Contractile deactivation and uncoupling of crossbridges: Effects of 2,3-butanedione monoxime on mammalian myocardium. Circulation Research. 1991;69:1280–1292. doi: 10.1161/01.res.69.5.1280. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Extramiana F, Hocini M, Cauchemez B, Jais P, Cabrera JA, et al. Mapping and ablation of ventricular fibrillation associated with Long-QT and Brugada syndromes. Circulation. 2003;108:925–928. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Miyauchi Y, Chou C-C, Karagueuzian HS, Chen P-S, Lin SF. Effects of cytochalasin D on electrical restitution and the dynamics of ventricular fibrillation in isolated rabbit heart. Journal of Cardiovascular Electrophysiology. 2003;14:1077–1084. doi: 10.1046/j.1540-8167.2003.03234.x. [DOI] [PubMed] [Google Scholar]
- Himel HD, IV, Knisley SB. Comparison of optical and electrical mapping of fibrillation. Physiological Measurement. 2007;28:707–719. doi: 10.1088/0967-3334/28/6/009. [DOI] [PubMed] [Google Scholar]
- Hwang GS, Hayashi H, Tang L, Ogawa M, Hernandez H, Tan AY, et al. Intracellular calcium and vulnerability to fibrillation and defibrillation in Langendorff-perfused rabbit ventricles. Circulation. 2006;114:2595–2603. doi: 10.1161/CIRCULATIONAHA.106.630509. [DOI] [PubMed] [Google Scholar]
- Ideker RE, Smith WM, Wolf PD. Cardiac mapping at Duke Medical Center. The American Journal of Cardiology. 1989;63:17F–30F. [PubMed] [Google Scholar]
- Jalife J, Morley GE, Tallini Y, Vaidya D. A fungal metabolite that eliminates motion artifacts. Journal of Cardiovascular Electrophysiology. 1998;9:1358–1362. doi: 10.1111/j.1540-8167.1998.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Jameson DM, Croney JC, Moens PD. Fluorescence: Basic concepts, practical aspects, and some anecdotes. Methods in Enzymology. 2003;360:1–43. doi: 10.1016/s0076-6879(03)60105-9. [DOI] [PubMed] [Google Scholar]
- Johnson ME, Landers JP. Fundamentals and practice for ultrasensitive laser-induced fluorescence detection in micoranalytical systems. Electrophoresis. 2004;25:3513–3527. doi: 10.1002/elps.200406086. [DOI] [PubMed] [Google Scholar]
- Jonkman JE, Swoger J, Kress H, Rohrbach A, Stelzer EH. Resolution in optical microscopy. Methods in Enzymology. 2003;360:416–446. doi: 10.1016/s0076-6879(03)60122-9. [DOI] [PubMed] [Google Scholar]
- Kamino K, Hirota A, Fujii S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature. 1981;290:595–597. doi: 10.1038/290595a0. [DOI] [PubMed] [Google Scholar]
- Katra RP, Laurita KR. Cellular mechanism of calcium-mediated triggered activity in the heart. Circulation Research. 2005;96:535–542. doi: 10.1161/01.RES.0000159387.00749.3c. [DOI] [PubMed] [Google Scholar]
- Katra RP, Pruvot E, Laurita KR. Intracellular calcium handling heterogeneities in intact guinea pig hearts. American Journal of Physiology. 2004;286:H648–H656. doi: 10.1152/ajpheart.00374.2003. [DOI] [PubMed] [Google Scholar]
- Kim Y-H, Xie F, Yashima M, Wu T-J, Valderrabano M, Lee M-H, et al. Role of papillary muscle in the generation and maintenance of reentry during ventricular tachycardia and fibrillation in isolated swine right ventricle. Circulation. 1999;100:1450–1459. doi: 10.1161/01.cir.100.13.1450. [DOI] [PubMed] [Google Scholar]
- Kohsaka S, Razavi M, Massumi A. Idiopathic ventricular fibrillation successfully terminated by radio-frequency ablation of the distal Purkinje fibers. PACE. 2007;30:701–704. doi: 10.1111/j.1540-8159.2007.00731.x. [DOI] [PubMed] [Google Scholar]
- Lakowicz JR. Principles of fluorescence spectroscopy. 3rd ed. Springer; Singapore: 2006. [Google Scholar]
- Laurita KR, Rosenbaum DS. Optical mapping of cellular repolarization in the intact heart. In: Shenasa M, Borggrefe M, Breithardt G, editors. Cardiac mapping. Blackwell Publishing Inc./Futura Division; New York: 2003. pp. 709–727. [Google Scholar]
- Lee H-C, Clusin WT. Cytosolic calcium staircase in cultured myocardial cells. Circulation Research. 1987;61:934–939. doi: 10.1161/01.res.61.6.934. [DOI] [PubMed] [Google Scholar]
- Lee H-C, Smith N, Mohabir R, Clusin WT. Cytosolic calcium transients from the beating mammalian heart. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7793–7797. doi: 10.1073/pnas.84.21.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-H, Lin S-F, Ohara T, Omichi C, Okuyama Y, Chudin E, et al. Effects of diacetyl monoxime and cytochalasin D on ventricular fibrillation in swine right ventricles. American Journal of Physiology. 2001;280:H2689–H2696. doi: 10.1152/ajpheart.2001.280.6.H2689. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Conchello J-A. Fluorescence microscopy. Nature Methods. 2005;2:910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- Lin S-F, Wikswo JP. Panoramic optical imaging of electrical propagation in isolated heart. Journal of Biomedical Optics. 1999;4:200–207. doi: 10.1117/1.429910. [DOI] [PubMed] [Google Scholar]
- Moe AE, Marx S, Banani N, Liu M, Marquardt B, Wilson DM. Improvement in LED-based fluorescence analysis system. Sensors and Actuators B. 2005;111–112:230–241. [Google Scholar]
- Nanthakumar K, Jalife J, Masse S, Downar E, Pop M, Asta J, et al. Optical mapping of Langendorff-perfused human hearts: establishing a model for the study of ventricular fibrillation in humans. American Journal of Physiology. 2007;293:H875–H880. doi: 10.1152/ajpheart.01415.2006. [DOI] [PubMed] [Google Scholar]
- Nygren A, Baczko I, Giles WR. Measurements of electrophysiological effects of components of acute ischemia in Langendorff-perfused rat hearts using voltage-sensitive dye mapping. Journal of Cardiovascular Electrophysiology. 2006;17:S113–S123. doi: 10.1111/j.1540-8167.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Omichi C, Lamp ST, Lin SF, Yang J, Baher A, Zhou S, et al. Intracellular Ca dynamics in ventricular fibrillation. American Journal of Physiology. 2004;286:H1836–H1844. doi: 10.1152/ajpheart.00123.2003. [DOI] [PubMed] [Google Scholar]
- Packer DL. Optical mapping of cardiac arrhythmias: Clinical insights and applications. In: Rosenbaum DS, Jalife J, editors. Optical mapping of cardiac excitation and arrhythmias. New York: Futura Publishing: 2001. pp. 295–305. [Google Scholar]
- Pak H-N, Oh Y-S, Liu Y-B, Wu T-J, Karagueuzian HS, Lin SF, et al. Catheter ablation of ventricular fibrillation in rabbit ventricle treated with B-Blockers. Circulation. 2003;108:3149–3156. doi: 10.1161/01.CIR.0000104563.12408.12. [DOI] [PubMed] [Google Scholar]
- Parker I. Photonics for biologist. Methods in Enzymology. 2003;360:345–382. doi: 10.1016/s0076-6879(03)60119-9. [DOI] [PubMed] [Google Scholar]
- Patrick MJ, Ernst LA, Waggoner AS, Thai D, Tai D, Salama G. Enhanced aqueous solubility of long wavelength voltage-sensitive dyes by covalent attachment of polyethylene glycol. Organic and Biomolecular Chemistry. 2007;5:3347–3353. doi: 10.1039/b711438a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Kay MW, Chattipakorn N, Redden DT, Ideker RE, Rogers JM. Effects of heart isolation, voltage-sensitive dye, and electromechanical uncoupling agents on ventricular fibrillation. American Journal of Physiology. 2003;284:H1818–H1828. doi: 10.1152/ajpheart.00923.2002. [DOI] [PubMed] [Google Scholar]
- Richter P, Hader DP. Calcium imaging in living cells. In: Hader DP, editor. Image analysis: Methods and applications. CRC Press; Florida: 2000. pp. 373–389. [Google Scholar]
- Rohr S. Optical mapping of microscopic impulse propagation. In: Cabo C, Rosenbaum DS, editors. Quantitative cardiac electrophysiology. Marcel Dekker; New York: 2002. pp. 507–554. [Google Scholar]
- Rosenbaum DS. Optical mapping of cardiac excitation and arrhythmias: A primer. In: Rosenbaum DS, Jalife J, editors. Optical mapping of cardiac excitation and arrhythmias. Futura Publishing; New York: 2001. pp. 2–7. [Google Scholar]
- Rothenberg F, Watanabe M, Eloff B, Rosenbaum D. Emerging patterns of cardiac conduction in the chick embryo: Waveform analysis with photodiode array-based optical imaging. Developmental Dynamics. 2005;233:456–465. doi: 10.1002/dvdy.20338. [DOI] [PubMed] [Google Scholar]
- Rubin SA. The principles of biomedical instrumentation. Year Book Medical Publishers; 1987. [Google Scholar]
- Rudolf R, Mongillo M, Rizzuto R, Pozzan T. Looking forward to seeing calcium. Nature Reviews, Molecular Cell Biology. 2003;4:579–586. doi: 10.1038/nrm1153. [DOI] [PubMed] [Google Scholar]
- Rueckschloss U, Isenberg G. Cytochalasin D reduces Ca+2 currents via cofilin-activated depolymerization of F-actin in guinea-pig 1cardiomyocytes. Journal of Physiology. 2001;537:363–370. doi: 10.1111/j.1469-7793.2001.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama G. Optical mapping: Background and historical perspective. In: Rosenbaum DS, Jalife J, editors. Optical mapping of cardiac excitation and arrhythmias. Futura Publishing; New York: 2001. pp. 9–31. [Google Scholar]
- Salama G, Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 1976;191:485–487. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- Sellin LC, McArdle JJ. Multiple effects of 2,3-butanedione monoxime. Journal of Pharmacology and Toxicology. 1994;74:305–313. doi: 10.1111/j.1600-0773.1994.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Sih HJ, Berbari EJ. Methodology of cardiac mapping. In: Shenasa M, Borggrefe M, Breithardt G, editors. Cardiac mapping. Blackwell Publishing Inc./Futura; New York: 2003. pp. 41–58. [Google Scholar]
- Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff-still viable in the new millennium. Journal of Pharmacological and Toxicological Methods. 2007;55:113–126. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Smith WM. Direct mapping of bioelectric activity. Critical Reviews in Biomedical Engineering. 1999;27:339–358. [PubMed] [Google Scholar]
- Stelzer EHK. Contrast, resolution, pixilation, dynamic range and signal to noise ratio: Fundamental limits to resolution in fluorescence light microscopy. Journal of Microscopy. 1998;189:15–24. [Google Scholar]
- Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacological Research. 2000;41:613–627. doi: 10.1006/phrs.1999.0653. [DOI] [PubMed] [Google Scholar]
- Tasaki I, Watanabe A, Sandling R, Carnay L. Changes in florescence, turbidity and birefringence associated with nerve excitation. Proceedings of the National Academy of Sciences of the United States of America. 1968;61:883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undrovinas AI, Shander GS, Makielski JC. Cytoskeleton modulates gating of voltage-dependent sodium channel in heart. American Journal of Physiology. 1995;269:H203–H214. doi: 10.1152/ajpheart.1995.269.1.H203. [DOI] [PubMed] [Google Scholar]
- Volders P, Vos MA, Szabo B, Sipido KR, Groot M, Gorgels A, et al. Progress in the understanding of cardiac early after depolarizations and torsades de pointes: Time to revise current concepts. Cardiovascular Research. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- Wellner M, Berenfeld O. Theory of reentry. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: From cell to bedside. Saunders; Philadephia: 2004. p. 317. [Google Scholar]
- Wu J, Biermann M, Rubart M, Zipes SP. Cytochalasin D as excitation-contraction uncoupler for optically mapping action potentials in wedges of ventricular myocardium. Journal of Cardiovascular Electrophysiology. 1998;9:1336–1347. doi: 10.1111/j.1540-8167.1998.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Qu Z, Chen P-S, Lin SF, Karagueuzian HS, Hayashi H, et al. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–1240. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- Wuskell JP, Boudreau D, Wei M-D, Jin L, Engl R, Chebolu R, et al. Synthesis, spectra, delivery and potentiometric responses of new styryl dyes with extended spectral ranges. Journal of Neuroscience Methods. 2006;151:200–215. doi: 10.1016/j.jneumeth.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Yuste R, Konnerth A. Imaging in neuroscience and development: A laboratory manual. Cold Spring Harbor Laboratory Press; New York: 2005. [Google Scholar]


