1. Introduction
Rat theilovirus (RTV), a Picornavirus belonging to the Cardiovirus genus and Theilovirus species, is a natural pathogen of rats for which little is known. Other Theilovirus strains include Theiler’s murine encephalomyelitis virus (TMEV), Vilyuisk human encephalomyelitis virus (VHEV), Saffold (SAV), and Saffold-like viruses. Notable, theiloviruses such as SAV have recently regained recognition as potential emerging human pathogens with evidence of infections worldwide; however, a direct association with clinical disease has yet to be demonstrated (Chiu et al., 2008; Liang et al., 2008; Zoll et al., 2009). The natural mode of transmission of Theiloviruses is by the fecal-oral route with the intestine being the primary site of infection (Olitsky, 1940; Theiler and Gard, 1940a). The TMEV isolates are the best characterized clade of Theilovirus and most infections are subclinical; however, in susceptible mouse strains a small percentage of mice develop overt neurologic disease due to viral dissemination from the intestinal tract (Olitsky, 1940; Rozengurt and Sanchez, 1993; Theiler, 1937; Theiler and Gard, 1940a; Thompson et al., 1951). As such, Theiler’s virus infections have been predominantly studied as a mouse model of viral induced neurologic disease where intracranial (IC) inoculation of mice results in disease ranging from acute fatal encephalitis to chronic demyelinating disease (Lipton, 1975; Theiler and Gard, 1940b).
The first report of a TMEV-like pathogen infecting rats occurred in 1964 when a small group of Sprague Dawley (SD) rats were identified with central nervous system deficits and histopathologic lesions that resembled those of mice infected with TMEV (McConnell et al., 1964). The original isolate, known as MHG, was reported to cause paralysis in suckling rats and mice following intracranial inoculation. It was further recognized that subclinically infected rats developed serum antibodies that cross-reacted with TMEV antigens (Hemelt et al., 1974). Recent reports document the presence of genetically unique Theiloviruses that infect rats and seroprevalence data indicate RTV, also referred to as Theiler’s-like rat virus and rat encephalomyelitis virus, is one of the most prevalent viral pathogens infecting research rat colonies (Drake et al., 2008; Jacoby and Lindsey, 1997; Livingston and Riley, 2003; Ohsawa et al., 2003; Rodrigues et al., 2005). In 2003, Japanese researchers documented a theilovirus infecting rats that was designated NSG910. The strain was isolated from Wistar Furth sentinel rats housed with TMEV-seropositive rats and the virus was sequenced to confirm its identity. The isolate has approximately 72% nucleotide identity and 79% amino acid identity to TMEV strains. Clinical disease was not reported throughout isolation or in vivo propagation of the virus (Ohsawa et al., 2003). Another strain of Rat theilovirus was isolated from the feces of infected SD rats in 2006 and designated RTV1. The isolate was sequenced and found to have 95% sequence identity to the NSG910 isolate but contained an additional 73 nucleotide segment on the 5′ end of the genome that shares homology with the 5′ genome ends of TMEV strains. Further, the complete RTV1 genome is comparable in size to genomes reported for strains of TMEV. In vivo oral inoculation studies have shown SD rats were susceptible to enteric RTV infection and produced a robust humoral response whereas related CD rats were relatively resistant to infection and developed little antibody response (Drake et al., 2008).
The literature amassed describing TMEV infections encompass the majority of what is known about theiloviruses. However, novel murine isolates have merit to contribute to an understanding of Theilovirus pathogenesis. The studies reported herein were designed to test the hypothesis that enterocytes of the upper small intestine are the primary cell population infected by RTV and thus identify in vivo enteric cellular tropisms of RTV. Additionally, susceptibility was evaluated in immunocompetent and immunocompromised rats common to many biomedical research colonies to test the hypothesis that the adaptive immune response is instrumental for clearance of enteric RTV infection. These data extend prior studies of rat theiloviruses and introduce a rat model to study the natural pathogenesis of related Theiloviruses.
2. Materials and Methods
2.1. RTV1 isolate, concentration and purification
RTV1 was previously isolated from neonatal SD rats exposed to dirty bedding from rats which had antibodies that reacted with TMEV GDVII antigens. The isolate was plaque purified and propagated in BHK21 cells as previously described (Drake et al., 2008). Supernatant containing the virus was harvested and stored at −80°C until used for animal inoculations or until further concentrated and purified. RTV1 was concentrated by adding 1M NaCl and 8% (wt/vol) polyethylene glycol (Fisher Scientific, Fair Lawn, NJ) to clarified supernatants and stirred overnight at 4°C. The precipitated material was pelleted by centrifugation at 10,000 × g at 4°C for 10 min and resuspended in PBS and 1% Nonidet P-40 substitute Octylphenoxypolyethoxyethanol (USB Corp., Cleveland, OH). A 10% to 40% cesium chloride gradient was used to purify the virus by centrifugation at 41,000 rpm for 1 h at 4°C in a SW41Ti rotor (Beckman Coulter, Inc.). Virus was harvested and dialyzed through a 10,000 Da cutoff membrane (Pierce Biotechnology, Inc., Rockford, IL) overnight at 4°C in 4 liters of PBS while stirring. Purified virus was aliquoted and stored at −80°C until used and protein concentrations were determined by bicinchoninic acid procedures.
2.2. Animals
Four-week-old male Sprague Dawley (SD), Crl:CD(SD), Hsd:RH-Fox1rnu, Brown Norway (BN), and Fischer (F344) rats and three-week-old female Lewis (LEW) rats were acquired from Harlan Laboratories (Indianapolis, IN). Four-week-old male NTac:NIH-Whn rats were acquired from Taconic (Cambridge City, IN). Health monitoring records indicated that all rats were free from adventitious pathogens. Additionally, at the start of each experiment, fecal samples from all rats were negative for RTV by RT-PCR and all immunocompetent rats tested negative for antibodies to RTV using a multiplex fluorescent immunoassay (MFI). A female, New Zealand White rabbit was also acquired from Harlan Laboratories (Indianapolis, IN) for polyclonal antibody production. All animal experiments were approved by the University of Missouri Animal Care and Use Committee.
2.3. Infection
For oral inoculation studies rats were inoculated by oral gavage with a 20 gauge ball-point needle. Rats were manually restrained and inoculated with 2.5 × 106 PFU of RTV1 or a similar volume of uninfected BHK cell lysates processed in the same manner as infected BHK cell cultures. For intracerebral inoculation studies, rats were anesthetized with vaporized isoflurane and 106 PFU (30 μl) of cesium chloride purified RTV1 or a similar volume of sterile saline was injected into the right cerebral hemisphere.
2.4. Sample collection
2.4.1. Oral inoculation experiments
Rats were euthanized at either 2 weeks or 8 weeks postinoculation by an inhaled overdose of carbon dioxide. Intestinal tracts were excised from rats from the pylorus to the ileocecal junction, flushed with sterile PBS then rolled and placed in zinc fixative. Samples were embedded in paraffin blocks for subsequent staining. Mesenteric lymph nodes, spleen, and fresh fecal pellets directly from rats were collected and immediately stored at −80ºC until processed for RT-PCR. Blood was collected from saphenous veins or by cardiocentesis and serum was separated and stored at −20ºC until antibody evaluation.
2.4.2. Intracranial inoculation experiment
All rats were monitored daily for clinical neurologic disease and sacrificed at 10 weeks post-inoculation. A section of the right cerebral hemisphere at the site of inoculation, left cerebral hemisphere, brainstem, and spinal cord between thoracic vertebra 6 and 7 were collected and stored at −80°C until processed for RT-PCR. The remainder of the brain and spinal cord were serially sectioned and fixed in 10% neutral buffered formalin for 24 h from all experimental and control rats. Blood was collected by cardiocentesis and serum was separated and stored at −20°C until antibody evaluation.
2.5. Histologic Methods
2.5.1. Immunohistochemisry
Rabbit anti-RTV polyclonal transudate was produced as previously described (Clemons et al., 1992). Briefly, a NZW rabbit was acclimated for one week prior to subcutaneous surgical implantation of sterile chambers. Transudate was extracted from the chambers prior to immunization to provide normal, negative control, transudate. Each chamber was injected with 250 μg of cesium chloride purified RTV1 and boostered approximately 1 month later with 50 μg of RTV1 prior to collection of hyperimmune transudate.
Paraffin-embedded blocks of small intestines from inoculated rats were sectioned 5 microns thick and processed for staining. To establish staining conditions for the assay, RTV1 infected and uninfected BHK cells were fixed in zinc and embedded in paraffin. Cell blocks were cut into sections 5 microns thick for staining. The EnVision + System (DAKO; Carpinteria, CA) was used for immunohistochemical labeling. Deparaffinized sections were incubated in 10% citrate solution at 95°C for approximately 40 min prior to staining using a Dako Universal Autostainer System (DAKO, Carpinteria, CA). Briefly, the staining protocol included a peroxidase block with 10% hydrogen peroxide for 5 min at room temperature (RT), a protein block with 5% BSA for 20 min at RT, incubation with 1:3000 to 1:4000 dilutions of rabbit polyclonal primary antibodies directed against RTV1 antigens for 60 min, incubation with a horseradish peroxidase-labeled polymer conjugated with goat anti-rabbit secondary antibodies (Envision + System, DAKO, Carpinteria, CA) for 30 min, and visualized using a 10 min Nova Red substrate reaction (Vector Laboratories; Burlingame, CA) followed by Mayer’s hematoxylin counterstain for 1 min. RTV-infected BHK cells probed with hyperimmune transudate served as positive controls. RTV-infected BHK cells probed with normal rabbit transudate or uninfected BHK cells probed with hyperimmune transudate were used as negative controls in subsequent experiments. Additional controls for the assay included tissue sections from rats probed with normal transudate from the preimmunized rabbit followed by goat anti-rabbit secondary antibodies and sections probed with secondary antibodies alone. All sections were visualized using a Zeiss Axiophot microscope and Olympus DP70 digital camera.
2.5.2. Histopathology
Histologic sections were processed and evaluated using standard techniques. Briefly, all tissues were fixed in 10% neutral buffered formalin, trimmed, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin or luxol fast blue. Stained sections were surveyed microscopically for lesions consistent with pathologic processes.
2.6. Molecular and serologic methods
2.6.1. Reverse transcriptase polymerase chain reaction
RNA was extracted from fecal and tissue samples with a MagAttract RNA tissue Mini M48 kit (QIAGEN Inc., Valencia, CA) and a BioRobot M48 Workstation (QIAGEN) according to the manufacturer’s protocol. Briefly, 70 mg of fresh feces collected directly from rats or 20 mg of mesenteric lymph node or spleen was placed in a sterile 2 ml tube with a 5 mm steel ball and 400 ul buffer RLT (QIAGEN). All mixtures were disrupted and homogenized in a tissue lyser (QIAGEN). Fecal samples were agitated at 30 Hz for 10 s and tissue samples 20 Hz for 2 min. Lysates from feces or spleen and lymph node were centrifuged for 10 min at 3000 × g or 13,000 × g, respectively, and RNA was extracted from the resultant supernatant. RTV1 RT-PCR was performed with a one-step RT-PCR kit with Q-Solution (QIAGEN) according to the manufacturer. Primers consisted of 212f (5′-ATTTTCCGGCCCAGGCTAAGAG-3′) and 397r (5′-TTTTAATCTCCAACCACGTCGC-3′) that amplified a 185-bp segment of 5′ UTR region of the RTV1 genome. RT-PCR products were subjected to electrophoresis in agarose gels containing ethidium bromide and visualized under UV light.
2.6.2. Real-time polymerase chain reaction
Primers consisted of 276f (5′-TCGCAAAGATAAGTCCTCCC -3′) and 385r (5′-ACCACGTCGCGTTGAAAGAG-3′) that amplified a 109 nucleotide sequence in the 5′ UTR region of the genome. Plasmids containing cloned amplicons were generated using TopoTA Cloning Kits (Invitrogen, Carlsbad, CA) and linearized to generate a standard curve using known concentrations of the plasmid from 100 to 107 copies in triplicate. Fluorescence from experimental samples was compared to fluorescence from the linearized plasmid standards that contained known concentrations of cloned amplicons. Viral copies were quantified using real-time PCR (LighyCycler, Roche Diagnostic; Indianapolis, IN). Briefly, RNA was extracted from 70 mg of feces using RNeasy Mini Kits (QIAGEN) and cDNA produced using SuperScript VILO cDNA Synthesis Kit (Invitrogen) according to manufacturers’ protocols. PCRs and melting curves were performed in a 20 μl volume in glass reaction capillaries that contained 3 mM MgCl, QuantiTect SYBR Green PCR Master Mix (QIAGEN), 0.5 mM concentration of each primer and 4 μl volume of cDNA. Cycling parameters consisted of a 95°C for 15 min to activate the DNA polymerase and 40 cycles consisting of denaturation at 94°C for 15 s, annealing at 58°C for 20 s, and extension at 72°C for 30 s with final 72°C extension for 10 min. Melting curves were generated to verify product specificity following amplification cycling. The analytical sensitivity of the assay was 10 viral copies as determined by testing log-fold dilutions of linearized plasmids containing the target RTV1 genome segment.
2.6.3. Multiplex fluorescent immunoassay
Rat serum samples were processed for detection of the anti-RTV antibodies as previously described with the following exception (Hsu et al., 2005). Cesium chloride purified RTV1 viral particles were covalently coupled to carboxylated polystyrene microspheres (Luminex Corp., Austin, TX) at a concentration of 2 μg protein per 106 microspheres.
2.7. Statistical Analysis
A nonlinear mixed modeling framework was used to analyze the serologic data with PROC NLMIXED in SAS/STAT software Version 9.2 of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA). Prior to modeling, a modified log2 transformation was applied to the raw response data y, such that the transformed data y′ ≡ log2(y+1). This transformation was used to stabilize the variance and to mitigate statistical concerns inherent with hetereoskedatic and non-additive random noise. Log transformation of serological data has additional benefits and its use not uncommon (Ndifon, 2011). A logistic model (S-shaped) fit the serological data and can be expressed as
where denotes the transformed fluorescence measurement of the kth mouse (k=1,… 10 or 11) in the ith group (i=1,..,4) taken at time tijk, where t ≥ 7 days. The fixed-effects parameters β1, β2, and β3 were estimated from the data for each of the four groups. The curve has a lower asymptote of 0 (when time is 0), and upper asymptote of β1 (when time is infinite). The random-effect parameters uk enter the model linearly, and were assumed to be independent and identically distributed . The residual errors, ε, were also assumed to be independent and identically distributed and independent of the random effects Prediction intervals were reported at the 95% confidence level assuming the mean random effect was zero.
A linear mixed modeling framework was used to model the fecal shedding data after applying the same modified log2-transform to the response measurement (virus copies/mg of feces) as for the serological data. The model, which was similar in form to an analysis of covariance (ACOVA) model with a random intercept, can be expressed as
where denotes the transformed virus copies/mg of feces measurement of the kth mouse (k=1,…,21) in the ith group (i=1 for immunocompromised, i=2 for immunocompetent) taken at time point j, where j = 1,..,4 corresponds to day 7, 14, 28, or 56. The variable xik is the day 7 measurement and was used as a covariate of the baseline response level. In other words, this variable serves as a proxy for whether the kth mouse in the ith group was really infected by the virus. The fixed-effects parameters (denoted by Greek letters in the model above) were estimated from the data by restricted maximum likelihood. The random-effect parameters uk allowed for the kth individual’s deviations from the population mean intercept (μ), after the other variables had been accounted for. Assumptions regarding the residuals were the same as for the serological data, except the within-mouse covariance matrix was modeled with a spatial power covariance structure based on the distance (in time) between measurements, with each mouse strain having its own set of parameters. For both the fecal shedding model and the serological model, model selection criteria (e.g., corrected Akaike Information Criteria) and diagnostic plots were used to guide model building in a parsimonious manner.
Fisher’s Exact test was performed to evaluate extraintestinal viral detection and fecal shedding when treated as a binary outcome as indicated, using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA). A P-value of less than 0.05 was considered significant.
3. Results
3.1. Enteric Cellular Tropisms of RTV1
To identify intestinal cellular tropisms of rat theilovirus, 4-week-old male SD rats were orally inoculated with either 2.5 × 106 PFU RTV1 or sham inoculated with uninfected BHK cell lysates. At 14 days post-inoculation (PI) all RTV1 dosed rats were shedding virus in the feces and all sections of small intestine, which included proximal small intestine near the pyloric region and segments 10 and 20 inches further aboral, were positive for RTV by RT-PCR. Conversely, virus was not detected by RT-PCR in the feces or intestinal sections of any sham inoculated rats (data not shown). The small intestine was processed at day 14 PI and evaluated by immunohistochemistry to identify RTV-infected cells using polyclonal RTV antibodies. Figure 1 shows a representative section of the proximal small intestine from RTV-inoculated rats. Multifocal areas of intracytoplasmic chromogen deposition indicative of RTV1 infection were detected in a small percentage of enterocytes in the duodenum of all RTV-inoculated rats. No chromogen deposition was observed in distal portions of the small bowel of RTV-inoculated rats and no chromogen deposition was present in enterocytes of any sham-inoculated rats or in IHC assay controls (data not shown).
Figure 1.
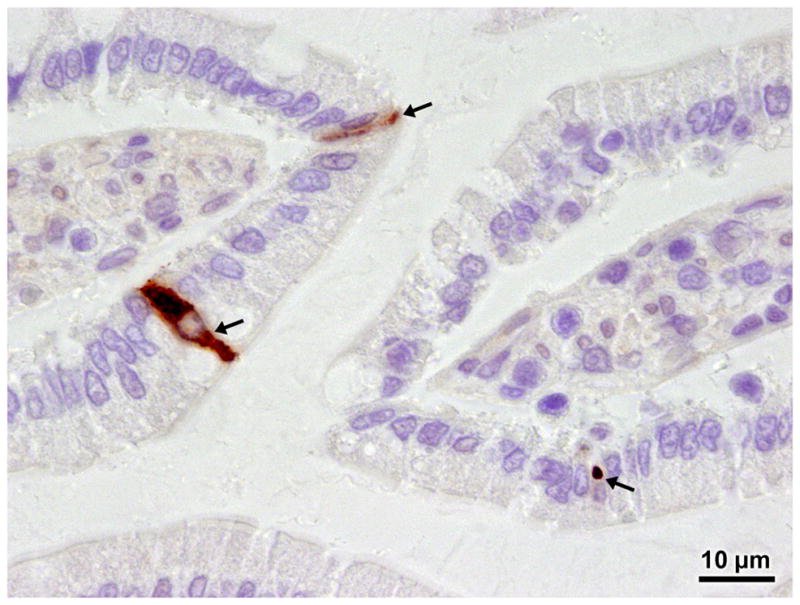
Representative photomicrograph of an immunohistochemically-stained section of proximal small intestine from an experimental Sprague Dawley rat. Chromogen deposition was apparent in the cytoplasm of isolated enterocytes (arrows) from all SD rats inoculated with 2.5 × 106 PFU RTV1 by oral gavage (n=10) and probed with polyclonal anti-RTV antibodies and goat anti-rabbit labeled secondary antibodies (1000×). No chromogen deposition was apparent in SD rats sham inoculated with uninfected BHK cell lysates (n=6). No chromogen deposition was apparent in experimental rats inoculated with 2.5 × 106 PFU RTV1 and probed with normal transudate from the preimmunized rabbit and goat anti-rabbit labeled secondary antibodies or probed with secondary antibodies alone (data not shown).
3.2. Fecal shedding and extraintestinal detection of RTV in immunocompromised and immunocompetent rats
Fecal shedding of RTV was compared throughout a 56 day study between groups of 4-week-old, male immunocompetent rats (BN and F344) and immunocompromised rats (Hsd:RH-Fox1rnu and NTac:NIH-Whn) orally inoculated with either 2.5 × 106 PFU RTV1 or sham inoculated with uninfected BHK cell lysates (Table 1). At day 7 PI 100% of RTV-inoculated rats shed RTV in the feces and had fecal viral loads from 1.24 × 105 to 3.14 × 106 copies of virus per mg of feces. Based on our linear mixed model, significant differences were not present between groups at this time point (p=0.957). At day 14 PI, 100% of RTV-inoculated rats continued to shed virus in the feces; however, viral loads in the immonocompetent group decreased by more than 36-fold compared to day 7 PI (p<0.001; 95% confidence interval 18 to 83 fold) based on the linear mixed model estimates. By day 28 PI 64% of BN and 54% of F344 rats had stopped shedding virus in the feces, whereas all rats from the Hsd:RH-Fox1rnu and NTac:NIH-Whn groups remained infected and continued to shed high levels of virus. As a result, viral loads shed at day 28 by the immunocompetent group were significantly less than that of immunocompromised group (p<0.001; 95% confidence interval 1600 to 14200 fold). By day 56 PI and termination of the study only 27% of BN rats shed virus in the feces with viral loads that ranged 8.3 × 101 to 3.13 × 102 copies/mg of feces and one F344 rat shed 7.13 × 102 copies of RTV per mg of feces. Conversely, 100% of Hsd:RH-Fox1rnu and NTac:NIH-Whn groups continued to shed virus with mean concentrations of 3.45 × 105 and 1.13 × 105 copies of virus per mg of feces, respectively. At termination of the study, fecal shedding in the immunocompetent group was significantly less than that of the immunocompromised group (P<0.001; 95% confidence interval 8100 to 74000 fold). RTV was not detected in any sham inoculated rats throughout the study.
Table 1.
Temporal and quantitative analysis of RTV viral loads shed in the feces of immunocompetent and immunocompromised rats.
| Rat strain/stocka |
Day 7 PI
|
Day 14 PI
|
Day 28 PI
|
Day 56 PI
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of rats shedding virusb |
Copies of virus shed in fecesc |
Min/Maxd |
Number of rats shedding virusb |
Copies of virus shed in fecesc |
Min/Maxd |
Number of rats shedding virusb |
Copies of virus shed in fecesc |
Min/Maxd |
Number of rats shedding virusb |
Copies of virus shed in fecesc |
Min/Maxd |
|
| BN | 11/11 | 4.13 × 105 | 1.24 × 105 9.26 × 105 |
11/11 | 8.6 × 103 | 5.43 × 102 3.68 × 104 |
4/11 | 5.78 × 102 | 0 2.89 × 103 |
3/11 | 4.5 × 101 | 0 3.13 × 102 |
| F344 | 11/11 | 4.91 × 105 | 1.37 × 105 1.10 × 106 |
11/11 | 7.39 × 104 | 6.64 × 103 5.06 × 105 |
5/11 | 5.89 × 102 | 0 4.46 × 103 |
1/11 | 6.6 × 101 | 0 7.13 × 102 |
| HSD:rnu | 10/10 | 1.47 × 106 | 8.40 × 105 3.14 × 106 |
10/10 | 1.08 × 106 | 1.37 × 105 2.43 × 106 |
10/10 | 2.61 × 105 | 1.08 × 104 5.15 × 105 |
10/10 | 3.45 × 105 | 7.03 × 104 6.39 × 105 |
| Tac:rnu | 10/10 | 9.0 × 105 | 5.70 × 105 1.42 × 106 |
10/10 | 2.26 × 105 | 3.63 × 104 7.85 × 105 |
10/10 | 1.33 × 105 | 4.87 × 104 2.31 × 105 |
10/10 | 1.13 × 105 | 2.42 × 104 2.58 × 105 |
BN-Brown Norway and F344-Fischer rats (immunocompetent strains, n=11/group) and Hsd:RH-Fox1rnu and NTac:NIH-Whn nude rats (immunocompromised stocks, n=10/group) were inoculated by oral gavage with 2.5 × 106 PFU RTV1. Sham inoculated rats (n=6 per group) were inoculated with uninfected BHK cell lysates by oral gavage.
Number of rats infected/number of rats inoculated with RTV.
Represents mean number of viral copies per mg of feces from rats actively shedding virus.
Values from rats shedding the lowest and highest number of viral copies within groups.
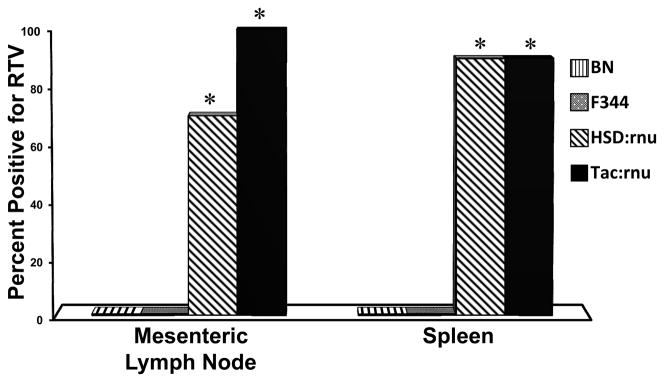
Upon completion of the study on day 56 PI, spleen and mesenteric lymph nodes from all rats were evaluated for RTV by RT-PCR. Virus was not detected in spleen or mesenteric lymph nodes of BN or F344 rats (Figure 2). Conversely, RTV was detected in the spleen and mesenteric lymph node of both immunocompromised rat strains. Mesenteric lymph nodes from 100% of NTac:NIH-Whn rats and 70% of Hsd:RH-Fox1rnu rats were positive for RTV. Similarly, 90% of the spleens from both immunocompromised rat strains were positive for RTV. Significant differences were present between BN or F344 rats and Hsd:RH-Fox1rnu or NTac:NIH-Whn rats (p≤0.001, Fisher’s).
Figure 2.
Detection of RTV1 in the spleen and mesenteric lymph nodes by RT-PCR. Brown Norway (BN) n=11, Fischer 344 (F344) n=11, Hsd:RH-Fox1rnu (Hsd:rnu) n=10, and NTac:NIH-Whn (Tac:rnu) n=10 rats were inoculated with 2.5 × 106 PFU RTV1 by oral gavage. Statistical analysis performed by Fischer’s Exact test with asterisk indicating statistical difference between immunocompromised and immunocompetent groups (P<0.05).
3.3. Serologic responses of inbred BN and F344 and outbred CD and SD rat strains to RTV1
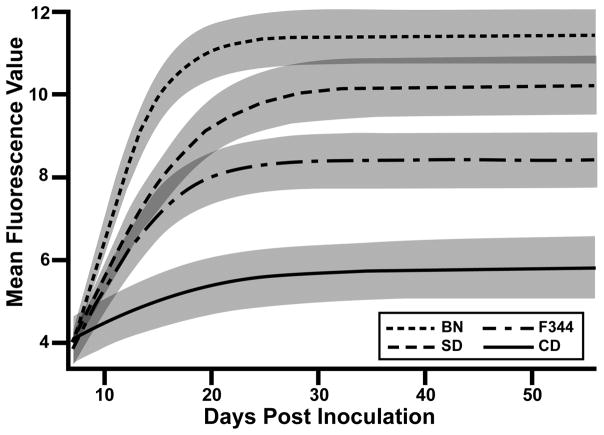
The magnitude of humoral responses to RTV1 was compared between BN, F344, CD and SD rats. Sera from rats inoculated with either 2.5 × 106 PFU RTV1 or sham inoculated with uninfected BHK cell lysates were evaluated at eight weekly time points to detect anti-RTV1 antibodies by MFI. Based on our nonlinear mixed model, at day 21 PI BN rats inoculated with RTV had a mean fluorescence value 3.4 times greater than SD rats (p<0.001,95% confidence interval 1.8 to 6.5) which were in turn 2.5 times greater than F344 (p=0.007,95% confidence interval 1.3 to 4.8). F344 rats had a mean fluorescence value 6.3 times greater than CD rats (p<0.001, 95% confidence interval 3.3 to 12.2). At day 56 PI both BN and SD groups had maintained robust humoral responses to RTV infection (>1000 mean fluorescence units) whereas CD and F344 groups had significantly less serologic response to the virus (<300 mean fluorescence units). These relationships can be seen in Figure 3, which is on a log2 fluorescence scale (i.e., 1 unit change corresponds to 21), and the shaded regions are the 95% confidence bands for a given group, based on the nonlinear mixed model.
Figure 3.
Detection of serum antibodies in rats inoculated with RTV1. Serum samples were tested in a microsphere fluorescent immunoassay using RTV1 as antigen to evaluate the magnitude of serologic response in Hsd:SD and CRL:CD (n=10 per group) and BN (n=11) and F344 (n=11) rats inoculated with RTV1 by oral gavage. Control rats were sham inoculated with uninfected BHK cell lysates (n=6 per group) and showed negligible activity. The relationships are based on a nonlinear mixed model. Solid lines represent mean fluorescent units on a log2 fluorescence scale and shaded regions are the 95% confidence bands for a given group.
3.4. Intracerebral inoculation of RTV1 in Lewis rats
To evaluate neurotropisms of RTV1, three-week-old female LEW rats were inoculated in the right cerebral hemisphere with 106 PFU RTV1 (n=10) or sham inoculated with sterile saline (n=4). Rats were observed for neurologic deficits affecting ambulation or postural compromise throughout a 10 week study. Sections of cerebrum from the right and left cerebral hemisphere, brainstem, and spinal cord between the sixth and seventh thoracic vertebra were evaluated by RT-PCR. Overt neurologic signs were not observed nor was virus detected in CNS tissues examined from any study rats. Additionally, serum was evaluated at week 10 for antibodies directed against RTV1 antigens. Anti-RTV serum antibodies were detected in virally inoculated rats but were absent in sham inoculated rats (data not shown).
4. Discussion
RTV has only recently been isolated in pure culture and little is known about its pathogenesis (Drake et al., 2008; Ohsawa et al., 2003). In the studies reported herein, enterocytes of the proximal small intestine were identified as in vivo cellular tropisms of RTV. Sections of proximal intestine examined from SD rats infected with RTV showed intracytoplasmic chromogen deposition consistent with viral antigens in a low percentage of enterocytes. This is also consistent with patterns of chromogen deposition reported in neuronal and non-neuronal cells within the central nervous system containing TMEV antigens (Ha-Lee et al., 1995; Zurbriggen and Fujinami, 1988). Interestingly, virus overlay assays have been used to suggest that certain strains of TMEV bind goblet cells within the intestinal tract of mice (Tsunoda et al., 2009). However, the anatomic location and morphologic characteristics of in vivo RTV infected cells in the rats in our studies are most consistent with enterocytes. A similar pattern of cytoplasmic chromogen deposition in a low percentage of enterocytes was also observed in immunocompetent Fischer 344 and Brown Norway rats and immunocompromised Hsd:RH-Fox1rnu and NTac:NIH-Whn rats (data not shown).
The kinetics of RTV shedding differed between the immunocompetent and immunodeficient rats evaluated. At early time points viral loads shed by all groups were equivalent, but as time progressed the immunodeficient rats continued to shed high amounts of virus in the feces while virus shedding in the immunocompetent rats declined or stopped by day 28 and 56 PI. Collectively, immunohistochemistry and virus shedding data suggest that persistently high fecal shedding of RTV in immunodeficient rats was due to sustained high levels of viral shedding of infected enterocytes and not due to increased numbers of virally-infected cells. The immunocompromised rats used in these studies have a defective winged-helix nude (Whn) gene and fail to develop an appropriate T cell population and subsequently have defective adaptive cell-mediated and humoral immune responses (Hanes, 2006; Nehls et al., 1994). It is likely viral loads shed by immunocompetent rats decreased due to initiation of the adaptive immune response whereas the lack of an adaptive immune response in the immunodeficient rats permitted persistently high levels of viral replication and fecal shedding of viral particles. Taken as a whole, these data support the importance of the adaptive immune response in limiting the magnitude of RTV infection within infected enterocytes over time, but not the extent of infection within the intestinal tract.
In addition to significant differences in fecal shedding kinetics, virus localization varied between rats with differing genetic backgrounds. Previous studies have shown RTV1 remained localized to the intestinal system at 8 weeks following oral inoculation of virus in SD and CD rats (Drake et al., 2008). Consistent with these findings, RTV remained in the intestinal tract of immunocompetent BN and F344 rats and most rats cleared the infection by termination of the study on day 56 PI. Conversely, RTV was also detected in the mesenteric lymph nodes and spleen of Hsd:RH-Fox1rnu and NTac:NIH-Whn nude rats on day 56 PI. These findings suggest RTV is capable of extraintestinal translocation and further support a role for T cells in limiting RTV propagation within the localized enteric environment. Interestingly, as depicted in figure 3, infected BN rats produced a more robust humoral response to RTV compared to infected F344 rats. The magnitude of this humoral response may account for the more rapid reduction in viral copies detected in the feces of BN rats at day 14 PI compared to F344 rats.
In immunocompetent rats RTV appears be an enteric virus that causes subclinical infection localized to the intestinal tract (Drake et al., 2008; Ohsawa et al., 2003). However, intracranial inoculation of RTV has been reported to cause CNS disease (McConnell et al., 1964; Rodrigues et al., 2005). In our studies intracranial inoculation of RTV1 did not cause clinical neurologic disease in Lewis rats nor was virus detected in the brain or spinal cord 10 weeks PI. Interestingly, while RTV1 was not overtly neurotropic in LEW rats a serologic response was elicited in intracranially-inoculated rats (data not shown). These data suggest infection may have occurred but the virus was recognized and cleared from the CNS prior to disease induction or collection of tissues. Accordingly, a rapid response by a competent immune system may be the integral component to prevent viral propagation within the CNS of Lewis rats. Although RTV1 is not neuropathogenic in LEW rats this does not preclude the possibility of RTV1 causing neurologic disease in other strains of rats or the possibility that other neurovirulent strains of RTV exist.
In conclusion, our results identify enterocytes of the proximal small intestine as a cellular tropism of RTV and suggest RTV1 is not overtly virulent. In immunocompetent rats, RTV1 is shed in the feces at high levels at acute time points with fecal viral concentrations decreasing over time and most rats clearing infection by day 56 PI. In contrast, immunocompromised rats persistently shed high concentrations of RTV in the feces and the virus can disseminate to local and regional lymphoid tissues. These results highlight an important role of the adaptive immune response to control RTV infection at the level of the intestinal mucosa and suggest this system may participate in Theilovirus infections in other mammals. RTV infection in rats has merit as a model for further study of Theilovirus pathogenesis.
Highlights.
Introduce a rat model to study
Theilovirus pathogenesis.
Rat Theilovirus 1 has tropisms for the epithelial cells of the small intestine.
Sustained high fecal viral shedding of RTV1 in rats deficient in T cells.
Immunocompetent rats have differing serum antibody responses to RTV infection.
Acknowledgments
We would like to thank Greg Purdy for assistance with cell culture and viral purification. Additional thanks to Don Connor and Howard Wilson for graphics. This work was supported by funds from the American College of Laboratory Animal Medicine Foundation, National Institutes of Health Postdoctoral Training in Comparative Medicine grant T32-RR07004 and the Research Animal Diagnostic Laboratory (RADIL).
Footnotes
Author Contributions:
MTD, CBW, MHM, and RSL conceived and designed the experiments. MTD performed the experiments. MTD and JWD analyzed the data. MTD, CBW, MHM, JWD, and RSL wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chiu, et al. Identification of cardioviruses related to Theiler’s murine encephalomyelitis virus in human infections. Proc Natl Acad Sci U S A. 2008;105(37):14124–9. doi: 10.1073/pnas.0805968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons, et al. Evaluation of a subcutaneously implanted chamber for antibody production in rabbits. Lab Anim Sci. 1992;42(3):307–11. [PubMed] [Google Scholar]
- Drake, et al. Differential susceptibility of SD and CD rats to a novel rat theilovirus. Comp Med. 2008;58(5):458–64. [PMC free article] [PubMed] [Google Scholar]
- Ha-Lee, et al. Mode of spread to and within the central nervous system after oral infection of neonatal mice with the DA strain of Theiler’s murine encephalomyelitis virus. J Virol. 1995;69(11):7354–61. doi: 10.1128/jvi.69.11.7354-7361.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes . The Nude Rat. In: Suckow, Weisbroth, Franklin, editors. The laboratory rat. 2. Elsevier Academic Press; Burlington, MA: 2006. pp. 733–755. [Google Scholar]
- Hemelt, et al. Comparison of MHG virus with mouse encephalomyelitis viruses. Lab Anim Sci. 1974;24(3):523–9. [PubMed] [Google Scholar]
- Hsu, et al. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin Diagn Lab Immunol. 2005;12(10):1145–51. doi: 10.1128/CDLI.12.10.1145-1151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby, Lindsey Health care for research animals is essential and affordable. Faseb J. 1997;11(8):609–14. doi: 10.1096/fasebj.11.8.9240962. [DOI] [PubMed] [Google Scholar]
- Liang, et al. Phylogenetic analysis of the species Theilovirus: emerging murine and human pathogens. J Virol. 2008;82(23):11545–54. doi: 10.1128/JVI.01160-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11(5):1147–55. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, Riley Diagnostic Testing of Mouse and Rat Colonies for Infectious Agents. Lab Anim. 2003;32(5):44–51. doi: 10.1038/laban0503-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell, et al. Isolation and Characterization of a Neurotropic Agent (Mhg Virus) from Adult Rats. Proc Soc Exp Biol Med. 1964;115:362–7. doi: 10.3181/00379727-115-28914. [DOI] [PubMed] [Google Scholar]
- Ndifon New methods for analyzing serological data with applications to influenza surveillance. Influenza Other Respi Viruses. 2011;5(3):206–12. doi: 10.1111/j.1750-2659.2010.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls, et al. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature. 1994;372(6501):103–7. doi: 10.1038/372103a0. [DOI] [PubMed] [Google Scholar]
- Ohsawa, et al. Genetic analysis of a Theiler-like virus isolated from rats. Comp Med. 2003;53(2):191–6. [PubMed] [Google Scholar]
- Olitsky A Transmissible Agent (Theiler’s Virus) in the Intestines of Normal Mice. J Exp Med. 1940;72(2):113–127. doi: 10.1084/jem.72.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, et al. Theiler’s murine encephalomyelitis virus in nonbarrier rat colonies. Comp Med. 2005;55(5):459–64. [PubMed] [Google Scholar]
- Rozengurt, Sanchez A spontaneous outbreak of Theiler’s encephalomyelitis in a colony of severe combined immunodeficient mice in the UK. Lab Anim. 1993;27(3):229–34. doi: 10.1258/002367793780745507. [DOI] [PubMed] [Google Scholar]
- Theiler Spontaneous Encephalomyelitis of Mice, A New Virus Disease. J Exp Med. 1937;65(5):705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler, Gard Encephalomyelitis of Mice: III. Epidemiology. J Exp Med. 1940a;72(1):79–90. doi: 10.1084/jem.72.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler, Gard Encephalomyelitis of Mice. I. Characteristics and pathogenesis of the virus. J Exp Med. 1940b;72:49–67. doi: 10.1084/jem.72.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, et al. A spontaneous epizootic of mouse encephalomyelitis. Proc Soc Exp Biol Med. 1951;77(2):262–6. doi: 10.3181/00379727-77-18744. [DOI] [PubMed] [Google Scholar]
- Tsunoda, et al. Theiler’s murine encephalomyelitis virus attachment to the gastrointestinal tract is associated with sialic acid binding. J Neurovirol. 2009;15(1):81–9. doi: 10.1080/13550280802380563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoll, et al. Saffold virus, a human Theiler’s-like cardiovirus, is ubiquitous and causes infection early in life. PLoS Pathog. 2009;5(5):e1000416. doi: 10.1371/journal.ppat.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen, Fujinami Theiler’s virus infection in nude mice: viral RNA in vascular endothelial cells. J Virol. 1988;62(10):3589–96. doi: 10.1128/jvi.62.10.3589-3596.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]