Abstract
Purpose
Epithelial homeostasis is critical for vocal fold health, yet little is known about the cells that support epithelial self-renewal. As a known characteristic of stem cells is that they are slow-cycling in vivo, the purpose of this prospective, controlled study was to identify and quantify slow-cycling cells or putative stem cells in murine vocal fold epithelium.
Method
Twelve mice were administered daily intraperitoneal injections of a nucleotide dye, bromodeoxyuridine (BrdU), over seven consecutive days. Under this pulse-chase paradigm, slow-cycling cells retain the dye (label-retaining cells; LRCs) while more rapidly cycling cells lose dye to dilution during multiple cell divisions. The percentage of label-retaining cells (%LRCs) was calculated following a chase period of two, four, and eight weeks post-injections.
Results
The %LRCs decreased significantly from 9.4% at two weeks to 3.1% at eight weeks following injections (p<.05). No statistically significant differences in the quantity of BrdU-positive cells were measured between the anterior, mid-membranous, or cartilaginous regions of the vocal fold (p>.05).
Conclusions
These findings are consistent with the presence and first report of a small population of putative stem cells along the length of murine vocal fold epithelium.
Keywords: larynx, stratified squamous epithelium, airway, stem cells, BrdU
Introduction
An intact epithelium provides a physical and biochemical barrier that protects vocal folds from external insults. When epithelial integrity is compromised, rapid and complete restoration of this integrity is critical for maintaining epithelial homeostasis. Adult stem cells maintain homeostasis by supplying the reserve of cells necessary for continuous self-renewal and repair of tissue throughout the body; they divide asymmetrically into one stem cell and one cell committed to differentiation (Lavker and Sun, 2000). The terminally differentiated cell provides epithelium with its particular characteristics (Potten, 1975). Mechanisms underlying preservation of epithelial homeostasis in vocal folds is presently unknown. Yet, the rate and manner of vocal fold epithelial self-renewal has important implications for pathogenesis of diseases such as cancer (Wodraz, 2007); tissue regeneration following phonotrauma and surgery; and treatment such as gene therapy (Blanpain, Horsely and Fuchs, 2007, Rawlins and Hogan, 2008). In this study, we sought to localize putative stem cells in the vocal fold epithelium, a necessary first step toward understanding how epithelial homeostasis is regulated.
Free margins of the vocal folds are covered by a stratified, squamous epithelium which is comprised of two compartments: the basal layer and the suprabasal layers. This epithelium is believed to undergo constant turnover across the lifespan. It has been proposed that cell division occurs in the basal layer of the vocal fold epithelium about every 30 hours in the rat under physiologic conditions (Savelli et al., 1991). Following cell division, the cells serve to replenish the epithelium as they differentiate and migrate through the suprabasal layers towards the surface. Superficial cells slough off and underlying cells take their place. Epithelial turnover occurs in about 96 hours (Savelli et al., 1991). This rapid rate of turnover is markedly faster than other airway epithelium such as that covering the trachea and bronchi where the life cycle of cells is at least 18 months (Rawlins and Hogan, 2008).
The duration of the epithelial cell cycle shortens following injury to permit rapid restoration of epithelium (Zhou, Leiberman, Xu and Lavker, 2006). Tateya and colleagues (2006) examined epithelial proliferation in vocal folds following acute mechanical injury. Mice were injected with bromodeoxyuridine (BrdU) at 1, 3, 7 and 14 days post injury. As BrdU is incorporated into DNA during cell division, quantification of the number of cells undergoing division could be determined by counting cells that stained positive for BrdU. The number of BrdU-positive cells in injured animals was compared to the number of BrdU-positive cells in uninjured control rats. Injury recruited the cells into a proliferative state that peaked at day 1. Positive cells were found at the site of injury and at the anterior and posterior portions of the vocal fold epithelium where no injury occurred. The number of BrdU-positive cells declined but remained significantly greater than control levels at day 3 post-injury. At day 3, these cells were found at high levels in the basal layer of the regenerated epithelium. By day 7, the density of epithelial cells fell to that of non-injured vocal fold epithelium.
While no specific marker of stem cells in the vocal fold epithelium is available, putative stem cells can be identified based on known characteristics of this class of cells. For example, a collection of cells that is rich in stem cells and progenitor cells, called side population (SP) cells, can be identified by their ability to efflux a DNA binding dye which the other cells lack. Side population cells were first identified in bone-marrow derived cells (Goodell, Brose, Paradis, Conner and Mulligan, 2006). They have since been identified in a variety of tissue including normal vocal fold mucosa (Yamashita et al., 2007) and laryngeal cancer cell lines (Wan, Zhou, Xie, Chen and Tian, 2010). Yamashita and colleagues (2007) identified a population of cells in vocal fold mucosa that demonstrated a characteristic feature of SP cells, exclusion of the DNA binding dye, Hoescht 33342. They reported that 0.2% of cells obtained from human vocal fold mucosa excluded Hoechst dye. In subsequent antibody labeling of the adenosine triphosphate-binding cassette transporter subfamily G member 2 (ABCG2) which is believed to give SP cells the capacity for Hoechst dye efflux, they found that 0.04% of cells had positive staining for ABCG2. Labeling was noted throughout the epithelial and subepithelial regions including the anterior and posterior macula flava and Reinke's space.
Hoechst dye effluxing SP cells that demonstrate cancer stem cell properties have been identified in laryngeal squamous cancer cell lines (Wan et al., 2010). Wan and colleagues reported that the SP cells demonstrated stem cell characteristics including high proliferation rates in vitro, radiotherapy resistance, and tumor-initiation potential; however, they noted that not all SP cells behaved like stem cells. For example, some SP did not initiate tumor formation in vivo. Further, some non-SP cells demonstrated cancer stem cell properties including an ability to expand in culture.
A second distinguishing feature of stem cells is that they are slow-cycling cells. This attribute has been exploited to identify stem cells in various other stratified squamous epithelium including esophageal (Croah, Philips, Redvers, Thomas & Kaur; Kalabis et al., 2008), endometrial (Chan and Gargett, 2006), urothelium (Kurzrock, Lieu, Degraffenreid, Chan and Isseroff, 2008), and palatal, buccal, and lingual epithelia (Bickenbach & Mackenzie, 1984). In these experiments, animals are exposed to a DNA label such as BrdU or 3H-thymidine via ingestion or injection over consecutive days. The label enters cells during the synthesis (S) phase of the cell cycle. The label is initially retained but becomes diluted over time during cell division. Following a chase period in which the animals are not exposed to the nucleotide label, tissues are harvested and the pattern of label retention in the epithelial cells is examined. Over the course of the chase period, the labeling of the more rapidly dividing cells, such as transit-amplifying cells, will be lost through dilution while the more slowly dividing stem cells, the “label-retaining cells” (LRCs), will maintain their labeling. Detection of LRCs following the chase period will be consistent with the presence of stem cells in tissue (Bickenbach, 1981; Lajtha, 1979).
The purpose of this prospective, controlled experiment was to identify the location and density of LRCs in murine vocal fold epithelium. Based on findings of previous studies highlighted above, we anticipated that LRCs would be found dispersed along the vocal folds. Instead, length of the entire vocal folds epithelium under physiologic conditions. Specifically, we hypothesized that the density of LRCs would be similar at the anterior commissure and mid-membranous and cartilaginous portions of the vocal folds. However, prior to conducting the experiment, we could not rule out the possibility that LRCs are located in discrete niches rather than along the full length of the stratified squamous epithelium. For example, it has been demonstrated that corneal epithelial stem cells are not found throughout the basal layer of the corneal epithelium; rather, their location is restricted to the limbal zone (Sun and Lavker, 2004). We considered, therefore, that vocal fold epithelial stem cells may be sparse or absent at the mid-membranous portion of the they may be located at the anterior commissure and cartilaginous portion of vocal fold epithelium where they would be protected against mechanical injury during phonation.
Methods
Animals
Fifteen two-month-old male C57BL/6N mice were purchased from Harlan Laboratories (Madison, WI). The animal protocols were implemented with approval from the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison.
BrdU Labeling
Twelve mice were given intraperitoneal injections with a 26-gauge needle daily at 10 am over the course of seven consecutive days with bromodeoxyuridine (BrdU; Sigma, St. Louis, MO) at a dose of 50mg/kg. Three mice, chosen at random, were sacrificed at each of four time points: 1, 14, 28 and 56 days following the week-long exposure to BrdU. An additional three mice received injections of phosphate buffered saline (PBS) to serve as controls. Following euthanasia, the larynges were harvested and transported to the laboratory in PBS for immunohistochemical analysis.
Immunohistochemistry
Larynges were prepared for immunohistochemical analysis using standard procedures. The larynges were fixed in 10% neutral phosphate-buffered formalin (pH of 7.0) at room temperature, processed, and embedded in paraffin. Five micron coronal sections were obtained across the length of the membranous and cartilaginous vocal folds for each mouse. A primary monoclonal antibody against BrdU (1:10 concentration) (BD Biosciences, San Jose, CA) was used to identify BrdU-positive epithelial cells. Labeling was detected using a biotinylated/steptavidin-horseradish peroxidase and visualized with diaminobenzidine (DAB) as the chromagen prior to counter staining with hematoxylin and mounting. Sections were viewed with a Nikon E600 microscope (Nikon, Melville, NY). Images of sections taken at 100μm increments across the entire length of the membranous and cartilaginous vocal folds (from the anterior commissure to the medial surface of the arytenoids) for each larynx at each time point were photographed using an Olympus DP71 microscope digital camera (Tokyo, Japan) (Figure 1).
Figure 1.
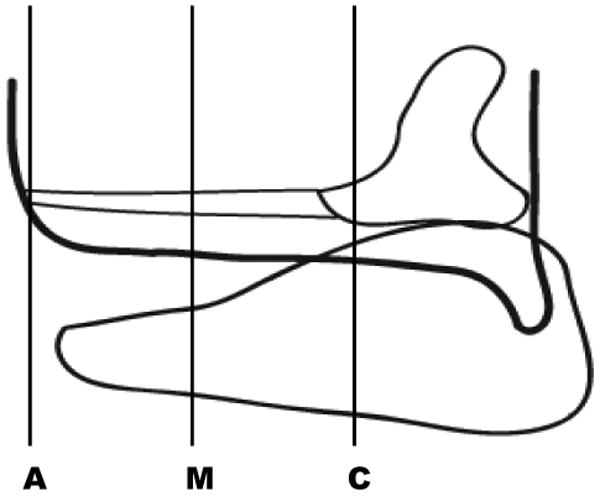
Schematic demonstrating orientation of the coronal sections obtained from the anterior (A), mid-membranous (M) and cartilaginous (C) aspects of the vocal fold for each animal.
Analysis
As a procedural control, the presence of BrdU-positive cells in vocal fold epithelium harvested one day following injections was examined qualitatively. Positive staining for BrdU would indicate successful administration of BrdU; however, as both fast and slowly dividing cells incorporate BrdU during injection of the dye, a chase period was needed to detect the slowly dividing, putative stem cells (LRCs). Two blinded raters counted the number of LRCs and unstained epithelial cells in each image obtained from vocal folds harvested following chase periods of 14, 28, and 56 days post-injections. The percentage of LRCs (%LRCs) was obtained by dividing the number of LRCs by the total number of cells for each section. Inter-rater reliability for %LRCs was obtained for 100% of the sections. Intra-rater reliability was completed on 20% of the sections selected at random. Utilizing Pearson Product Moment Correlation Coefficient inter-rater reliability for %LRCs was r = 0.89; intra-rater reliability was above r = 0.97 for both raters.
We were interested in determining whether the quantity of %LRCs was dependent on the number of days post-surgery or the region of the vocal fold from which the tissue was obtained. Since tissue samples were obtained from serial sections within each animal, a split-plot analysis of variance (ANOVA) was used to assess associations. Pair-wise comparisons of the %LRC across the three time points were performed with Fisher's protected least significant difference tests. Prior to analysis, data were rank-transformed to better meet the assumptions of ANOVA. P-values less than 0.05 were considered significant. All results were obtained using SAS statistical software version 9.1 (SAS Institute, Inc., Cary, NC).
To permit a comparison of the quantity of LRCs identified at locations along the length of vocal folds, images were classified by two authors (RB and CL) into three regions: anterior commissure (A), mid-membranous portion (M), and cartilaginous portion (C). We used the thryroid cartilage to identify images of the anterior commissure (A). Images depicting the thickest part of the lamina propria and epithelium were considered mid-membranous (M). Images of epithelium overlying the vocal process of the arytenoid cartilage were designated as cartilaginous (C). Use of these anatomical landmarks permitted consistent identification of the three regions of the vocal folds across rats. Inter-rater agreement for classifying images according to location along the vocal folds (A, M, or C) was 100%.
Results
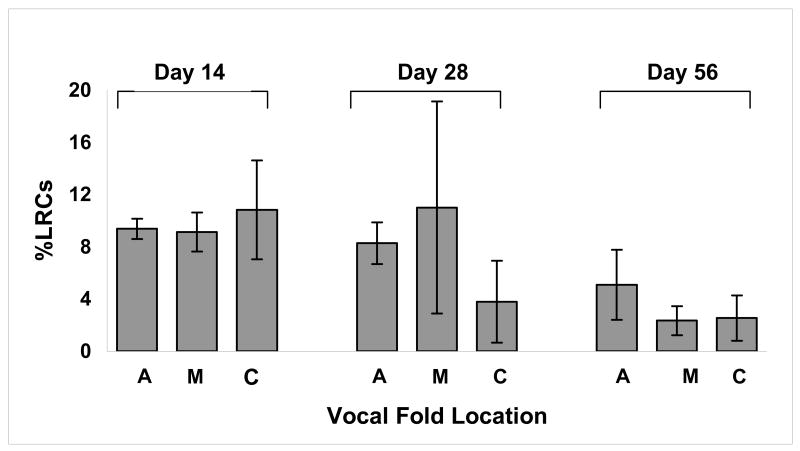
Positive staining for BrdU was observed in stratified squamous epithelium obtained from mice that were exposed to daily injections of BrdU but not from negative controls. Labeling was noted in the basal and suprabasal layers in vocal folds harvested one day following BrdU injections (Figure 2). Figure 3 shows the average percentage of LRC (%LRC) staining at the anterior (A), mid-membranous (M) and cartilaginous (C) portions of the vocal folds at 14, 28, and 56 days post-injury.
Figure 2.
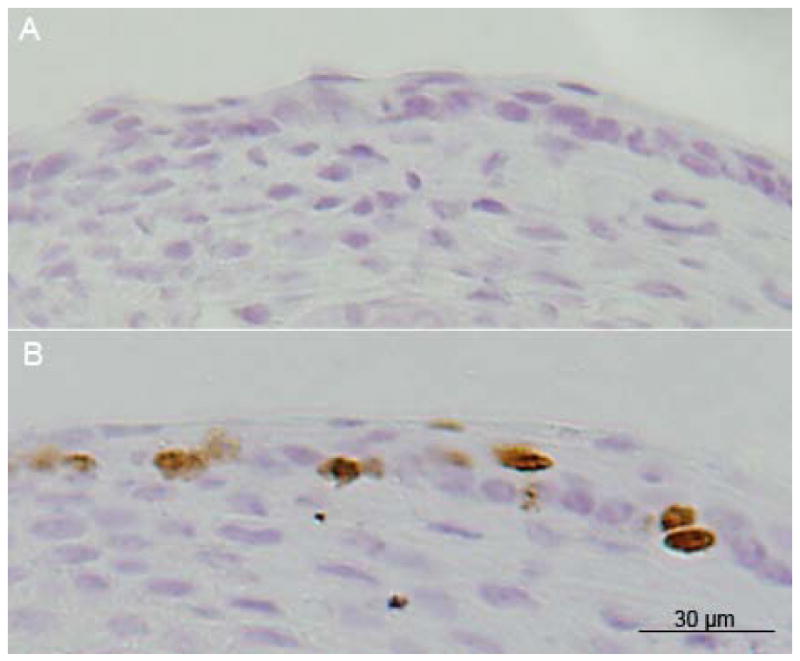
A: Bromodeoxyuridine (BrdU)-positive cells were not observed in negative control murine epithelium. B: BrdU-positive cells (brown staining) were observed in basal and suprabasal layers one day post-injections.
Figure 3.
The mean percentage of label-retaining cells (%LRCs) at the anterior (A), mid-membranous (M), and cartilaginous (C) portions of the vocal folds are shown at Days 14, 28 and 56 post-injections. Error bars indicate ± 1 SD of the mean. No statistically significant difference in %LRCs was found between locations (A, M and C) along the vocal folds.
Figure 4 shows positive staining for BrdU at three locations (A, M and C) along the vocal folds at 14, 28, and 56 days post-injury. A split-plot analysis of variance (ANOVA) of the pattern of distribution of labeled cells along the length of the vocal folds revealed no difference in amount of BrdU-positive cells regardless of position along the vocal fold at each time point(F=1.71, p > 0.05). Consequently, the ratings for location (A,M and C) were collapsed and a mean %LRCs was obtained for the each group at each time point.
Figure 4.
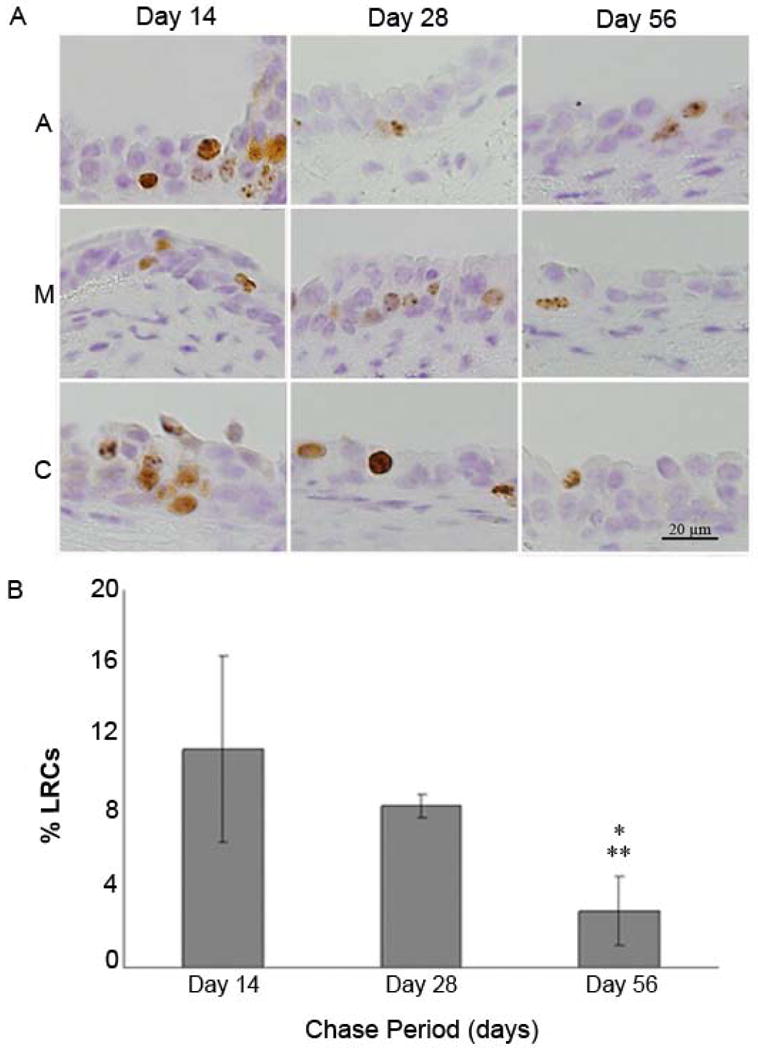
A: Label-retaining cells (LRCs; brown staining) were present in basal and suprabasal layers at the anterior (A), membranous (M), and cartilaginous (C) portions of vocal fold epithelium at 14 and 28 days following injections. Minimal LRC staining was present at the A, M, and C portions of a representative vocal epithelium at 56 days post-injections. B: Mean percent label-retaining cells (%LRCs) at three points (14, 28 and 56 days post-injections). Error bars indicate ± 1 SD of the mean. * Statistical difference between day 14 and day 56 (t=4.58, p<0.05) ** Statistical difference between day 28 and day 56 (t=2.76, p<0.05).
The mean %LRCs decreased over the course of the chase period. After 14 days, a mean of 9.4% (SD=2.7) of LRCs per vocal fold was observed. This percentage decreased to 8.6% (SD=4.7) at 28 days and 3.1% (SD=2.1) at 56 days post-injections (Figure 4). A split-plot analysis of variance (ANOVA) revealed a significant effect of time point on %LRCs (F=10.49, p<0.01). Fisher's protected least significant difference tests demonstrated significant differences between Day 14 and Day 56 (t=4.58, p<0.05) and between Day 28 and Day 56 (t=2.76, p<0.05).
Discussion
The purpose of this study was to determine the location and percentage of label-retaining cells (%LRCs) in stratified squamous vocal fold epithelium, in an effort to provide a preliminary characterization of the resident putative stem cells. To that end, mice were exposed to daily injections of a nucleotide label, BrdU, over seven consecutive days. Larynges were harvested at various time points following the injections. The %LRCs was determined at each time point. We found that BrdU was taken up by cells throughout the epithelium over the week-long injection period. After 14 days, the %LRCs decreased. Overall, the LRCs represented a small fraction of all cells; the percentage of labeled cells ranged from 9.4% at 14 days to 3.1% at 56 days post-injections. Identification of LRCs in our samples establishes the presence of a small population of putative stem cells in murine vocal fold epithelium.
A small population of BrdU-positive cells (3.1%) was identified following the eight-week chase period. This percentage was in agreement with reports from other epithelium including the endometrium (3%; Chan and Gargett, 2006), colon (3.8%; Kim, Cheung & Hellerstein, 2006), and tongue (3.71%; Huang, Tao, Xia, Li and Cheng, 2009) epithelia. However, the %LRCs reported here differed from findings observed in some other epithelia. For example, it was less than the amount observed in buccal and palatal epithelium following a 10 week chase period (4.1% and 6.4%, respectively) and exceeded the 1% of label-retaining cells in the esophagus (Kalabis et al., 2008). While we speculate that the discrepancies in findings may be attributable to differences in tissue type, it is also possible that inconsistent methodologies (i.e., differences in dosages and delivery of BrdU) may have played a contributing role. For example, in the present study 50mg/kg BrdU was injected into mice once daily. In contrast, 50ug/kg injections were administered twice daily in the study reported by Huang and colleagues (2009) and 0.8mg/ml BrdU in drinking water offered ad libitum was administerd by Kalabis and colleagues (2008). Additionally, the animal model used differed across studies; while a mouse model was used in the present study, a rat model was used by Huang and colleagues (2009).
We found that the LRCs in the stratified squamous epithelium of vocal fold tissue, which is consistent with reports of LRC staining for epithelium in other parts of the body, including the esophagus (Croagh et al., 2007); palate, buccal cavity, and tongue (Bickenbach and Mackenzie, 1984); and urinary tract (Kurzrock et al., 2008). We also observed that the LRCs were located along the length of the vocal fold rather than in niches at the anterior and posterior portions of the vocal fold. A secondary statistical analysis was performed to confirm this finding. Using a split-plot ANOVA, we compared the %LRCs between the membranous (anterior commissure (A) and midmebranous portion (M)) and cartilaginous (C) portions of the vocal fold. This analysis revealed that, despite likely differences in mechanical stress experienced by membranous versus cartilaginous regions of vocal folds, significant differences were not noted in % LRCs in the two regions (p>.05). This result provided additional support for our hypothesis that the distribution of LRCs in vocal fold epithelium may have important implications of maintenance of vocal fold epithelial homeostasis; a supply of LRCs is available to replenish and repair epithelium along the length of the vocal fold that may be damaged by subclinical microtrauma associated with normal phonation.
Examination of SP cells and LRCs offer two methods to identify cells with stem cell-like features in vocal fold epithelium. The %LRCs found in the present study exceeded the 0.4% side population cells located in the epithelium and subepithelium of human vocal folds by Yamashita et al. (2007). While the Hoechst 33342 efflux method for identifying SP cells does not allow for identifying the anatomical localization of the SP cells because of the use of flow cytometry to identify the cells, immunolocalization of the transporter purported to be responsible for Hoechst efflux, the ABCG2 antibody, allows localization of these cells. Consistent with these the ABCG2-positive cells, the LRCs were located along the length of vocal folds. Future studies that simultaneously identifies SP cells and LRCs in vocal fold epithelium are warranted to better understand the relationship between the two cell types.
We are mindful of the limitations of the nucleotide labeling methodology used in the present study to identify slow-cycling cells. It is likely that we have underestimated the true population of slow-cycling cells for two reasons. First, the label is only taken up by the cell when it is dividing. Consequently, only those cells that are mitotically active during exposure to BrdU will incorporate the label. Given that we performed the experiment under physiologic conditions, a portion of the stem cells in the vocal fold epithelium may have been quiescent during the experimental period. It is possible that had we injured the vocal folds we could have recruited the stem cells residing in the epithelium into a proliferative state, thus shortening the cell cycle time and increasing the likelihood of cell division and thus incorporation of BrdU during the experimental period. Second, the true population of slow-cycling cells may have been underestimated due to the length of the chase periods (14, 28 and 56 days). Savelli and colleagues (6) have proposed that cells in the basal layer of epithelium divide every 30 hours under physiologic conditions. Bonhoeffer and colleagues (2000) reported that BrdU could be detected in human lymphocytes using flow cytometry for up to five or six cell divisions. If BrdU labeling is detectable for up to five or six divisions of vocal fold epithelial cells using immunohistochemistry, possible stem cells would have lost their labeling within a time frame of 150-200 hours (6.25-8.3 days) which is substantially less than our shortest chase period of 14 days. However, the presence of BrdU-positive stem cells at 56 days suggests there is heterogeneity in the basal cell population; a subpopulation of cells divide less frequently than previously suggested.
In the present study, we demonstrated the presence of a small population of LRCs dispersed along the length of the vocal fold epithelium. Future studies are needed to evaluate the functionality of LRCs, including assessment of their potential for proliferation and differentiation, as well as to determine the nature and extent of their contribution to epithelial homeostasis. Additionally, elucidation of the mechanisms underlying epithelial self-renewal and repair is warranted to advance our understanding of normal epithelial development, maintenance of epithelial homeostasis under normal conditions, epithelial proliferation following phonotrauma, surgery and benign lesions and abnormal proliferation including hyperplasia and tumorigenesis.
Acknowledgments
The authors would like to acknowledge the National Institute of Deafness and Other Communication Disorders (R01 DC004336) for supporting this research project. We also extend our gratitude to Glen E. Leverson for assistance with statistical analysis and to Supriya D. Hayer and Michael Stitgen for assistance with data collection.
Contributor Information
Ciara Leydon, Department of Speech Communication Arts and Sciences, Brooklyn College.
Rebecca S. Bartlett, Division of Otolarynology – Head and Neck Surgery, University of Wisconsin Madison.
Drew A. Roenneburg, Division of Transplant, University of Wisconsin Madison.
Susan L. Thibeault, Division of Otolarynology – Head and Neck Surgery, University of Wisconsin Madison.
References
- Bickenbach JR. Identification and behavior of label-retaining cells in oral mucosa and skin. Journal of Dental Research. 1981;60:1611–20. doi: 10.1177/002203458106000311011. [DOI] [PubMed] [Google Scholar]
- Bickenbach JR, Mackenzie IC. Identification and localization of label-retaining cells in hamster epithelia. The Journal of Investigative Dermatology. 1984;82:618–22. doi: 10.1111/1523-1747.ep12261460. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–58. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer S, Mohri H, Ho D, Perelson AS. Quantification of cell turnover kinetics using 5-bromo-2′-deoxyuridine. Journal of Immunology. 2000;164:5049–54. doi: 10.4049/jimmunol.164.10.5049. [DOI] [PubMed] [Google Scholar]
- Chan RWS, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–38. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- Croagh D, Phillips WA, Redvers R, Thomas JST, Kaur P. Identification of candidate murine esophageal stem cells using a combination of cell kinetic studies and cell surface markers. Stem Cells. 2007;25:313–8. doi: 10.1634/stemcells.2006-0421. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopietic stem cells that replicating in vivo. Journal of Experimental Medicine. 2006;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Tao X, Xia J, Li CY, Cheng B. Distribution and Quantity of Label-Retaining Cells in Rat Oral Epithelium. Oral Pathology & Medicine. 2009;38:663–7. doi: 10.1111/j.1600-0714.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- Kalabis J, Oyama K, Okawa T, Naaagawa H, Michaylira CZ, et al. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. Journal of clinical investigation. 2008;118:3860–9. doi: 10.1172/JCI35012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kwon HJ, Shinozaki N, Hashimoto S, Shimono M, Cho SW, Jung HS. Comparative analysis of ABCG2-expressing and label-retaining cells in mouse submandibular gland. Cell and Tissue Research. 2008;334:47–53. doi: 10.1007/s00441-008-0667-8. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelium stem cells. American Journal of Physiology. Renal Physiology. 2008;294:F1415–21. doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- Lajtha LG. Stem cell concepts. Differentiation. 1979;14:23–4. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proceeding of the National Academy of Sciences of the United States of America. 2000;97:13473–5. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Control of epidermal proliferation units (EPUs). A hypothesis based on the arrangement of neighbouring differentiated cells. Differentiation. 1975;3:161–5. doi: 10.1111/j.1432-0436.1975.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. American Journal of Physiology. Lung Cell and Molecular Physiology. 2008;295:L231–4. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli V, Rizzoli R, Rizzi E, Galanzi A, Buffa A, Rana R, Lattanzi G, Baratta B. Cell kinetics of vocal fold epithelium in rats. Bollettino della Società Italiana di Biologia Sperimentale. 1991;67:1091–8. [PubMed] [Google Scholar]
- Sun TT, Lavker RM. Corneal epithelial stem cells: past, present, and future. Journal of Investigative Dermatology. 2004;9:202–7. doi: 10.1111/j.1087-0024.2004.09311.x. [DOI] [PubMed] [Google Scholar]
- Tateya I, Tateya T, Lim X, Sohn JH, Bless DM. Cell production in injured vocal folds: A rat study. Annals of Otology, Rhinology & Laryngology. 2006;115:135–43. doi: 10.1177/000348940611500210. [DOI] [PubMed] [Google Scholar]
- Wan G, Zhou L, Xie M, Chen H, Tian J. Characterization of side population cells from laryngeal cancer cell lines. Head & Neck. 2010 doi: 10.1002/hed.21325. [DOI] [PubMed] [Google Scholar]
- Wodraz D. Effect of stem cell turnover rates on protection against cancer and aging. Journal of Theoretical Biology. 2007;3:449–58. doi: 10.1016/j.jtbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Hirano S, Kanemaru S, Tsuji S, Suehiro A, Ito J. Side population cells in the human vocal fold. Annals of Otology, Rhinology & Laryngology. 2007;116:847–52. doi: 10.1177/000348940711601110. [DOI] [PubMed] [Google Scholar]
- Zhou M, Leiberman J, Xu J, Lavker RM. A hierarchy of proliferative cells exists in mouse lens epithelium: implications for lens maintenance. Investigative Ophthalmology & Visual Science. 2006;47:2997–3003. doi: 10.1167/iovs.06-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]