Abstract
Thirty years after its discovery, the hepatitis E virus (HEV) continues to represent a major public health problem in developing countries. In developed countries, it has emerged as a significant cause of non-travel-associated acute hepatitis. HEV infects a wide range of mammalian species and a key reservoir worldwide appears to be swine. Genomic sequence similarity between some human HEV genotypes and swine HEV strains has been identified and we know that humans can acquire HEV infection from animals. Although for the most part the clinical course of HEV infection is asymptomatic or mild, significant risk of serious disease exists in pregnant women and those with chronic liver disease. In addition, there are data on the threat of chronic infections in immunocompromised patients. Beyond management of exposure by public health measures, recent data support that active immunisation can prevent hepatitis E, highlighting the need for vaccination programmes. Here we review the current knowledge on HEV, its epidemiology, and the management and prevention of human disease.
Introduction
First recognised in Asia almost 30 years ago as the main cause of non-A, non-B enterically transmitted hepatitis,1, 2 hepatitis E virus (HEV) is now acknowledged to have worldwide distribution.
In countries with poor sanitation, HEV is endemic and typically causes explosive outbreaks of acute hepatitis, usually associated with faecal contamination of the water supply. The disease is generally mild, yet pregnant women suffer significant morbidity and mortality.3–5
In contrast, in countries with high standards of sanitation, hepatitis E occurs sporadically, initially identified as an imported disease in travellers from highly endemic regions, but subsequently diagnosed in patients with no travel history as well; this latter form has been named ‘hepatitis E indigenous to developed countries’.6–26
Phylogenetic analysis of HEV genome from different isolates has led to the identification of four main genotypes, with genotypes 1 and 2 circulating in Africa and Asia, genotype 3 showing a broad distribution worldwide, and genotype 4 being restricted to Asia.
Genotypes 3 and 4 are enzootic in a variety of wild and domestic animals, particularly pigs,8, 27–36 which gave rise to the question of whether human HEV infection is a zoonosis? Evidence from Japan37–40 and China28 now confirms that humans can acquire HEV infection from animals.
Hepatitis E represents a significant public health and economic burden particularly in countries where the absence of sanitation infrastructures, or their breakdown as a consequence of wars or natural disasters, brings the hygienic conditions below a safe level.4, 41, 42 The development of an effective vaccine is expected to dramatically reduce the incidence of the disease, particularly in the most susceptible individuals such as pregnant women.
Virology
Taxonomy and virus structure
In the early 1980s, the observation that individuals involved in epidemics of jaundice were seronegative for markers of acute hepatitis A and B suggested the existence of an unrecognised aetiological agent of enterically transmitted hepatitis.1, 2 The confirmation came in 1983, when small, virus-like particles were identified by immune electron microscopy in stool specimens from a volunteer experimentally infected with pooled faecal extracts from human cases of epidemic non-A, non-B hepatitis.43 The pathogen, HEV, is a non-enveloped virus, 27–30nm in diameter, with an icosahedral capsid. In the early 1990s, the virus genome was cloned44, 45 and diagnostic antibody assays were developed. 46 After being provisionally assigned to the Caliciviridae family,47 HEV was re-classified as the sole member of the genus Hepevirus, family Hepeviridae, in 2004.48
Genomic organisation and viral proteins
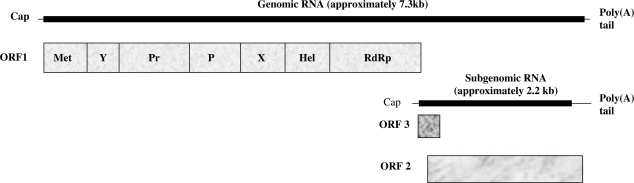
The HEV genome consists of a single-stranded RNA molecule with positive polarity approximately 7300 nucleotides in length.45, 49, 50 It comprises a short 5’ noncoding region (28 nucleotides), followed by three open reading frames (ORF), a 30 noncoding region (65–74 nucleotides), and a poly(A) tail (Figure 1).45, 53 The genome is capped at its 5’ terminus54 and capping is required for virus viability.55
Figure 1.
Schematic organisation of genomic and subgenomic HEV RNAs. ORF1 encodes a nonstructural polyprotein; ORF2 encodes the capsid protein; ORF3 encodes a phosphoprotein. Met, methyltransferase; Y, no function assigned at present; Pr, putative papain-like cysteine protease; P, proline ‘hinge’; X, no function assigned at present; Hel, RNA helicase; RdRp, RNA-dependent RNA polymerase.51, 52
ORF1 (approximately 5 kb) encodes a large nonstructural polyprotein with key functions for viral genome replication and viral protein processing. ORF2 (approximately 2 kb) occupies the 3’ end of the coding region and encodes the capsid protein.45, 51, 56–58 The N-terminal region of ORF2 protein binds the 5’ noncoding region of the HEV genome59 and is possibly involved in viral encapsidation.41 Only the ORF2 recombinant protein truncated at its N-termini can efficiently self-assemble in vitro into empty, virus-like particles. These share antigenic properties with the native HEV capsid protein, although they are smaller than the native virions.57, 58
ORF3 is a small reading frame (372 bases) with the 5’ end overlapping ORF1 by four nucleotides and the 3’ end overlapping ORF2 by 331 nucleotides;45, 53, 51 it encodes a small phosphoprotein that associates with the cytoskeleton and the capsid protein.60, 61 The product of ORF3 is possibly involved in modulation of cell signalling62, 63 and in the assembly of the HEV nucleocapsid;60 recently, it has been shown that this protein is essential for infectivity in vivo 64 but not in vitro.65 The poly(A) tail is necessary for binding of RNA-dependent RNA polymerase (RdRp) to the 3’ noncoding region.41, 66
Compared with genotypes 1–3, the genome of genotype 4 contains a nucleotide insertion (U) just after the second AUG codon of ORF3, which changes the downstream reading frames so that, for genotype 4, different AUG codons were initially expected to initiate translation in both ORF2 and ORF3.52,53 However, a recent study by Graff et al., 51 using a replicon, demonstrated that ORF2 and ORF3 proteins are produced from a single subgenomic RNA of approximately 2.2 kb, which initiates downstream of the first two AUG codons of ORF3 in genotypes 1–3 (downstream of the insertion of U in genotype 4), using two closely spaced start codons. It is therefore expected that both ORF2 and ORF3 proteins are similar in size in all four genotypes.51
Resistance to physical and chemical agents
Boiling and chlorination of water represent the main measures to control and prevent infection. However, Emerson et al.67 reported that HEV is moderately resistant to heat inactivation. This finding was further corroborated in a more recent study that showed how HEV was still infectious on incubation at 56°C for 30 min.68
HEV genetic diversity
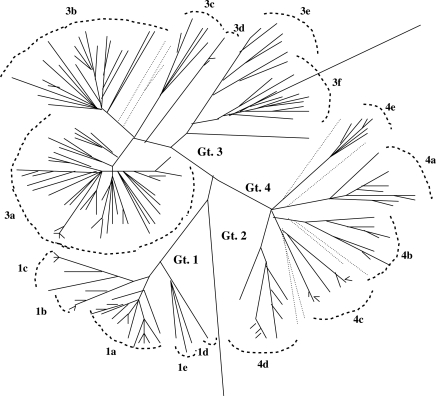
Comparative nucleotide sequence analysis of whole genomes of HEV isolates has revealed extensive genomic diversity leading to the identification of four major genotypes and several subtypes within each genotype (Figure 2). However, although the separation of HEV into four major genogroups is widely accepted, so far there is no agreement about the number of subtypes within each genotype.69 Genomic regions that have been used for phylogenetic purposes include a 301-nucleotide-long sequence at the 5’ end of the ORF2 region (Figure 2)69 and a 306-nucleotidelong sequence in the RdRp of ORF1.70
Figure 2.
Phylogeny of HEV genotypes (Gt.) based on the 301-nucleotide-long 5’ end of the ORF2 region, the most conserved in all HEV isolates. Sequences determined from this region accounted for the majority of HEV sequences.69
Replication in cell culture
The lack of efficient cell culture systems has hampered detailed studies on HEV biology, critical for helping to develop diagnostic assays and vaccine research.68 Replication and propagation of HEV was attempted with limited success by using continuous cell lines71, 72 and primary hepatocytes from nonhuman primates.73 Recently, more efficient cell-culture systems were developed in cell lines,67, 68, 74 allowing studies on HEV thermal stability and improving neutralisation tests. It was shown that anti-HEV antibodies are broadly crossreactive because HEV genotype 3 was neutralised by convalescent serum samples from patients infected with HEV genotypes 1, 3, and 4.68 Similarly, genotype 1 was neutralised by convalescent serum samples obtained from rhesus monkey infected with any of the four mammalian genotypes.74 In addition, serum specimens obtained 24 years after the onset of HEV infection could prevent the propagation of HEV in cell culture, suggesting that long-lasting HEV antibodies with neutralisation activity are induced.68
Epidemiology
Geographical distribution of HEV according to genotype
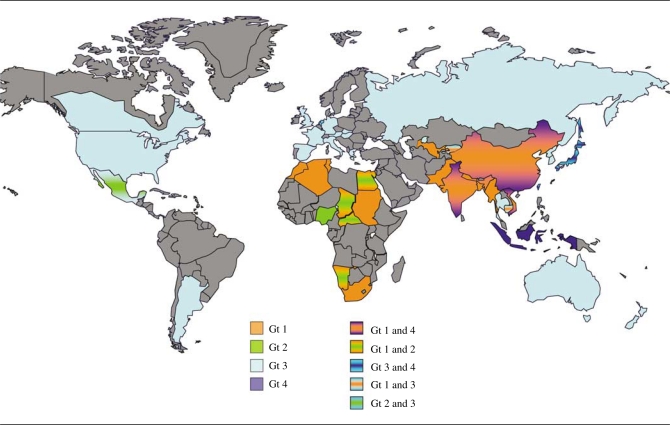
The geographical distribution of HEV genotypes is complex and continuously evolving (Figure 3). Genotype 1 extensively circulates in Asia (including India,75–78 Pakistan,79 Nepal,80, 81 Bangladesh,82 China,49, 83–85 Kyrgyzstan,86 and Uzbekistan87 and Africa (including Egypt,88 Algeria,89 Morocco,87 Namibia,90 Sudan,91 and Chad91, 92), whereas genotype 2 has been isolated only in Mexico93 and in some African countries (Nigeria,94 Namibia,95 Chad,91 and Sudan91. Genotype 3 has been detected worldwide (America, 13, 26, 32, 33, 96–99 Europe,9, 10, 12, 15, 16, 18, 20, 21, 27, 34, 35, 100–102 Asia,25, 33, 37, 39, 40, 103–111 Australia,69 and New Zealand11 with the exception of Africa, whereas genotype 4 is restricted to India112, 113 and East Asia.39, 84, 103, 114–118
Figure 3.
Geographical distribution of HEV isolates according to genotypes (Gt). HEV Gt 1 and 2: epidemic strains causing human infection. HEV Gt 3 and 4: zoonotic strains isolated from humans and a variety of animals, particularly pigs. In some countries, different genotypes co-circulate in distinct ecological niches: Gt 1 and 4 in China, India, and Vietnam; Gt 1 and 2 in several African countries, including Namibia, Chad, and Sudan; Gt 3 and 4 in Japan; Gt 1 and 3 in Cambodia; Gt 2 and 3 in Mexico.
Although genotypes 1 and 2 are considered human viruses, genotypes 3 and 4 have been isolated from both humans 10, 11, 16, 17, 20, 25, 97, 119–122 and animals.32, 33, 123–126 Of significant interest is the unique distribution of HEV isolates in India, as human and swine HEVs belong to different genotypes (genotype 1 and genotype 4, respectively);113 genotype 4 appears to be specifically restricted to swine and it has never been isolated from humans, in spite of extensive investigations.127, 128 Phylogenetic analysis shows that the Indian HEV genotype 4 represents a distinct variant among HEV genotype 4 isolates with 26 unique amino-acid substitutions (16 in ORF1, 8 in ORF2, and 2 in ORF3).129 Whether the difference in sequence, particularly in ORF2, determines tropism and explains the lack of infectivity in humans remains to be determined.
Mode of transmission
Waterborne
In developing countries, HEV is transmitted through the faecal–oral route, mainly by the consumption of water contaminated with sewage disposal.130 In developed countries, HEV RNA has been detected in human sewage only occasionally.19, 20 Interestingly, HEV rescued from sewage in Spain was infectious for rhesus monkey,131 raising the possibility that HEV might occasionally contaminate the environment and shellfish even in nonepidemic regions.16, 131, 132
Foodborne
Evidence that food can transmit HEV came from Japan, where acute hepatitis E was diagnosed in patients who consumed raw or undercooked pig liver and intestine,23 wild boar meat and liver,38, 110, 133 and deer meat40 contaminated with the virus. HEV with identical nucleotide sequence was detected both in the blood of affected patients and in batches of meat and liver not consumed.40, 109, 133 A higher HEV IgG seroprevalence in Japanese individuals with frequent dietary consumption of raw deer meat, in comparison with a control group, indirectly supports the foodborne route.134 Food as a vehicle of infection has not yet been proven in other developed countries.
Person-to-person transmission
In contrast to hepatitis A virus (HAV) infection, secondary transmission among household members of patients with acute hepatitis E is an uncommon event,135, 136 both in the context of outbreaks137 and sporadic infections.138, 139
Parenteral transmission
HEV-infected individuals can transmit the infection by donating blood during the viraemic period. Viraemia can be detected even in asymptomatic infections and during the incubation period,140, 141 even in the absence of aminotransferase elevation.140 Transmission of HEV via blood transfusion has been documented in several countries, including Saudi Arabia,142 Japan,105, 143, 144 and the UK,145 where matching RNA sequences were found in blood donors and their recipients.
Mother-to-child transmission
Mother-to-child transmission of HEV has been scarcely documented; however, the available data suggest a significant rate of HEV vertical transmission among the HEV RNA-positive mothers with worsening liver disease.146–149
Epidemic versus sporadic forms and seasonality
In the developing world, HEV infection represents the most common aetiological agent of periodic outbreaks of acute hepatitis.42, 130, 136, 150 Epidemics are most frequent during the monsoon season when flooding causes faecal contamination of drinking water.41 Between epidemics, HEV is transmitted in a discrete manner leading to the onset of sporadic forms of acute hepatitis. In India, 30–70% of all cases of acute sporadic hepatitis are caused by HEV infection.41, 151
In economically developed regions, indigenous hepatitis E is largely a sporadic disease. However, in Japan, small outbreaks have been described as a consequence of consumption of the same contaminated food.152, 153 A small outbreak involving a family with two children has also been reported in France.139 No clear seasonality has been observed in developed countries.24, 154
Age- and sex-specific clinical attack rates and case fatality rates
Clinical infection concentrates among adolescents and young adults in countries of high endemicity. During outbreaks, the clinical attack rate (3–30%)136, 150, 155–157 is highest among pregnant women.4, 158, 159 The mortality rate, which is usually low (0.07–0.6%),136, 150, 160, 161 can reach values as high as 25–31% in pregnant women, particularly during the third trimester of pregnancy.4, 136, 150, 155, 162–164
A distinct characteristic of hepatitis E indigenous to developed countries is its high attack rate among older male adults.13, 26, 100 This has been observed in both Europe10, 16, 18, 20, 100, 101 and Japan,24 which experienced the greatest number of detected cases. No documented case of hepatitis E has been identified in pregnant women in this different epidemiological setting.
A high mortality, ranging between 25 and 70%, has been recently documented among patients already suffering from chronic liver disease of different aetiologies. These observations were made both in highly endemic countries, such as India,165–168 Pakistan,169 and Nepal,170 where hepatitis E is caused by genotype 1, and in Europe,171 where indigenous hepatitis E is linked to genotype 3.
Seroprevalence of HEV infection
HEV seroprevalence studies have been conducted using several antibody assays based on different recombinant antigens, which do not always include the most relevant Bcell epitopes, leading to lack of sensitivity and poor reciprocal concordance.172 In particular, a greater sensitivity of currently available antibody assays has been reported in detecting overt disease in comparison with subclinical infections,173 which may hamper seroepidemiological investigations. Antibody assays based on the ORF2 protein, which exposes at least one major crossreactive epitope shared by all HEV genotypes,56, 62, 174 should be used for investigating seroepidemiology46, 56, 175, 176 (see ‘Diagnosis’).
Seroprevalence studies in HEV epidemic countries, such as India, have revealed that HEV infection is rare in children, reaching peak prevalence (33–40%) only in early adulthood. 177 These data are in striking contrast with HAV serosurveys, showing anti-HAV antibodies in the majority of children by the age of three years.177 The reason for the difference in age-specific seroprevalence between HAV and HEV, both transmitted by the faecal–oral route, remains unanswered.
In contrast, HEV seroepidemiology closely mirrors that of HAV in Egypt, a country highly endemic for HEV infection (similar to India), where anti-HEV antibodies are detectable in 65% of children younger than 10 years.178 Although the reason for the earlier exposure in life in Egypt remains unknown, the widespread immunity of the population at an early age might account for the absence of large outbreaks of hepatitis E in the general population179 and among pregnant women.180
In rural southern China, where the majority of HEV infections are zoonotic (genotype 4), anti-HEV IgG is rarely detected in children, rapidly increases in young adults, and peaks (60–80%) at the age of 60 years.181 Interestingly, after 30 years of age, but not in younger age groups, the seroprevalence is two times higher for men than for women, suggesting a link with different social roles adopted by men and women once families are established.181
Seroprevalence investigations in developed regions have revealed the ubiquitous presence of anti-HEV antibodies in the analysed populations, although with significant differences between and within countries.11, 172, 182–186 A significant concern is the high seroprevalence among US blood donors (18–21%)184, 187 compared with individuals professionally exposed to swine HEV, such as veterinarians (23–26%).184
Some studies have documented an increasing seroprevalence with age in both sexes, suggesting a continuous ongoing exposure to HEV.172, 182, 187, 188 In Japan, age-specific profiles of anti-HEV and anti-HAV antibodies suggest silent HEV infection in the last few decades, during which HAV infection rates declined.188
Zoonosis and host range
The possibility that HEV infection can be a zoonosis was raised when a virus, closely related to the HEV human strains, was isolated from pigs, initially in the USA189 and, subsequently, worldwide.28, 32–36, 96, 112, 183, 190–193
Experimental cross-species infections between swine and primates have shown that swine HEV could infect primates (surrogates for human infection), and the US-2 strain of human HEV (genotype 3) could infect specific pathogen-free pigs.194 In contrast, US pigs could not support replication of human epidemic strains (genotypes 1 and 2).195 Similarly, Indian pigs could be infected with swine HEV (genotype 4) but not with human HEV (genotype 1).112
These findings are supported by phylogenetic analysis data showing a high degree of nucleotide and amino-acid sequence homology between swine and human HEV isolates of genotypes 3 and 4 from the same geographical regions, suggesting that pigs may act as a reservoir for human infection.69, 35, 109, 115, 116, 125 On the contrary, swine HEV strains are highly divergent from the human strains of HEV classified within genotypes 1 and 2.69
Pigs are infected via the faecal–oral route196 and develop a self-limiting subclinical infection189, 197, 198 with transient viraemia (one to two weeks) but prolonged viral shedding (three to four weeks) in faeces. Current pig-raising practices perpetuate exposure of pigs to their waste, promoting viral transmission.196
Seroprevalence studies in pigs have shown the presence of HEV IgG in an unexpectedly high proportion of animals, 36, 199–201 with peaks up to 85% in the UK,34 95–98% in India,127 and 70–100% in Japan.109, 202, 203 Although pigs are infected primarily at the early stage of production (1–3 months), HEV can still be detected by PCR at slaughter age, meaning that swine HEV can enter the food chain.28, 36, 98 This has been shown in Japan, where infectious HEV was found in 2% of the pig liver packages ready for sale,39 and more recently in the USA30 and in The Netherlands.204 HEV RNA was also detected in 3.1% of bile samples from swine in abattoirs in eastern China.28
HEV replicates in different visceral organs.192, 205, 206 This explains the common foodborne transmission in Japan owing to the gastronomic habit of eating rarely, or poorly cooked, pig liver and intestines. Despite absence of evidence for HEV replication in muscles, HEV infection has been transmitted by consumption of meat from boar and deer as well.38, 40, 133
The finding of infectious HEV in pig-farm manure slurry samples193 suggests that human exposure to swine waste may represent an alternative mode of transmission of zoonotic strains, particularly in regions where the water supply comes from wells, rivers, and streams, and where sewage treatment is not generally available.181 In rural eastern China, where 9.6% of pig herds were found to be HEV RNA-positive by stool samples, a 74% higher risk of infection among people professionally engaged in swine farming was observed. Seroprevalence increased with the duration of occupational exposure to swine.28 There was also a 29% higher risk of infection in people without occupational exposure to swine and residing in communities downstream of the Chinese swine farms, compared with those living in communities upstream.28 Compared with control individuals, increased HEV IgG seroprevalence was also detected in people with occupational exposure to pigs in other countries, including Sweden and The Netherlands. 183, 207
Seroprevalence studies have shown that HEV natural infection is widespread in many species of wild and domestic mammals, including rats,208–212 cattle,115, 209 goats,127 wild mongooses,213 monkeys,214 dogs,215 and pet cats,216 with antibody positivity increasing with age in Japanese macaques and Japanese domestic pet cats.214, 216 Although these data show the prevalence of HEV circulation among animals, they have not established the possible role of these animals in transmitting HEV infection to humans. Of particular interest is the high HEV seroprevalence among wild rats,208–212 as these rodents, ubiquitous worldwide, have the potential to be infected with swine and human HEV strains. Rats may therefore be an important intermediate host between pigs and humans or, alternatively, a reservoir for both human and swine infection.
The majority of studies on animals other than swine have been more successful in detecting HEV antibodies than viral RNA. Although HEV RNA from genotypes 3 and 4 has been systematically found in pigs, boars, and deer, it has not yet been defined with certainty which genotypes circulate within other species. Two studies document HEV genotype 1 in horses217 and pigs.218 The finding of HEV genotype 1 in rats219 has subsequently been found to be a laboratory error.220 More studies are necessary to assess if HEV genotype 1 and 2 strains can induce sustainable infection in some animal species. In view of the large number of animal species that are potentially involved, further exploration of zoonotic transmission of HEV is warranted.23
HEV strains were identified in poultry as well as mammals; phylogenetic analysis indicates that avian HEV is genetically related to, but distinct from, mammalian HEV strains221, 222 and does not represent a risk for cross-infection to humans.
Pathogenesis, immune response, and time course of infection
It is thought that HEV infection initiates via cells lining the alimentary tract (primary site of virus replication) (Figure 4).223 The virus then reaches the liver through the portal vein41 and replicates in the cytoplasm of hepatocytes without causing direct cytolytic damage.223 Several observations suggest that, in analogy with other hepatitis viruses, liver injury is largely immune-mediated:150, 223, 224 first, viraemia precedes the onset of alanine transaminase elevation and liver histopathological changes;, 225–227 secondly, experimental infection of nonhuman primates has shown how the liver damage coincides with the detection of serum anti-HEV antibodies and with a decreasing level of HEV antigens in the hepatocytes;150, 225 and finally, the lymphocytes infiltrating the liver have a cytotoxic/suppressor immunophenotype.150, 225
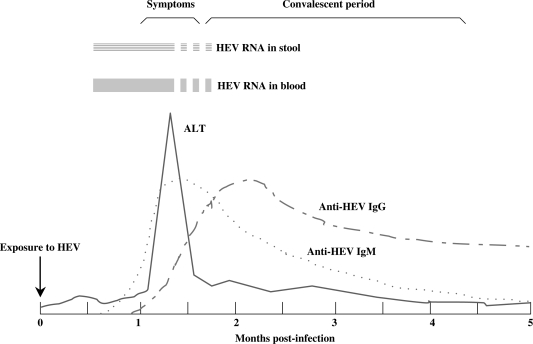
Figure 4.
Time course of HEV infection and specific humoral immune response. This figure shows the correlation of HEV RNA in stool and blood, sign of liver damage and development of anti-HEV antibody response with time. ALT, alanine aminotransferase; IgG, immunoglobulin G; IgM, immunoglobulin M.150, 153, 173
HEV RNA is detectable in blood from as early as two weeks before173 and for two to four weeks after the onset of symptoms.228–230 HEV faecal excretion shows a similar temporal pattern.229 Once liver function has normalised, HEV RNA is usually undetectable in blood and stool.229 Viraemia and faecal shedding beyond the duration of biochemical hepatitis are uncommon,229–231 suggesting that prolonged faecal shedding is not important in maintaining the environmental reservoir of HEV.229
The antibody responses are directed primarily against epitopes in the ORF2 and ORF3 proteins and are typically detectable at the onset of the disease, with IgM antibodies persisting for two to six months.223, 228, 232 Anti-HEV IgG appears soon after IgM, and persists for a longer period of time.41, 223, 228, 232, 233 However, the possibility of repeated infections being the cause of IgG persistence cannot be excluded.41
Overt disease in young adults is commonly the result of primary infection.234 The importance of antibodies in protecting from clinical hepatitis E has been proven experimentally in primates, in which passive immunisation with anti-HEV antibodies was able to protect them against overt disease after challenge with virulent HEV.235
Limited data are available on anti-HEV cellular immune response. Evidence for anti-HEV T-cell response was provided by a study on patients with acute hepatitis E whose Tlymphocytes showed sensitisation to HEV peptides.236 The same group was recently able to map CD4 T-cell epitopes in the ORF2 and ORF3 proteins of HEV using lymphocyte proliferation assays in patients with acute hepatitis E,237 providing the basis for future studies on the immunopathogenesis of hepatitis E. No data are currently available regarding anti-HEV-specific CD8 T-cell responses or the role of cellular responses in the protection against viral infection.
Of great interest are the mechanisms determining the severity of disease during pregnancy, in which fulminant hepatitis is a common complication. HEV infection studied in pregnant and nonpregnant healthy women has shown that infection in pregnancy is associated with a shift in the Th cell type 1/Th cell type 2 balance toward Th cell type 2 response.238 However, at this time it is difficult to link the clinical severity of the illness to this observation because the mechanism of liver injury in HEV infection has not yet been clarified.239 A recent Indian study suggested that a subset of CD4-positive interferon-γ-secreting cells, which do not belong to either the helper Th cell type 1 or type 2 phenotype, might be involved in liver damage during acute HEV infection.240
Clinical features
Acute infection
The incubation period ranges from two to 10 weeks with an average of 40 days., 43, 163, 228 Hepatitis E is indistinguishable from other forms of viral hepatitis. Typical clinical features are one- to 10-day prodrome of malaise, fever, gastrointestinal symptoms (abdominal pain, anorexia, nausea, vomiting), followed by the onset of jaundice.41, 150, 155, 161, 241 Once jaundice appears, prodromal symptoms subside. Clinical jaundice is not a constant feature and anicteric forms of hepatitis are well recognised.242 Serum investigations reveal raised levels of bilirubin (predominantly conjugated) and alanine aminotransferase. The magnitude of the alanine aminotransferase elevation does not correlate with the severity of the liver injury, better expressed by the liver synthetic function, as determined by coagulation function estimation. Acute infection resolves in one to four weeks; however, some patients develop a more prolonged clinical illness with cholestasis (cholestatic hepatitis).150 HEV infection is not known to progress to chronicity or cirrhosis2, 243 in immunocompetent patients.
Complications
A small proportion of patients develop fulminant or subacute hepatic failure with high mortality as a result of massive liver necrosis.150, 244, 245 Fulminant hepatitis E has been described worldwide but it is particularly common in developing countries among pregnant women, mainly during the third trimester.164, 239, 246–249 In this setting, HEV adversely affects both pregnant women and foetal outcome, with high mortality rate, increased frequency of abortions, preterm delivery, stillbirth, and neonatal death.23, 99, 148, 150, 239, 250
In Japan, HEV genotype 4 appears to cause severe hepatitis more frequently than genotype 3,23, 121, 251 possibly as a consequence of specific genomic mutations.119 In Argentina, a country not endemic for hepatitis E, fulminant hepatitis has been recently diagnosed in three children infected with HEV genotype 3.99
Severe forms of hepatitis E have also been increasingly documented among patients, mainly men, with stable chronic liver disease of different aetiologies, including chronic hepatitis B and C, autoimmune hepatitis, alcoholic liver disease, cryptogenetic hepatitis, and Wilson's disease.165–171, 252, 253
Prolonged mild hepatitis with viral shedding has been described in immunocompromised patients during chemotherapy for T-cell lymphoma.143, 254 More recently, the evolution of HEV infection into chronic hepatitis E has been reported in solid organ transplant patients in France:255, 256 they not only persistently shed the virus in the presence of deranged alanine aminotransferase values, but also showed histopathological changes similar to those observed in chronic hepatitis C.
Uncommon HEV infection complications, described in anecdotal reports, include the Guillain–Barré syndrome,257 acute transverse myelitis,258 acute pancreatitis,259 nonimmune haemolytic anaemia,260 lymphocytic destructive cholangitis,261 and prolonged polyarthritis.262
Asymptomatic infections
The number of asymptomatic infections far exceeds that of icteric hepatitis, as a large proportion of individuals who test positive for anti-HEV antibodies in highly endemic countries, such as India,150 China,28, 181 and Egypt,179 do not recall having suffered from jaundice. Similar data, based on seroprevalence, have been obtained from a variety of population profiles in developed countries: blood donors in Japan and the USA,140, 184 prisoners and drug users in Denmark,172 and individuals living in the community in Spain.182
Direct evidence of ongoing subclinical HEV infection in the general population comes from a study conducted in Honshu (Japan) on 6700 asymptomatic blood donors with elevated aminotranferase levels during a three-year period: about 3% of the individuals with an aminotransferase level of ≥201 IU/l (normal value <60 IU/l) were HEV RNA-positive. 140 Based on the number of asymptomatic viraemic individuals and the incidence of clinical hepatitis E in Honshu, the authors estimate that less than 0.1% of HEV-infected cases exhibit clinical manifestation of the infection.140 Asymptomatic viraemia has been detected in about 0.3% of individuals in rural eastern China.28
Diagnosis
Antibody detection
HEV antibody assays represent the routine diagnostic tool for acute hepatitis E cases. Test formats mostly consist of indirect enzyme-linked immunosorbent assays (EIA), with recombinant HEV proteins or peptides as detecting antigens. Currently available commercial assays are based mainly on HEV epidemic strains but EIA tests specific for genotypes 3 and 4 have also been developed.25, 128, 135 The specificity and sensitivity of these tests have not been established with precision, limiting the reliability of laboratory results.263
One of the most widely available commercial antibody assays, the Genelabs-EIA, uses short recombinant proteins derived from the 3’ termini of ORF2 (42 amino acids) and ORF3 (33 amino acids) from the Burmese (genotype 1) and Mexican (genotype 2) prototype sequences.263 However, according to Zhou et al.,175 a truncated form of the ORF2 protein, encompassing amino acids 112–607, contains the neutralisation epitopes (with amino acids 458–607 representing the major neutralisation site) and elicits the greatest and most long-lasting anti-HEV antibody response, being therefore suitable both for diagnostic and seroprevalence estimation purposes. In contrast, the amino acids 1–111 (Nterminus) and 607–660 (C-terminus) of the ORF2 protein and the ORF3 recombinant antigens elicit a weaker and transient antibody response, and are consequently of limited value in diagnosing acute HEV infection, and are not useful for seroprevalence studies.46, 176 Importantly, all genotypes share at least one major serologically crossreactive epitope, despite substantial genomic variability.62, 174
Diagnosis of acute hepatitis E is made by detecting HEV-specific IgM in acute-phase sera or by detecting a rise in anti-HEV IgG titre between acute and convalescent serum samples. Cases of aberrant IgM and IgG serological profiles, including those with immunologically silent acute hepatitis E, have been documented.84, 173 Although atypical serological profiles from patients with proven HEV RNA viraemia may be the expression of a modified immune response, the insensitivity of diagnostic assays should be taken into account when interpreting these data.101, 264, 265
Molecular detection
Reverse transcription-polymerase chain reaction (RT-PCR) assays represent the most commonly used molecular investigation for HEV genome detection. The usage of RT-PCR as a diagnostic tool has become feasible since the development of the real-time PCR platforms, closed systems that minimise the risk of contamination by the amplified target.
The majority of HEV RT-PCR assays used for diagnosis were developed as in-house assays by choosing different conserved HEV genomic regions as the target for amplification.230, 266–268 Considering the wide genetic heterogeneity of HEV isolates, it is critical to design primers and probes that guarantee the development of highly sensitive and broadly reactive assays.268
RT-PCR is a useful complementary diagnostic tool for the diagnosis of acute HEV infection, as it can confirm cases of hepatitis E with atypical serological profiles. RT-PCR assays are also critically important for public health purposes when used for detecting HEV-contaminated environmental samples.
Prevention and control of the infection
Active immunization
The observation that passive immune prophylaxis with convalescent serum samples prevented hepatitis E in primates has indicated that vaccination against HEV based on humoral immunity is feasible.55, 235, 269 This has prompted HEV immunisation studies based mainly on recombinant proteins, because the unavailability of an efficient cell culture system for HEV replication270 has precluded the development of vaccines based on inactivated or attenuated whole-virus particles. However, other approaches, such as DNA-based vaccines,271, 272 able to induce both cellular and antibody response, are also under evaluation.
The ORF2 protein has been considered the best candidate for HEV vaccine because it contains the neutralisation epitope located between amino acids 458 and 60756 and is crossreactive with all mammalian HEV.56, 273 Animal studies have shown that ORF2 recombinant proteins274–278 elicit neutralising antibodies and mediate protective immunity in vaccinated primates.277, 279
One such vaccine with a 56 kDa protein encompassing amino acids 112–60755, 280 was recently evaluated in young adults in Nepal in a phase 2, randomised, doubleblind, placebo-controlled trial.281 The study had encouraging results establishing that three doses of hepatitis E vaccine were 95.5% effective in protecting against clinical hepatitis E after a median of 804 days. The primary endpoint of this study was the prevention of clinically overt HEV infection, but the ability of the vaccine to prevent asymptomatic infection and asymptomatic virus shedding was not investigated.174 Asymptomatic HEV shedding in vaccine recipients, shown previously in primates,280 may be relevant in maintaining the environmental reservoir of HEV for human infection.174 Another aspect that this trial could not clarify is the duration of the induced immunity. Based on currently available data, this vaccine may be useful for travellers to highly endemic areas and for susceptible pregnant women,174 particularly during outbreaks. However, its use in children and adolescents in hepatitis E endemic countries,174 or in individuals with chronic liver disease, requires further study assessing the duration of its protective efficacy.
Protection of the environment and control of the outbreaks
The most important measure to prevent HEV infection is the protection of water supply from faecal contamination. Chlorination and filtration systems are generally inadequate if the source water is heavily contaminated.223 Travellers to highly endemic regions should strictly consume only bottled or boiled water.
During outbreaks, it is critical to provide clean water to all pregnant women. The isolation of individuals affected by acute hepatitis E is not justified because person-to-person transmission is uncommon. Infected people should refrain from food handling and food preparation.223
Conclusion
HEV infection has complex, and not yet completely clarified, clinical-epidemiological characteristics, which are summarised in Table 1. Two forms of infection have been identified: hepatitis E caused by epidemic strains, affecting mainly young adults and particularly pregnant women, and hepatitis E caused by zoonotic strains, which mostly affect older males.282 These differences in sex- and age-specific attack rates are puzzling because the route of transmission in developed countries, apart from Japan, has not yet been identified.
Table 1.
Clinical–epidemiological characteristics of HEV infection
| HEV Genotypes | Geographical distribution | Hosts | Mode of transmission | Epidemic versus sporadic forms | Clinical attack rate | Disease severity |
|---|---|---|---|---|---|---|
| 1 | Asia Africa | Humans? Animals | Contamination of water supply | Sporadic and epidemic forms | Highest in young adults | Highest in pregnant.women and individuals with chronic liver disease |
| 2 | Central America, Africa | |||||
| 3 | Asia, America, Europe, Oceania | Humans and a variety of animals, particularly swine | Food (Japan) | Sporadic and small outbreaks | Highest in older males | Highest in individuals with chronic liver disease |
| 4 | Asia | Food (Japan), Environmental contamination by swine waste |
Abbreviation: HEV, hepatitis E virus. Zoonotic strains (HEV genotype 3 and 4) cause infection both in developed and in developing countries (in italics), such as China.
More extensive epidemiological studies are needed, not only to assess the HEV seroprevalence in humans, but also in several animal species. For this purpose, the development of broadly reactive reliable antibody assays, which include immunodominant neutralisation antigens, is critical. Equally important is to establish which HEV genotypes circulate among the different animal species, and their role in human infections.
A high level of suspicion is needed in developed countries where the increasing number of recognised cases of hepatitis E16, 18, 100–102 suggests significant underdiagnosis.100 Awareness of HEV infection should exist in immunocompromised patients with signs of liver damage in view of the recent finding that hepatitis E can evolve to a chronic infection.255, 256
The results of a HEV vaccination phase 2 trial have provided encouraging preliminary data for the prevention of hepatitis E.281 Data are now awaited regarding the duration of the immune response induced by the vaccine before routine immunisation of children can be promoted in epidemic countries. It will also be important to establish the vaccine efficacy among older adults and the elderly, who are targets of overt HEV infection and in whom most cases of chronic liver disease, a risk factor for severe hepatitis E, concentrate.
Acknowledgements
Competing interest
The authors state no competing interests.
Provenance and peer review
Commissioned without payment, externally peer-reviewed.
References
- 1.Wong DC, Purcell RH, Sreenivasan MA, Prasad SR, Pavri KM. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet. 1980;2:876–9. doi: 10.1016/s0140-6736(80)92045-0. [DOI] [PubMed] [Google Scholar]
- 2.Khuroo MS, Saleem M, Teli MR, Sofi MA. Failure to detect chronic liver disease after epidemic non-A, non-B hepatitis. Lancet. 1980;2:97–8. doi: 10.1016/s0140-6736(80)92984-0. [DOI] [PubMed] [Google Scholar]
- 3.Jary C. Hepatitis E and meat carcasses. Br J Gen Pract. 2005;55:557–558. [PMC free article] [PubMed] [Google Scholar]
- 4.Boccia D, Guthmann JP, Klovstad H, Hamid N, Tatay M, Ciglenecki I, et al. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin Infect Dis. 2006;42:1679–84. doi: 10.1086/504322. [DOI] [PubMed] [Google Scholar]
- 5.Banait VS, Sandur V, Parikh F, Murugesh M, Ranka P, Ramesh VS, et al. Outcome of acute liver failure due to acute hepatitis E in pregnant women. Indian J Gastroenterol. 2007;26:6–10. [PubMed] [Google Scholar]
- 6.Reuter G, Fodor D, Szucs G. Molecular detection of hepatitis E virus in non-imported hepatitis E cases: identification of a potential new human hepatitis E virus lineage in Hungary. Orv ‘Hetil. 2005;146:2389–94. article in Hungarian. [PubMed] [Google Scholar]
- 7.Preiss JC, Plentsz A, Engelman E, Scneider T, Jilq W, Zeitz M, et al. Autochthonous hepatitis E virus infection in Germany with sequence similarities to other European isolates. Infection. 2006;24:173–5. doi: 10.1007/s15010-006-4132-x. [DOI] [PubMed] [Google Scholar]
- 8.Widdowson MA, Jaspers WJ, van der Poel WH, Verschoor F, de Roda Husman AM, Winter HL, et al. Cluster of cases of acute hepatitis associated with hepatitis E virus infection acquired in The Netherlands. Clin Infect Dis. 2003;36:29–33. doi: 10.1086/345439. [DOI] [PubMed] [Google Scholar]
- 9.Worm HC, Wurzer H, Frosner G. Sporadic hepatitis E in Austria. N Engl J Med. 1998;339:1554–5. doi: 10.1056/NEJM199811193392115. [DOI] [PubMed] [Google Scholar]
- 10.Mansuy JM, Peron JM, Abravanel F, Poirson H, Dubois M, Miedouge M, et al. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J Med Virol. 2004;74:419–24. doi: 10.1002/jmv.20206. [DOI] [PubMed] [Google Scholar]
- 11.Dalton HR, Fellows HJ, Gane EJ, Wong P, Gerred S, Schroeder B, et al. Hepatitis E in New Zealand. J Gastroenterol Hepatol. 2007;22:1236–40. doi: 10.1111/j.1440-1746.2007.04894.x. [DOI] [PubMed] [Google Scholar]
- 12.Schlauder GG, Desai SM, Zanetti AR, Tassopoulos NC, Mushahwar IK. Novel hepatitis E virus (HEV) isolates from Europe: evidence for additional genotypes of HEV. J Med Virol. 1999;57:243–51. doi: 10.1002/(sici)1096-9071(199903)57:3<243::aid-jmv6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Schlauder GG, Dawson GJ, Erker JC, Kwo PY, Knigge MF, Smalley DL, et al. The sequence and phylogenetic analysis of a novel hepatitis E virus isolated from a patient with acute hepatitis reported in the United States. J Gen Virol. 1998;79(Pt 3):447–56. doi: 10.1099/0022-1317-79-3-447. [DOI] [PubMed] [Google Scholar]
- 14.Teo CG. Hepatitis E indigenous to economically developed countries: to what extent a zoonosis? Curr Opin Infect Dis. 2006;19:460–6. doi: 10.1097/01.qco.0000244052.61629.49. [DOI] [PubMed] [Google Scholar]
- 15.Zanetti AR, Schlauder GG, Romano L, Tanzi E, Fabris P, Dawson GJ, et al. Identification of a novel variant of hepatitis E virus in Italy. J Med Virol. 1999;57:356–60. doi: 10.1002/(sici)1096-9071(199904)57:4<356::aid-jmv5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Ijaz S, Arnold E, Banks M, Bendall RP, Cramp ME, Cunningham R, et al. Non-travel-associated hepatitis E in England and Wales: demographic, clinical, and molecular epidemiological characteristics. J Infect Dis. 2005;192:1166–72. doi: 10.1086/444396. [DOI] [PubMed] [Google Scholar]
- 17.Amon JJ, Drobeniuc J, Bower WA, Magana JC, Escobedo MA, Williams IT, et al. Locally acquired hepatitis E virus infection, El Paso, Texas. J Med Virol. 2006;78:741–6. doi: 10.1002/jmv.20617. [DOI] [PubMed] [Google Scholar]
- 18.Dalton HR, Thurairajah PH, Fellows HJ, Hussaini HS, Mitchell J, Bendall R, et al. Autochthonous hepatitis E in southwest England. J Viral Hepat. 2007;14:304–9. doi: 10.1111/j.1365-2893.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 19.Clemente-Casares P, Pina S, Buti M, Jardi R, MartIn M, Bofill-Mas S, et al. Hepatitis E virus epidemiology in industrialized countries. Emerg Infect Dis. 2003;9:448–54. doi: 10.3201/eid0904.020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buti M, Clemente-Casares P, Jardi R, Formiga-Cruz M, Schaper M, Valdes A, et al. Sporadic cases of acute autochthonous hepatitis E in Spain. J Hepatol. 2004;41:126–31. doi: 10.1016/j.jhep.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 21.McCrudden R, O'Connell S, Farrant T, Beaton S, Iredale JP, Fine D. Sporadic acute hepatitis E in the United Kingdom: an underdiagnosed phenomenon? Gut. 2000;46:732–3. doi: 10.1136/gut.46.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine DF, Bendall RP, Teo CG. Hepatitis E acquired in the UK. Gut. 2000;47:740. doi: 10.1136/gut.47.5.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuo H, Yazaki Y, Sugawara K, Tsuda F, Takahashi M, Nishizawa T, et al. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol. 2005;76:341–9. doi: 10.1002/jmv.20364. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto H, Takahashi M, Nishizawa T. Features of hepatitis E virus infection in Japan. Intern Med. 2003;42:1065–71. doi: 10.2169/internalmedicine.42.1065. [DOI] [PubMed] [Google Scholar]
- 25.Mizuo H, Suzuki K, Takikawa Y, Sugai Y, Tokita H, Akahane Y, et al. Polyphyletic strains of hepatitis E virus are responsible for sporadic cases of acute hepatitis in Japan. J Clin Microbiol. 2002;40:3209–18. doi: 10.1128/JCM.40.9.3209-3218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwo PY, Schlauder GG, Carpenter HA, Murphy PJ, Rosenblatt JE, Dawson GJ, et al. Acute hepatitis E by a new isolate acquired in the United States. Mayo Clin Proc. 1997;72:1133–6. doi: 10.4065/72.12.1133. [DOI] [PubMed] [Google Scholar]
- 27.Herremans M, Vennema H, Bakker J, van der Veer B, Duizer E, Benne CA, et al. Swine-like hepatitis E viruses are a cause of unexplained hepatitis in The Netherlands. J Viral Hepat. 2007;14:140–6. doi: 10.1111/j.1365-2893.2006.00786.x. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Ge S, Zhang J, Guo Q, Ng MH, Wang F, et al. Swine as a principal reservoir of hepatitis E virus that infects humans in eastern China. J Infect Dis. 2006;193:1643–9. doi: 10.1086/504293. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y, Takahashi K, Orito E, Karino Y, Kang JH, Suzuki K, et al. Molecular tracing of Japan-indigenous hepatitis E viruses. J Gen Virol. 2006;87(Pt 4):949–54. doi: 10.1099/vir.0.81661-0. [DOI] [PubMed] [Google Scholar]
- 30.Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Detection and characterization of infectious hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol. 2007;88(Pt 3):912–17. doi: 10.1099/vir.0.82613-0. [DOI] [PubMed] [Google Scholar]
- 31.Jung K, Kang B, Song DS, Chae C. Prevalence and genotyping of hepatitis E virus in swine population in Korea between 1995 and 2004: a retrospective study. Vet J. 2007;173:683–7. doi: 10.1016/j.tvjl.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Munne MS, Vladimirsky S, Otegui L, Castro R, Brajterman L, Soto S, et al. Identification of the first strain of swine hepatitis E virus in South America and prevalence of anti-HEV antibodies in swine in Argentina. J Med Virol. 2006;78:1579–83. doi: 10.1002/jmv.20741. [DOI] [PubMed] [Google Scholar]
- 33.Cooper K, Huang FF, Batista L, Rayo CD, Bezanilla JC, Toth TE, et al. Identification of genotype 3 hepatitis E virus (HEV) in serum and fecal samples from pigs in Thailand and Mexico, where genotype 1 and 2 HEV strains are prevalent in the respective human populations. J Clin Microbiol. 2005;43:1684–8. doi: 10.1128/JCM.43.4.1684-1688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banks M, Heath GS, Grierson SS, King DP, Gresham A, Girones R, et al. Evidence for the presence of hepatitis E virus in pigs in the United Kingdom. Vet Rec. 2004;154:223–7. doi: 10.1136/vr.154.8.223. [DOI] [PubMed] [Google Scholar]
- 35.van der Poel WH, Verschoor F, van der Heide R, Herrera MI, Vivo A, Kooreman M, et al. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg Infect Dis. 2001;7:970–6. doi: 10.3201/eid0706.010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seminati C, Mateu E, Peralta B, de Deus N, Martin M. Distribution of hepatitis E virus infection and its prevalence in pigs on commercial farms in Spain. Vet J. 2008;175:130–2. doi: 10.1016/j.tvjl.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or nearcomplete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–5. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Masuda J, Yano K, Tamada Y, Takii Y, Ito M, Omagari K, et al. Acute hepatitis E of a man who consumed wild boar meat prior to the onset of illness in Nagasaki, Japan. Hepatol Res. 2005;31:178–83. doi: 10.1016/j.hepres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, et al. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol. 2003;84(Pt 9):2351–7. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- 40.Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–3. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 41.Panda SK, Thakral D, Rehman S. Hepatitis E virus. Rev Med Virol. 2007;17:151–80. doi: 10.1002/rmv.522. [DOI] [PubMed] [Google Scholar]
- 42.Corwin AL, Tien NT, Bounlu K, Winarno J, Putri MP, Laras K, et al. The unique riverine ecology of hepatitis E virus transmission in South-East Asia. Trans R Soc Trop Med Hyg. 1999;93:255–60. doi: 10.1016/s0035-9203(99)90014-7. [DOI] [PubMed] [Google Scholar]
- 43.Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal–oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 44.Reyes GR, Purdy MA, Kim JP, Luk KC, Young LM, Fry KE, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247:1335–9. doi: 10.1126/science.2107574. [DOI] [PubMed] [Google Scholar]
- 45.Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, et al. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–31. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawson GJ, Chau KH, Cabal CM, Yarbough PO, Reyes GR, Mushahwar IK. Solid-phase enzyme-linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides. J Virol Methods. 1992;38:175–86. doi: 10.1016/0166-0934(92)90180-l. [DOI] [PubMed] [Google Scholar]
- 47.Berke T, Golding B, Jiang X, Cubitt DW, Wolfaardt M, Smith AW, et al. Phylogenetic analysis of the Caliciviruses. J Med Virol. 1997;52:419–24. doi: 10.1002/(sici)1096-9071(199708)52:4<419::aid-jmv13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 48.Emerson SU, Anderson D, Arankalle A, Meng XJ, Purdy M, Schlauder GG, et al. Hepevirus. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: VIIIth Report of the ICTV. London: Elsevier/Academic Press; 2004. pp. 851–5. [Google Scholar]
- 49.Aye TT, Uchida T, Ma XZ, Iida F, Shikata T, Zhuang H, et al. Complete nucleotide sequence of a hepatitis E virus isolated from the Xinjiang epidemic (1986–1988) of China. Nucleic Acids Res. 1992;20:3512. doi: 10.1093/nar/20.13.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes GR, Huang CC, Tam AW, Purdy MA. Molecular organization and replication of hepatitis E virus (HEV) Arch Virol Suppl. 1993;7:15–25. doi: 10.1007/978-3-7091-9300-6_2. [DOI] [PubMed] [Google Scholar]
- 51.Graff J, Torian U, Nguyen H, Emerson SU. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J Virol. 2006;80:5919–26. doi: 10.1128/JVI.00046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamoto H. Genetic variability and evolution of hepatitis E virus. Virus Res. 2007;127:216–28. doi: 10.1016/j.virusres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Zhang H, Ling R, Li H, Harrison TJ. The complete sequence of hepatitis E virus genotype 4 reveals an alternative strategy for translation of open reading frames 2 and 3. J Gen Virol. 2000;81(Pt 7):1675–86. doi: 10.1099/0022-1317-81-7-1675. [DOI] [PubMed] [Google Scholar]
- 54.Kabrane-Lazizi Y, Meng XJ, Purcell RH, Emerson SU. Evidence that the genomic RNA of hepatitis E virus is capped. J Virol. 1999;73:8848–50. doi: 10.1128/jvi.73.10.8848-8850.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emerson SU, Purcell RH. Recombinant vaccines for hepatitis E. Trends Mol Med. 2001;7:462–6. doi: 10.1016/s1471-4914(01)02106-2. [DOI] [PubMed] [Google Scholar]
- 56.Zhou YH, Purcell RH, Emerson SU. A truncated ORF2 protein contains the most immunogenic site on ORF2: antibody responses to non-vaccine sequences following challenge of vaccinated and non-vaccinated macaques with hepatitis E virus. Vaccine. 2005;23:3157–65. doi: 10.1016/j.vaccine.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Xing L, Kato K, Li T, Takeda N, Miyamura T, Hammar L, et al. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T¼1 particle presenting native virus epitopes. Virology. 1999;265:35–45. doi: 10.1006/viro.1999.0005. [DOI] [PubMed] [Google Scholar]
- 58.Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, et al. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J Virol. 1997;71:7207–13. doi: 10.1128/jvi.71.10.7207-7213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surjit M, Jameel S, Lal SK. The ORF2 protein of hepatitis E virus binds the 50 region of viral RNA. J Virol. 2004;78:320–8. doi: 10.1128/JVI.78.1.320-328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tyagi S, Korkaya H, Zafrullah M, Jameel S, Lal SK. The phosphorylated form of the ORF3 protein of hepatitis E virus interacts with its non-glycosylated form of the major capsid protein, ORF2. J Biol Chem. 2002;277:22759–67. doi: 10.1074/jbc.M200185200. [DOI] [PubMed] [Google Scholar]
- 61.Zafrullah M, Ozdener MH, Panda SK, Jameel S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol. 1997;71:9045–53. doi: 10.1128/jvi.71.12.9045-9053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emerson SU, Purcell RH. Hepatitis E virus. Rev Med Virol. 2003;13:145–54. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- 63.Korkaya H, Jameel S, Gupta D, Tyagi S, Kumar R, Zafrullah M, et al. The ORF3 protein of hepatitis E virus binds to Src homology 3 domains and activates MAPK. J Biol Chem. 2001;276:42389–400. doi: 10.1074/jbc.M101546200. [DOI] [PubMed] [Google Scholar]
- 64.Huang YW, Opriessnig T, Halbur PG, Meng XJ. Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo . J Virol. 2007;81:3018–26. doi: 10.1128/JVI.02259-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Emerson SU, Nguyen H, Torian U, Purcell RH. ORF3 protein of hepatitis E virus is not required for replication, virion assembly, or infection of hepatoma cells in vitro . J Virol. 2006;80:10457–64. doi: 10.1128/JVI.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agrawal S, Gupta D, Panda SK. The 30 end of hepatitis E virus (HEV) genome binds specifically to the viral RNA-dependent RNA polymerase (RdRp) Virology. 2001;282:87–101. doi: 10.1006/viro.2000.0819. [DOI] [PubMed] [Google Scholar]
- 67.Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis. 2005;192:930–3. doi: 10.1086/432488. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka T, Takahashi M, Kusano E, Okamoto H. Development and evaluation of an efficient cell-culture system for hepatitis E virus. J Gen Virol. 2007;88(Pt 3):903–11. doi: 10.1099/vir.0.82535-0. [DOI] [PubMed] [Google Scholar]
- 69.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 70.Zhai L, Dai X, Meng J. Hepatitis E virus genotyping based on full-length genome and partial genomic regions. Virus Res. 2006;120:57–69. doi: 10.1016/j.virusres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 71.Huang R, Li D, Wei S, Li Q, Yuan X, Geng L, et al. Cell culture of sporadic hepatitis E virus in China. Clin Diagn Lab Immunol. 1999;6:729–33. doi: 10.1128/cdli.6.5.729-733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wei S, Walsh P, Huang R, To SS. 93G, a novel sporadic strain of hepatitis E virus in South China isolated by cell culture. J Med Virol. 2000;61:311–18. doi: 10.1002/1096-9071(200007)61:3<311::aid-jmv5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 73.Tam AW, White R, Reed E, Short M, Zhang Y, Fuerst TR, et al. In vitro propagation and production of hepatitis E virus from in vivo-infected primary macaque hepatocytes. Virology. 1996;215:1–9. doi: 10.1006/viro.1996.0001. [DOI] [PubMed] [Google Scholar]
- 74.Emerson SU, Clemente-Casares P, Moiduddin N, Arankalle VA, Torian U, Purcell RH. Putative neutralization epitopes and broad cross-genotype neutralization of hepatitis E virus confirmed by a quantitative cell-culture assay. J Gen Virol. 2006;87(Pt 3):697–704. doi: 10.1099/vir.0.81545-0. [DOI] [PubMed] [Google Scholar]
- 75.Jameel S, Zafrullah M, Chawla YK, Dilawari JB. Reevaluation of a North India isolate of hepatitis E virus based on the full-length genomic sequence obtained following long RT-PCR. Virus Res. 2002;86:53–8. doi: 10.1016/s0168-1702(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 76.Aggarwal R, McCaustland KA, Dilawari JB, Sinha SD, Robertson BH. Genetic variability of hepatitis E virus within and between three epidemics in India. Virus Res. 1999;59:35–48. doi: 10.1016/s0168-1702(98)00123-3. [DOI] [PubMed] [Google Scholar]
- 77.Arankalle VA, Paranjape S, Emerson SU, Purcell RH, Walimbe AM. Phylogenetic analysis of hepatitis E virus isolates from India (1976–1993) J Gen Virol. 1999;80(Pt 7):1691–700. doi: 10.1099/0022-1317-80-7-1691. [DOI] [PubMed] [Google Scholar]
- 78.Vaidya SR, Chitambar SD, Arankalle VA. Polymerase chain reaction-based prevalence of hepatitis A, hepatitis E and TT viruses in sewage from an endemic area. J Hepatol. 2002;37:131–136. doi: 10.1016/s0168-8278(02)00106-x. [DOI] [PubMed] [Google Scholar]
- 79.Tsarev SA, Emerson SU, Reyes GR, Tsareva TS, Legters LJ, Malik IA, et al. Characterization of a prototype strain of hepatitis E virus. Proc Natl Acad Sci USA. 1992;89:559–63. doi: 10.1073/pnas.89.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shrestha SM, Shrestha S, Tsuda F, Nishizawa T, Gotanda Y, Takeda N, et al. Molecular investigation of hepatitis E virus infection in patients with acute hepatitis in Kathmandu, Nepal. J Med Virol. 2003;69:207–14. doi: 10.1002/jmv.10276. [DOI] [PubMed] [Google Scholar]
- 81.Shrestha SM, Shrestha S, Tsuda F, Nishizawa T, Takahashi M, Gotanda Y, et al. Genetic changes in hepatitis E virus of subtype 1a in patients with sporadic acute hepatitis E in Kathmandu, Nepal, from 1997 to 2002. J Gen Virol. 2004;85(Pt 1):97–104. doi: 10.1099/vir.0.19571-0. [DOI] [PubMed] [Google Scholar]
- 82.Drabick JJ, Gambel JM, Gouvea VS, Caudill JD, Sun W, Hoke CH, Jr, et al. A cluster of acute hepatitis E infection in United Nations Bangladeshi peacekeepers in Haiti. Am J Trop Med Hyg. 1997;57:449–54. doi: 10.4269/ajtmh.1997.57.449. [DOI] [PubMed] [Google Scholar]
- 83.Bi SL, Purdy MA, McCaustland KA, Margolis HS, Bradley DW. The sequence of hepatitis E virus isolated directly from a single source during an outbreak in China. Virus Res. 1993;28:233–47. doi: 10.1016/0168-1702(93)90024-h. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Zhang H, Li Z, Gu W, Lan H, Hao W, et al. Detection of sporadic cases of hepatitis E virus (HEV) infection in China using immunoassays based on recombinant open reading frame 2 and 3 polypeptides from HEV genotype 4. J Clin Microbiol. 2001;39:4370–4379. doi: 10.1128/JCM.39.12.4370-4379.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin S, Purcell RH, Emerson SU. A new Chinese isolate of hepatitis E virus: comparison with strains recovered from different geographical regions. Virus Genes. 1994;9:23–32. doi: 10.1007/BF01703432. [DOI] [PubMed] [Google Scholar]
- 86.Lu L, Drobeniuc J, Kobylnikov N, Usmanov RK, Robertson BH, Favorov MO, et al. Complete sequence of a Kyrgyzstan swine hepatitis E virus (HEV) isolated from a piglet thought to be experimentally infected with human HEV. J Med Virol. 2004;74:556–562. doi: 10.1002/jmv.20214. [DOI] [PubMed] [Google Scholar]
- 87.Chatterjee R, Tsarev S, Pillot J, Coursaget P, Emerson SU, Purcell RH. African strains of hepatitis E virus that are distinct from Asian strains. J Med Virol. 1997;53:139–44. [PubMed] [Google Scholar]
- 88.Tsarev SA, Binn LN, Gomatos PJ, Arthur RR, Monier MK, van Cuyck-Gandre H, et al. Phylogenetic analysis of hepatitis E virus isolates from Egypt. J Med Virol. 1999;57:68–74. doi: 10.1002/(sici)1096-9071(199901)57:1<68::aid-jmv10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 89.Grandadam M, Tebbal S, Caron M, Siriwardana M, Larouze B, Koeck JL, et al. Evidence for hepatitis E virus quasispecies. J Gen Virol. 2004;85(Pt 11):3189–94. doi: 10.1099/vir.0.80248-0. [DOI] [PubMed] [Google Scholar]
- 90.He J, Binn LN, Tsarev SA, Hayes CG, Frean JA, Isaacson M, et al. Molecular characterization of a hepatitis E virus isolate from Namibia. J Biomed Sci. 2000;7:334–8. doi: 10.1007/BF02253253. [DOI] [PubMed] [Google Scholar]
- 91.Nicand E, Armstrong GL, Enouf V, Guthmann JP, Guerin JP, Caron M, et al. Genetic heterogeneity of hepatitis E virus in Darfur, Sudan, and neighboring Chad. J Med Virol. 2005;77:519–21. doi: 10.1002/jmv.20487. [DOI] [PubMed] [Google Scholar]
- 92.van Cuyck H, Juge F, Roques P. Phylogenetic analysis of the first complete hepatitis E virus (HEV) genome from Africa. FEMS Immunol Med Microbiol. 2003;39:133–9. doi: 10.1016/S0928-8244(03)00241-4. [DOI] [PubMed] [Google Scholar]
- 93.Huang CC, Nguyen D, Fernandez J, Yun KY, Fry KE, Bradley DW, et al. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV) Virology. 1992;191:550–8. doi: 10.1016/0042-6822(92)90230-m. [DOI] [PubMed] [Google Scholar]
- 94.Buisson Y, Grandadam M, Nicand E, Cheval P, van Cuyck- Gandre H, Innis B, et al. Identification of a novel hepatitis E virus in Nigeria. J Gen Virol. 2000;81(Pt 4):903–9. doi: 10.1099/0022-1317-81-4-903. [DOI] [PubMed] [Google Scholar]
- 95.Maila HT, Bowyer SM, Swanepoel R. Identification of a new strain of hepatitis E virus from an outbreak in Namibia in 1995. J Gen Virol. 2004;85(Pt 1):89–95. doi: 10.1099/vir.0.19587-0. [DOI] [PubMed] [Google Scholar]
- 96.Huang FF, Haqshenas G, Guenette DK, Halbur PG, Schommer SK, Pierson FW, et al. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J Clin Microbiol. 2002;40:1326–32. doi: 10.1128/JCM.40.4.1326-1332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kabrane-Lazizi Y, Zhang M, Purcell RH, Miller KD, Davey RT, Emerson SU. Acute hepatitis caused by a novel strain of hepatitis E virus most closely related to United States strains. J Gen Virol. 2001;82(Pt 7):1687–93. doi: 10.1099/0022-1317-82-7-1687. [DOI] [PubMed] [Google Scholar]
- 98.Leblanc D, Ward P, Gagne MJ, Poitras E, Muller P, Trottier YL, et al. Presence of hepatitis E virus in a naturally infected swine herd from nursery to slaughter. Int J Food Microbiol. 2007;177:160–166. doi: 10.1016/j.ijfoodmicro.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 99.Munne MS, Vladimirsky S, Otegui L, Brajterman L, Castro R, Soto S, et al. Molecular characterization of hepatitis E virus in three acute liver failure cases in children in Argentina. Acta Gastroenterol Latinoam. 2006;36:125–30. [PubMed] [Google Scholar]
- 100.Lewis H, Morgan D, Ijaz S, Boxall E. Indigenous hepatitis E virus infection in England and Wales. BMJ. 2006;332:1509–10. doi: 10.1136/bmj.332.7556.1509-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Silva AN, Muddu AK, Iredale JP, Sheron N, Khakoo SI, Pelosi E. Unexpectedly high incidence of indigenous acute hepatitis E within South Hampshire: time for routine testing? J Med Virol. 2008;80:283–8. doi: 10.1002/jmv.21062. [DOI] [PubMed] [Google Scholar]
- 102.Peron JM, Mansuy JM, Poirson H, Bureau C, Dupuis E, Alric L, et al. Hepatitis E is an autochthonous disease in industrialized countries. Analysis of 23 patients in South-West France over a 13-month period and comparison with hepatitis A. Gastroenterol Clin Biol. 2006;30:757–62. doi: 10.1016/s0399-8320(06)73310-3. [DOI] [PubMed] [Google Scholar]
- 103.Takahashi K, Kang JH, Ohnishi S, Hino K, Mishiro S. Genetic heterogeneity of hepatitis E virus recovered from Japanese patients with acute sporadic hepatitis. J Infect Dis. 2002;185:1342–5. doi: 10.1086/340023. [DOI] [PubMed] [Google Scholar]
- 104.Takahashi K, Iwata K, Watanabe N, Hatahara T, Ohta Y, Baba K, et al. Full-genome nucleotide sequence of a hepatitis E virus strain that may be indigenous to Japan. Virology. 2001;287:9–12. doi: 10.1006/viro.2001.1017. [DOI] [PubMed] [Google Scholar]
- 105.Matsubayashi K, Nagaoka Y, Sakata H, Sato S, Fukai K, Kato T, et al. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion. 2004;44:934–40. doi: 10.1111/j.1537-2995.2004.03300.x. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi M, Nishizawa T, Okamoto H. Identification of a genotype III swine hepatitis E virus that was isolated from a Japanese pig born in 1990 and that is most closely related to Japanese isolates of human hepatitis E virus. J Clin Microbiol. 2003;41:1342–3. doi: 10.1128/JCM.41.3.1342-1343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okamoto H, Takahashi M, Nishizawa T, Fukai K, Muramatsu U, Yoshikawa A. Analysis of the complete genome of indigenous swine hepatitis E virus isolated in Japan. Biochem Biophys Res Commun. 2001;289:929–36. doi: 10.1006/bbrc.2001.6088. [DOI] [PubMed] [Google Scholar]
- 108.Tokita H, Harada H, Gotanda Y, Takahashi M, Nishizawa T, Okamoto H. Molecular and serological characterization of sporadic acute hepatitis E in a Japanese patient infected with a genotype III hepatitis E virus in 1993. J Gen Virol. 2003;84(Pt 2):421–7. doi: 10.1099/vir.0.18802-0. [DOI] [PubMed] [Google Scholar]
- 109.Takahashi M, Nishizawa T, Miyajima H, Gotanda Y, Iita T, Tsuda F, et al. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J Gen Virol. 2003;84(Pt 4):851–62. doi: 10.1099/vir.0.18918-0. [DOI] [PubMed] [Google Scholar]
- 110.Tamada Y, Yano K, Yatsuhashi H, Inoue O, Mawatari F, Ishibashi H. Consumption of wild boar linked to cases of hepatitis E. J Hepatol. 2004;40:869–70. doi: 10.1016/j.jhep.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 111.Ning H, Yu S, Zhu Y, Dong S, Yu R, Shen S, et al. Genotype 3 hepatitis E has been widespread in pig farms of Shanghai suburbs. Vet Microbiol. 2008;126:257–63. doi: 10.1016/j.vetmic.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 112.Arankalle VA, Chobe LP, Joshi MV, Chadha MS, Kundu B, Walimbe AM. Human and swine hepatitis E viruses from Western India belong to different genotypes. J Hepatol. 2002;36:417–25. doi: 10.1016/s0168-8278(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 113.Arankalle VA, Chobe LP, Walimbe AM, Yergolkar PN, Jacob GP. Swine HEV infection in south India and phylogenetic analysis (1985–1999) J Med Virol. 2003;69:391–6. doi: 10.1002/jmv.10301. [DOI] [PubMed] [Google Scholar]
- 114.Li K, Zhuang H, Zhu W. Partial nucleotide sequencing of hepatitis E viruses detected in sera of patients with hepatitis E from 14 cities in China. Chin Med J (Engl) 2002;115:1058–63. [PubMed] [Google Scholar]
- 115.Wang YC, Zhang HY, Xia NS, Peng G, Lan HY, Zhuang H, et al. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J Med Virol. 2002;67:516–21. doi: 10.1002/jmv.10131. [DOI] [PubMed] [Google Scholar]
- 116.Nishizawa T, Takahashi M, Mizuo H, Miyajima H, Gotanda Y, Okamoto H. Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99% identity over the entire genome. J Gen Virol. 2003;84(Pt 5):1245–51. doi: 10.1099/vir.0.19052-0. [DOI] [PubMed] [Google Scholar]
- 117.Wibawa ID, Muljono DH, Mulyanto , Suryadarma IG, Tsuda F, Takahashi M, et al. Prevalence of antibodies to hepatitis E virus among apparently healthy humans and pigs in Bali, Indonesia: identification of a pig infected with a genotype 4 hepatitis E virus. J Med Virol. 2004;73:38–44. doi: 10.1002/jmv.20059. [DOI] [PubMed] [Google Scholar]
- 118.Hijikata M, Hayashi S, Trinh NT, Ha le D, Ohara H, Shimizu YK, et al. Genotyping of hepatitis E virus from Vietnam. Intervirology. 2002;45:101–4. doi: 10.1159/000063231. [DOI] [PubMed] [Google Scholar]
- 119.Inoue J, Nishizawa T, Takahashi M, Aikawa T, Mizuo H, Suzuki K, et al. Analysis of the full-length genome of genotype 4 hepatitis E virus isolates from patients with fulminant or acute selflimited hepatitis E. J Med Virol. 2006;78:476–84. doi: 10.1002/jmv.20565. [DOI] [PubMed] [Google Scholar]
- 120.Ahn JM, Kang SG, Lee DY, Shin SJ, Yoo HS. Identification of novel human hepatitis E virus (HEV) isolates and determination of the seroprevalence of HEV in Korea. J Clin Microbiol. 2005;43:3042–8. doi: 10.1128/JCM.43.7.3042-3048.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suzuki K, Aikawa T, Okamoto H. Fulminant hepatitis E in Japan. N Engl J Med. 2002;347:1456. doi: 10.1056/NEJM200210313471819. [DOI] [PubMed] [Google Scholar]
- 122.Takahashi M, Nishizawa T, Yoshikawa A, Sato S, Isoda N, Ido K, et al. Identification of two distinct genotypes of hepatitis E virus in a Japanese patient with acute hepatitis who had not travelled abroad. J Gen Virol. 2002;83(Pt 8):1931–40. doi: 10.1099/0022-1317-83-8-1931. [DOI] [PubMed] [Google Scholar]
- 123.Michitaka K, Takahashi K, Furukawa S, Inoue G, Hiasa Y, Horiike N, et al. Prevalence of hepatitis E virus among wild boar in the Ehime area of western Japan. Hepatol Res. 2007;37:214–20. doi: 10.1111/j.1872-034X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 124.Martelli F, Caprioli A, Zengarini M, Marata A, Fiegna C, Di Bartolo I, et al. Detection of hepatitis E virus (HEV) in a demographic managed wild boar (Sus scrofa scrofa) population in Italy. Vet Microbiol. 2008;126:74–81. doi: 10.1016/j.vetmic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Banks M, Bendall R, Grierson S, Heath G, Mitchell J, Dalton H. Human and porcine hepatitis E virus strains, United Kingdom. Emerg Infect Dis. 2004;10:953–5. doi: 10.3201/eid1005.030908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pei Y, Yoo D. Genetic characterization and sequence heterogeneity of a Canadian isolate of swine hepatitis E virus. J Clin Microbiol. 2002;40:4021–9. doi: 10.1128/JCM.40.11.4021-4029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shukla P, Chauhan UK, Naik S, Anderson D, Aggarwal R. Hepatitis E virus infection among animals in northern India: an unlikely source of human disease. J Viral Hepat. 2007;14:310–17. doi: 10.1111/j.1365-2893.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 128.Arankalle VA, Lole KS, Deshmukh TM, Chobe LP, Gandhe SS. Evaluation of human (genotype 1) and swine (genotype 4)- ORF2-based ELISAs for anti-HEV IgM and IgG detection in an endemic country and search for type 4 human HEV infections. J Viral Hepat. 2007;14:435–45. doi: 10.1111/j.1365-2893.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 129.Chobe LP, Lole KS, Arankalle VA. Full genome sequence and analysis of Indian swine hepatitis E virus isolate of genotype 4. Vet Microbiol. 2006;114:240–51. doi: 10.1016/j.vetmic.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 130.Jothikumar N, Aparna K, Kamatchiammal S, Paulmurugan R, Saravanadevi S, Khanna P. Detection of hepatitis E virus in raw and treated wastewater with the polymerase chain reaction. Appl Environ Microbiol. 1993;59:2558–62. doi: 10.1128/aem.59.8.2558-2562.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pina S, Jofre J, Emerson SU, Purcell RH, Girones R. Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Appl Environ Microbiol. 1998;64:4485–8. doi: 10.1128/aem.64.11.4485-4488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li TC, Miyamura T, Takeda N. Detection of hepatitis E virus RNA from the bivalve Yamato-Shijimi (Corbicula japonica) in Japan. Am J Trop Med Hyg. 2007;76:170–2. [PubMed] [Google Scholar]
- 133.Li TC, Chijiwa K, Sera N, Ishibashi T, Etoh Y, Shinohara Y, et al. Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis. 2005;11:1958–60. doi: 10.3201/eid1112.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tei S, Kitajima N, Ohara S, Inoue Y, Miki M, Yamatani T, et al. Consumption of uncooked deer meat as a risk factor for hepatitis E virus infection: an age- and sex-matched case–control study. J Med Virol. 2004;74:67–70. doi: 10.1002/jmv.20147. [DOI] [PubMed] [Google Scholar]
- 135.Arankalle VA, Chadha MS, Mehendale SM, Tungatkar SP. Epidemic hepatitis E: serological evidence for lack of intrafamilial spread. Indian J Gastroenterol. 2000;19:24–8. [PubMed] [Google Scholar]
- 136.Naik SR, Aggarwal R, Salunke PN, Mehrotra NN. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597–604. [PMC free article] [PubMed] [Google Scholar]
- 137.Aggarwal R, Naik SR. Hepatitis E: intrafamilial transmission versus waterborne spread. J Hepatol. 1994;21:718–23. doi: 10.1016/s0168-8278(94)80229-7. [DOI] [PubMed] [Google Scholar]
- 138.Somani SK, Aggarwal R, Naik SR, Srivastava S, Naik S. A serological study of intrafamilial spread from patients with sporadic hepatitis E virus infection. J Viral Hepat. 2003;10:446–9. doi: 10.1046/j.1365-2893.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 139.Ducancelle A, Payan C, Nicand E, Le Guillou H, Cales P, Lunel- Fabiani F. Intrafamilial hepatitis E in France. J Clin Virol. 2007;39:51–3. doi: 10.1016/j.jcv.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 140.Gotanda Y, Iwata A, Ohnuma H, Yoshikawa A, Mizoguchi H, Endo K, et al. Ongoing subclinical infection of hepatitis E virus among blood donors with an elevated alanine aminotransferase level in Japan. J Med Virol. 2007;79:734–42. doi: 10.1002/jmv.20834. [DOI] [PubMed] [Google Scholar]
- 141.Mitsui T, Tsukamoto Y, Suzuki S, Yamazaki C, Masuko K, Tsuda F, et al. Serological and molecular studies on subclinical hepatitis E virus infection using periodic serum samples obtained from healthy individuals. J Med Virol. 2005;76:526–33. doi: 10.1002/jmv.20393. [DOI] [PubMed] [Google Scholar]
- 142.Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J Gastroenterol Hepatol. 2004;19:778–84. doi: 10.1111/j.1440-1746.2004.03437.x. [DOI] [PubMed] [Google Scholar]
- 143.Tamura A, Shimizu YK, Tanaka T, Kuroda K, Arakawa Y, Takahashi K, et al. Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol Res. 2007;37:113–20. doi: 10.1111/j.1872-034X.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 144.Mitsui T, Tsukamoto Y, Yamazaki C, Masuko K, Tsuda F, Takahashi M, et al. Prevalence of hepatitis E virus infection among hemodialysis patients in Japan: evidence for infection with a genotype 3 HEV by blood transfusion. J Med Virol. 2004;74:563–72. doi: 10.1002/jmv.20215. [DOI] [PubMed] [Google Scholar]
- 145.Boxall E, Herborn A, Kochethu G, Pratt G, Adams D, Ijaz S, et al. Transfusion-transmitted hepatitis E in a ‘nonhyperendemic’ country. Transfus Med. 2006;16:79–83. doi: 10.1111/j.1365-3148.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 146.Kumar A, Beniwal M, Kar P, Sharma JB, Murthy NS. Hepatitis E in pregnancy. Int J Gynaecol Obstet. 2004;85:240–4. doi: 10.1016/j.ijgo.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 147.Kumar RM, Uduman S, Rana S, Kochiyil JK, Usmani A, Thomas L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur J Obstet Gynecol Reprod Biol. 2001;100:9–15. doi: 10.1016/s0301-2115(01)00448-1. [DOI] [PubMed] [Google Scholar]
- 148.Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet. 1995;345:1025–6. doi: 10.1016/s0140-6736(95)90761-0. [DOI] [PubMed] [Google Scholar]
- 149.Singh S, Mohanty A, Joshi YK, Deka D, Mohanty S, Panda SK. Mother-to-child transmission of hepatitis E virus infection. Indian J Pediatr. 2003;70:37–9. doi: 10.1007/BF02722743. [DOI] [PubMed] [Google Scholar]
- 150.Aggarwal R, Krawczynski K. Hepatitis E: an overview and recent advances in clinical and laboratory research. J Gastroenterol Hepatol. 2000;15:9–20. doi: 10.1046/j.1440-1746.2000.02006.x. [DOI] [PubMed] [Google Scholar]
- 151.Arankalle VA, Chobe LP, Jha J, Chadha MS, Banerjee K, Favorov MO, et al. Aetiology of acute sporadic non-A, non-B viral hepatitis in India. J Med Virol. 1993;40:121–5. doi: 10.1002/jmv.1890400208. [DOI] [PubMed] [Google Scholar]
- 152.Ishida S, Yoshizumi S, Miyoshi M, Okui T, Ishida A, Abe S, et al. A cluster of hepatitis E virus infection in Hokkaido, Japan. Jpn J Infect Dis. 2006;59:135–6. [PubMed] [Google Scholar]
- 153.Nakano Y, Yamauchi A, Yano T, Nakayama O, Sakai H, Nagasaka Y, et al. A diffuse outbreak of hepatitis E in Mie Prefecture, 2005. Jpn J Infect Dis. 2006;59:136–8. [PubMed] [Google Scholar]
- 154.Sainokami S, Abe K, Kumagai I, Miyasaka A, Endo R, Takikawa Y, et al. Epidemiological and clinical study of sporadic acute hepatitis E caused by indigenous strains of hepatitis E virus in Japan compared with acute hepatitis A. J Gastroenterol. 2004;39:640–8. doi: 10.1007/s00535-003-1359-5. [DOI] [PubMed] [Google Scholar]
- 155.Khuroo MS. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from posttransfusion non-A, non-B type. Am J Med. 1980;68:818–24. doi: 10.1016/0002-9343(80)90200-4. [DOI] [PubMed] [Google Scholar]
- 156.Kane MA, Bradley DW, Shrestha SM, Maynard JE, Cook EH, Mishra RP, et al. Epidemic non-A, non-B hepatitis in Nepal. Recovery of a possible etiologic agent and transmission studies in marmosets. JAMA. 1984;252:3140–5. doi: 10.1001/jama.252.22.3140. [DOI] [PubMed] [Google Scholar]
- 157.Tsega E, Hansson BG, Krawczynski K, Nordenfelt E. Acute sporadic viral hepatitis in Ethiopia: causes, risk factors, and effects on pregnancy. Clin Infect Dis. 1992;14:961–5. doi: 10.1093/clinids/14.4.961. [DOI] [PubMed] [Google Scholar]
- 158.Guthmann JP, Klovstad H, Boccia D, Hamid N, Pinoges L, Nizou JY, et al. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: the role of water treatment methods. Clin Infect Dis. 2006;42:1685–91. doi: 10.1086/504321. [DOI] [PubMed] [Google Scholar]
- 159.Rab MA, Bile MK, Mubarik MM, Asghar H, Sami Z, Siddiqi S, et al. Water-borne hepatitis E virus epidemic in Islamabad, Pakistan: a common source outbreak traced to the malfunction of a modern water treatment plant. Am J Trop Med Hyg. 1997;57:151–7. doi: 10.4269/ajtmh.1997.57.151. [DOI] [PubMed] [Google Scholar]
- 160.Sanyal MC. Epidemic of infectious hepatitis amongst personnel of the armed forces, Delhi (1955–56): epidemiology. Indian J Med Res ; (Suppl) 1957;45:91–9. [PubMed] [Google Scholar]
- 161.Viswanathan R, Sidhu AS. Infectious hepatitis; clinical findings. Indian J Med Res ; (Suppl) 1957;45:49–58. [PubMed] [Google Scholar]
- 162.Balayan MS. Epidemiology of hepatitis E virus infection. J Viral Hepat. 1997;4:155–65. doi: 10.1046/j.1365-2893.1997.00145.x. [DOI] [PubMed] [Google Scholar]
- 163.Vishwanathan Infectious hepatitis in Delhi (1955–1956). A critical study: epidemiology. Indian J Med Res. 1957;45:49–58. [Google Scholar]
- 164.Jilani N, Das BC, Husain SA, Baweja UK, Chattopadhya D, Gupta RK, et al. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676–82. doi: 10.1111/j.1440-1746.2007.04913.x. [DOI] [PubMed] [Google Scholar]
- 165.Kumar AS, Kumar SP, Singh R, Kumar MS, Madan K, Kumar JJ, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Heat. 2007;46:387–94. doi: 10.1016/j.jhep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 166.Kumar A, Aggarwal R, Naik SR, Saraswat V, Ghoshal UC, Naik S. Hepatitis E virus is responsible for decompensation of chronic liver disease in an endemic region. Indian J Gastroenterol. 2004;23:59–62. [PubMed] [Google Scholar]
- 167.Monga R, Garg S, Tyagi P, Kumar N. Superimposed acute hepatitis E infection in patients with chronic liver disease. Indian J Gastroenterol. 2004;23:50–2. [PubMed] [Google Scholar]
- 168.Ramachandran J, Eapen CE, Kang G, Abraham P, Hubert DD, Kurian G, et al. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134–8. doi: 10.1111/j.1440-1746.2004.03188.x. [DOI] [PubMed] [Google Scholar]
- 169.Hamid SS, Atiq M, Shehzad F, Yasmeen A, Nissa T, Salam A, et al. Hepatitis E virus superinfection in patients with chronic liver disease. Hepatology. 2002;36:474–8. doi: 10.1053/jhep.2002.34856. [DOI] [PubMed] [Google Scholar]
- 170.Kc S, Mishra AK, Shrestha R. Hepatitis E virus infection in chronic liver disease causes rapid decompensation. JNMA J Nepal Med Assoc. 2006;45:212–15. [PubMed] [Google Scholar]
- 171.Dalton HR, Hazeldine S, Banks M, Ijaz S, Bendall R. Locally acquired hepatitis E in chronic liver disease. Lancet. 2007;369:1260. doi: 10.1016/S0140-6736(07)60595-9. [DOI] [PubMed] [Google Scholar]
- 172.Christensen PB, Engle RE, Jacobsen SE, Krarup HB, Georgsen J, Purcell RH. High prevalence of hepatitis E antibodies among Danish prisoners and drug users. J Med Virol. 2002;66:49–55. doi: 10.1002/jmv.2110. [DOI] [PubMed] [Google Scholar]
- 173.Nicand E, Grandadam M, Teyssou R, Rey JL, Buisson Y. Viraemia and faecal shedding of HEV in symptom-free carriers. Lancet. 2001;357:68–9. doi: 10.1016/S0140-6736(05)71568-3. [DOI] [PubMed] [Google Scholar]
- 174.Krawczynski K. Hepatitis E vaccineFready for prime time? N Engl J Med. 2007;356:949–51. doi: 10.1056/NEJMe068311. [DOI] [PubMed] [Google Scholar]
- 175.Zhou YH, Purcell RH, Emerson SU. An ELISA for putative neutralizing antibodies to hepatitis E virus detects antibodies to genotypes 1, 2, 3, and 4. Vaccine. 2004;22:2578–85. doi: 10.1016/j.vaccine.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 176.Ghabrah TM, Tsarev S, Yarbough PO, Emerson SU, Strickland GT, Purcell RH. Comparison of tests for antibody to hepatitis E virus. J Med Virol. 1998;55:134–7. [PubMed] [Google Scholar]
- 177.Arankalle VA, Tsarev SA, Chadha MS, Alling DW, Emerson SU, Banerjee K, et al. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–50. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 178.Fix AD, Abdel-Hamid M, Purcell RH, Shehata MH, Abdel-Aziz F, Mikhail N, et al. Prevalence of antibodies to hepatitis E in two rural Egyptian communities. Am J Trop Med Hyg. 2000;62:519–23. doi: 10.4269/ajtmh.2000.62.519. [DOI] [PubMed] [Google Scholar]
- 179.Stoszek SK, Engle RE, Abdel-Hamid M, Mikhail N, Abdel-Aziz F, Medhat A, et al. Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans R Soc Trop Med Hyg. 2006;100:89–94. doi: 10.1016/j.trstmh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 180.Stoszek SK, Abdel-Hamid M, Saleh DA, El Kafrawy S, Narooz S, Hawash Y, et al. High prevalence of hepatitis E antibodies in pregnant Egyptian women. Trans R Soc Trop Med Hyg. 2006;100:95–101. doi: 10.1016/j.trstmh.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 181.Li RC, Ge SX, Li YP, Zheng YJ, Nong Y, Guo QS, et al. Seroprevalence of hepatitis E virus infection, rural southern People's Republic of China. Emerg Infect Dis. 2006;12:1682–8. doi: 10.3201/eid1211.060332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Buti M, Dominguez A, Plans P, Jardi R, Schaper M, Espunes J, et al. Community-based seroepidemiological survey of hepatitis E virus infection in Catalonia, Spain. Clin Vaccine Immunol. 2006;13:1328–32. doi: 10.1128/CVI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Olsen B, Axelsson-Olsson D, Thelin A, Weiland O. Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scand J Infect Dis. 2006;38:55–8. doi: 10.1080/00365540500321470. [DOI] [PubMed] [Google Scholar]
- 184.Thomas DL, Yarbough PO, Vlahov D, Tsarev SA, Nelson KE, Saah AJ, et al. Seroreactivity to hepatitis E virus in areas where the disease is not endemic. J Clin Microbiol. 1997;35:1244–7. doi: 10.1128/jcm.35.5.1244-1247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kikuchi K, Yoshida T, Kimata N, Sato C, Akiba T. Prevalence of hepatitis E virus infection in regular hemodialysis patients. Ther Apher Dial. 2006;10:193–7. doi: 10.1111/j.1744-9987.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 186.Cheng PN, Wang RH, Wu IC, Wu JC, Tseng KC, Young KC, et al. Seroprevalence of hepatitis E virus infection among institutionalized psychiatric patients in Taiwan. J Clin Virol. 2007;38:44–8. doi: 10.1016/j.jcv.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 187.Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–22. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tanaka E, Matsumoto A, Takeda N, Li TC, Umemura T, Yoshizawa K, et al. Age-specific antibody to hepatitis E virus has remained constant during the past 20 years in Japan. J Viral Hepat. 2005;12:439–42. doi: 10.1111/j.1365-2893.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 189.Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860–5. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Karetnyi YV, Gilchrist MJ, Naides SJ. Hepatitis E virus infection prevalence among selected populations in Iowa. J Clin Virol. 1999;14:51–5. doi: 10.1016/s1386-6532(99)00037-2. [DOI] [PubMed] [Google Scholar]
- 191.Nakai I, Kato K, Miyazaki A, Yoshii M, Li TC, Takeda N, et al. Different fecal shedding patterns of two common strains of hepatitis E virus at three Japanese swine farms. Am J Trop Med Hyg. 2006;75:1171–7. [PubMed] [Google Scholar]
- 192.Choi C, Chae C. Localization of swine hepatitis E virus in liver and extrahepatic tissues from naturally infected pigs by in situ hybridization. J Hepatol. 2003;38:827–32. doi: 10.1016/s0168-8278(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 193.Kasorndorkbua C, Opriessnig T, Huang FF, Guenette DK, Thomas PJ, Meng XJ, et al. Infectious swine hepatitis E virus is present in pig manure storage facilities on United States farms, but evidence of water contamination is lacking. Appl Environ Microbiol. 2005;71:7831–7. doi: 10.1128/AEM.71.12.7831-7837.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, et al. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol. 1998;72:9714–21. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Meng XJ, Halbur PG, Haynes JS, Tsareva TS, Bruna JD, Royer RL, et al. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch Virol. 1998;143:1405–15. doi: 10.1007/s007050050384. [DOI] [PubMed] [Google Scholar]
- 196.Kasorndorkbua C, Guenette DK, Huang FF, Thomas PJ, Meng XJ, Halbur PG. Routes of transmission of swine hepatitis E virus in pigs. J Clin Microbiol. 2004;42:5047–52. doi: 10.1128/JCM.42.11.5047-5052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Halbur PG, Kasorndorkbua C, Gilbert C, Guenette D, Potters MB, Purcell RH, et al. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol. 2001;39:918–23. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Martin M, Segales J, Huang FF, Guenette DK, Mateu E, de Deus N, et al. Association of hepatitis E virus (HEV) and postweaning multisystemic wasting syndrome (PMWS) with lesions of hepatitis in pigs. Vet Microbiol. 2007;122:16–24. doi: 10.1016/j.vetmic.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 199.Rutjes SA, Lodder WJ, Bouwknegt M, de Roda Husman AM. Increased hepatitis E virus prevalence on Dutch pig farms from 33 to 55% by using appropriate internal quality controls for RTPCR. J Virol Methods. 2007;143:112–16. doi: 10.1016/j.jviromet.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 200.Chandler JD, Riddell MA, Li F, Love RJ, Anderson DA. Serological evidence for swine hepatitis E virus infection in Australian pig herds. Vet Microbiol. 1999;68:95–105. doi: 10.1016/s0378-1135(99)00065-6. [DOI] [PubMed] [Google Scholar]
- 201.Meng XJ, Dea S, Engle RE, Friendship R, Lyoo YS, Sirinarumitr T, et al. Prevalence of antibodies to the hepatitis E virus in pigs from countries where hepatitis E is common or is rare in the human population. J Med Virol. 1999;59:297–302. [PubMed] [Google Scholar]
- 202.Takahashi M, Nishizawa T, Tanaka T, Tsatsralt-Od B, Inoue J, Okamoto H. Correlation between positivity for immunoglobulin A antibodies and viraemia of swine hepatitis E virus observed among farm pigs in Japan. J Gen Virol. 2005;86(Pt 6):1807–13. doi: 10.1099/vir.0.80909-0. [DOI] [PubMed] [Google Scholar]
- 203.Yoo D, Willson P, Pei Y, Hayes MA, Deckert A, Dewey CE, et al. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin Diagn Lab Immunol. 2001;8:1213–19. doi: 10.1128/CDLI.8.6.1213-1219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bouwknegt M, Lodder-Verschoor F, van der Poel WH, Rutjes SA, de Roda Husman AM. Hepatitis E virus RNA in commercial porcine livers in The Netherlands. J Food Prot. 2007;70:2889–95. doi: 10.4315/0362-028x-70.12.2889. [DOI] [PubMed] [Google Scholar]
- 205.Williams TP, Kasorndorkbua C, Halbur PG, Haqshenas G, Guenette DK, Toth TE, et al. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J Clin Microbiol. 2001;39:3040–6. doi: 10.1128/JCM.39.9.3040-3046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ha SK, Chae C. Immunohistochemistry for the detection of swine hepatitis E virus in the liver. J Viral Hepat. 2004;11:263–7. doi: 10.1111/j.1365-2893.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 207.Bouwknegt M, Engel B, Herremans MM, Widdowson MA, Worm HC, Koopmans MP, et al. Bayesian estimation of hepatitis E virus seroprevalence for populations with different exposure levels to swine in The Netherlands. Epidemiol Infect. 2007;20:1–0. doi: 10.1017/S0950268807008941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hirano M, Ding X, Li TC, Takeda N, Kawabata H, Koizumi N, et al. Evidence for widespread infection of hepatitis E virus among wild rats in Japan. Hepatol Res. 2003;27:1–5. doi: 10.1016/s1386-6346(03)00192-x. [DOI] [PubMed] [Google Scholar]
- 209.Arankalle VA, Joshi MV, Kulkarni AM, Gandhe SS, Chobe LP, Rautmare SS, et al. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J Viral Hepat. 2001;8:223–7. doi: 10.1046/j.1365-2893.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 210.Favorov MO, Kosoy MY, Tsarev SA, Childs JE, Margolis HS. Prevalence of antibody to hepatitis E virus among rodents in the United States. J Infect Dis. 2000;181:449–55. doi: 10.1086/315273. [DOI] [PubMed] [Google Scholar]
- 211.Kabrane-Lazizi Y, Fine JB, Elm J, Glass GE, Higa H, Diwan A, et al. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am J Trop Med Hyg. 1999;61:331–5. doi: 10.4269/ajtmh.1999.61.331. [DOI] [PubMed] [Google Scholar]
- 212.Easterbrook JD, Kaplan JB, Vanasco NB, Reeves WK, Purcell RH, Kosoy MY, et al. A survey of zoonotic pathogens carried by Norway rats in Baltimore, Maryland, USA. Epidemiol Infect. 2007;15:1–8. doi: 10.1017/S0950268806007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nakamura M, Takahashi K, Taira K, Taira M, Ohno A, Sakugawa H, et al. Hepatitis E virus infection in wild mongooses of Okinawa, Japan: demonstration of anti-HEV antibodies and a full-genome nucleotide sequence. Hepatol Res. 2006;34:137–40. doi: 10.1016/j.hepres.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 214.Hirano M, Ding X, Tran HT, Li TC, Takeda N, Sata T, et al. Prevalence of antibody against hepatitis E virus in various species of non-human primates: evidence of widespread infection in Japanese monkeys (Macaca fuscata) Jpn J Infect Dis. 2003;56:8–11. [PubMed] [Google Scholar]
- 215.Vitral CL, Pinto MA, Lewis-Ximenez LL, Khudyakov YE, dos Santos DR, Gaspar AM. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Mem Inst Oswaldo Cruz. 2005;100:117–22. doi: 10.1590/s0074-02762005000200003. [DOI] [PubMed] [Google Scholar]
- 216.Okamoto H, Takahashi M, Nishizawa T, Usui R, Kobayashi E. Presence of antibodies to hepatitis E virus in Japanese pet cats. Infection. 2004;32:57–8. doi: 10.1007/s15010-004-3078-0. [DOI] [PubMed] [Google Scholar]
- 217.Saad MD, Hussein HA, Bashandy MM, Kamel HH, Earhart KC, Fryauff DJ, et al. Hepatitis E virus infection in work horses in Egypt. Infect Genet Evol. 2007;7:368–73. doi: 10.1016/j.meegid.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 218.Caron M, Enouf V, Than SC, Dellamonica L, Buisson Y, Nicand E. Identification of genotype 1 hepatitis E virus in samples from swine in Cambodia. J Clin Microbiol. 2006;44:3440–2. doi: 10.1128/JCM.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.He J, Innis BL, Shrestha MP, Clayson ET, Scott RM, Linthicum KJ, et al. Evidence that rodents are a reservoir of hepatitis E virus for humans in Nepal. J Clin Microbiol. 2002;40:4493–8. doi: 10.1128/JCM.40.12.4493-4498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 220.He J, Innis BL, Shrestha MP, Clayson ET, Scott RM, Linthicum KJ, et al. Evidence that rodents are a reservoir of hepatitis E virus for humans in Nepal. J Clin Microbiol. 2006;44:1208. doi: 10.1128/JCM.44.3.1208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitissplenomegaly syndrome in the United States. J Gen Virol. 2001;82(Pt 10):2449–62. doi: 10.1099/0022-1317-82-10-2449. [DOI] [PubMed] [Google Scholar]
- 222.Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, et al. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J Gen Virol. 2004;85(Pt 6):1609–18. doi: 10.1099/vir.0.79841-0. [DOI] [PubMed] [Google Scholar]
- 223.Anderson DA, Shrestha IL. Hepatitis E Virus. Washington DC: ASM Press; 2002. [Google Scholar]
- 224.Zhang J, Ge SX, Huang GY, Li SW, He ZQ, Wang YB, et al. Evaluation of antibody-based and nucleic acid-based assays for diagnosis of hepatitis E virus infection in a rhesus monkey model. J Med Virol. 2003;71:518–26. doi: 10.1002/jmv.10523. [DOI] [PubMed] [Google Scholar]
- 225.Soe S, Uchida T, Suzuki K, Komatsu K, Azumi J, Okuda Y, et al. Enterically transmitted non-A, non-B hepatitis in cynomolgus monkeys: morphology and probable mechanism of hepatocellular necrosis. Liver. 1989;9:135–45. doi: 10.1111/j.1600-0676.1989.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 226.Longer CF, Denny SL, Caudill JD, Miele TA, Asher LV, Myint KS, et al. Experimental hepatitis E: pathogenesis in cynomolgus macaques (Macaca fascicularis) J Infect Dis. 1993;168:602–9. doi: 10.1093/infdis/168.3.602. [DOI] [PubMed] [Google Scholar]
- 227.Tsarev SA, Emerson SU, Tsareva TS, Yarbough PO, Lewis M, Govindarajan S, et al. Variation in course of hepatitis E in experimentally infected cynomolgus monkeys. J Infect Dis. 1993;167:1302–6. doi: 10.1093/infdis/167.6.1302. [DOI] [PubMed] [Google Scholar]
- 228.Chauhan A, Jameel S, Dilawari JB, Chawla YK, Kaur U, Ganguly NK. Hepatitis E virus transmission to a volunteer. Lancet. 1993;341:149–50. doi: 10.1016/0140-6736(93)90008-5. [DOI] [PubMed] [Google Scholar]
- 229.Aggarwal R, Kini D, Sofat S, Naik SR, Krawczynski K. Duration of viraemia and faecal viral excretion in acute hepatitis E. Lancet. 2000;356:1081–2. doi: 10.1016/S0140-6736(00)02737-9. [DOI] [PubMed] [Google Scholar]
- 230.Zhao ZY, Ruan B, Shao H, Chen ZJ, Liu SL. Detection of hepatitis E virus RNA in sera of patients with hepatitis E by polymerase chain reaction. Hepatobiliary Pancreat Dis Int. 2007;6:38–42. [PubMed] [Google Scholar]
- 231.Nanda SK, Ansari IH, Acharya SK, Jameel S, Panda SK. Protracted viremia during acute sporadic hepatitis E virus infection. Gastroenterology. 1995;108:225–30. doi: 10.1016/0016-5085(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 232.Arankalle VA, Chadha MS, Tsarev SA, Emerson SU, Risbud AR, Banerjee K, et al. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically transmitted hepatitis agent. Proc Natl Acad Sci USA. 1994;91:3428–32. doi: 10.1073/pnas.91.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Clayson ET, Myint KS, Snitbhan R, Vaughn DW, Innis BL, Chan L, et al. Viremia, fecal shedding, and IgM and IgG responses in patients with hepatitis E. J Infect Dis. 1995;172:927–33. doi: 10.1093/infdis/172.4.927. [DOI] [PubMed] [Google Scholar]
- 234.Bryan JP, Tsarev SA, Iqbal M, Ticehurst J, Emerson S, Ahmed A, et al. Epidemic hepatitis E in Pakistan: patterns of serologic response and evidence that antibody to hepatitis E virus protects against disease. J Infect Dis. 1994;170:517–21. doi: 10.1093/infdis/170.3.517. [DOI] [PubMed] [Google Scholar]
- 235.Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, et al. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci USA. 1994;91:10198–202. doi: 10.1073/pnas.91.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Naik S, Aggarwal R, Naik SR, Dwivedi S, Talwar S, Tyagi SK, et al. Evidence for activation of cellular immune responses in patients with acute hepatitis E. Indian J Gastroenterol. 2002;21:149–52. [PubMed] [Google Scholar]
- 237.Aggarwal R, Shukla R, Jameel S, Agrawal S, Puri P, Gupta VK, et al. T-cell epitope mapping of ORF2 and ORF3 proteins of human hepatitis E virus. J Viral Hepat. 2007;14:283–92. doi: 10.1111/j.1365-2893.2006.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pal R, Aggarwal R, Naik SR, Das V, Das S, Naik S. Immunological alterations in pregnant women with acute hepatitis E. J Gastroenterol Hepatol. 2005;20:1094–101. doi: 10.1111/j.1440-1746.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 239.Aggarwal R. Hepatitis E and pregnancy. Indian J Gastroenterol. 2007;26:3–5. [PubMed] [Google Scholar]
- 240.Srivastava R, Aggarwal R, Jameel S, Puri P, Gupta VK, Ramesh VS, et al. Cellular immune responses in acute hepatitis E virus infection to the viral open reading frame 2 protein. Viral Immunol. 2007;20:56–65. doi: 10.1089/vim.2006.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tsega E, Krawczynski K, Hansson BG, Nordenfelt E, Negusse Y, Alemu W, et al. Outbreak of acute hepatitis E virus infection among military personnel in northern Ethiopia. J Med Virol. 1991;34:232–6. doi: 10.1002/jmv.1890340407. [DOI] [PubMed] [Google Scholar]
- 242.Krawczynski K, Kamili S, Aggarwal R. Global epidemiology and medical aspects of hepatitis E. Forum (Genova) 2001;11:166–79. [PubMed] [Google Scholar]
- 243.Purcell RH, Emerson SU. Hepatitis E virus in Fields Virology. Philadelphia: Lippincott, William and Wilkins; 2001. [Google Scholar]
- 244.Nanda SK, Yalcinkaya K, Panigrahi AK, Acharya SK, Jameel S, Panda SK. Etiological role of hepatitis E virus in sporadic fulminant hepatitis. J Med Virol. 1994;42:133–7. doi: 10.1002/jmv.1890420207. [DOI] [PubMed] [Google Scholar]
- 245.Arora NK, Nanda SK, Gulati S, Ansari IH, Chawla MK, Gupta SD, et al. Acute viral hepatitis types E, A, and B singly and in combination in acute liver failure in children in north India. J Med Virol. 1996;48:215–21. doi: 10.1002/(SICI)1096-9071(199603)48:3<215::AID-JMV1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 246.Tsega E, Krawczynski K, Hansson BG, Nordenfelt E. Hepatitis E virus infection in pregnancy in Ethiopia. Ethiop Med J. 1993;31:173–81. [PubMed] [Google Scholar]
- 247.Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252–5. doi: 10.1016/0002-9343(81)90758-0. [DOI] [PubMed] [Google Scholar]
- 248.Mirghani OA, Saeed OK, Basama FM. Viral hepatitis in pregnancy. East Afr Med J. 1992;69:445–9. [PubMed] [Google Scholar]
- 249.Hussaini SH, Skidmore SJ, Richardson P, Sherratt LM, Cooper BT, O'Grady JG. Severe hepatitis E infection during pregnancy. J Viral Hepat. 1997;4:51–4. doi: 10.1046/j.1365-2893.1997.00123.x. [DOI] [PubMed] [Google Scholar]
- 250.Peron JM, Bureau C, Poirson H, Mansuy JM, Alric L, Selves J, et al. Fulminant liver failure from acute autochthonous hepatitis E in France: description of seven patients with acute hepatitis E and encephalopathy. J Viral Hepat. 2007;14:298–303. doi: 10.1111/j.1365-2893.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 251.Ohnishi S, Kang JH, Maekubo H, Arakawa T, Karino Y, Toyota J, et al. Comparison of clinical features of acute hepatitis caused by hepatitis E virus (HEV) genotypes 3 and 4 in Sapporo, Japan. Hepatol Res. 2006;36:301–7. doi: 10.1016/j.hepres.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 252.Coursaget P, Buisson Y, N'Gawara MN, Van Cuyck-Gandre H, Roue R. Role of hepatitis E virus in sporadic cases of acute and fulminant hepatitis in an endemic area (Chad) Am J Trop Med Hyg. 1998;58:330–4. doi: 10.4269/ajtmh.1998.58.330. [DOI] [PubMed] [Google Scholar]
- 253.Sallie R, Chiyende J, Tan KC, Bradley D, Portmann B, Williams R, et al. Fulminant hepatic failure resulting from coexistent Wilson's disease and hepatitis E. Gut. 1994;35:849–53. doi: 10.1136/gut.35.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Peron JM, Mansuy JM, Recher C, Bureau C, Poirson H, Alric L, et al. Prolonged hepatitis E in an immunocompromised patient. J Gastroenterol Hepatol. 2006;21:1223–4. doi: 10.1111/j.1440-1746.2006.04209.x. [DOI] [PubMed] [Google Scholar]
- 255.Gerolami R, Moal V, Colson P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med. 2008;358:859–60. doi: 10.1056/NEJMc0708687. [DOI] [PubMed] [Google Scholar]
- 256.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–17. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 257.Kamani P, Baijal R, Amarapurkar D, Gupte P, Patel N, Kumar P, et al. Guillain–Barre syndrome associated with acute hepatitis E. Indian J Gastroenterol. 2005;24:216. [PubMed] [Google Scholar]
- 258.Mandal K, Chopra N. Acute transverse myelitis following hepatitis E virus infection. Indian Pediatr. 2006;43:365–6. [PubMed] [Google Scholar]
- 259.Jaroszewicz J, Flisiak R, Kalinowska A, Wierzbicka I, Prokopowicz D. Acute hepatitis E complicated by acute pancreatitis: a case report and literature review. Pancreas. 2005;30:382–4. doi: 10.1097/01.mpa.0000160962.06333.17. [DOI] [PubMed] [Google Scholar]
- 260.Zamvar V, McClean P, Odeka E, Richards M, Davison S. Hepatitis E virus infection with nonimmune hemolytic anemia. J Pediatr Gastroenterol Nutr. 2005;40:223–5. doi: 10.1097/00005176-200502000-00027. [DOI] [PubMed] [Google Scholar]
- 261.Wendum D, Nachury M, Yver M, Lemann M, Flejou JF, Janin A, et al. Acute hepatitis E: a cause of lymphocytic destructive cholangitis. Hum Pathol. 2005;36:436–8. doi: 10.1016/j.humpath.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 262.Serratrice J, Disdier P, Colson P, Ene N, de Roux CS, Weiller PJ. Acute polyarthritis revealing hepatitis E. Clin Rheumatol. 2007;20:1973–5. doi: 10.1007/s10067-007-0595-0. [DOI] [PubMed] [Google Scholar]
- 263.Worm HC, van der Poel WH, Brandstatter G. Hepatitis E: an overview. Microbes Infect. 2002;4:657–66. doi: 10.1016/s1286-4579(02)01584-8. [DOI] [PubMed] [Google Scholar]
- 264.Myint KS, Endy TP, Gibbons RV, Laras K, Mammen MP, Jr, Sedyaningsih ER, et al. Evaluation of diagnostic assays for hepatitis E virus in outbreak settings. J Clin Microbiol. 2006;44:1581–3. doi: 10.1128/JCM.44.4.1581-1583.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mast EE, Alter MJ, Holland PV, Purcell RH. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatitis E Virus Antibody Serum Panel Evaluation Group. Hepatology. 1998;27:857–61. doi: 10.1002/hep.510270331. [DOI] [PubMed] [Google Scholar]
- 266.Orru G, Masia G, Orru G, Romano L, Piras V, Coppola RC. Detection and quantitation of hepatitis E virus in human faeces by real-time quantitative PCR. J Virol Methods. 2004;118:77–82. doi: 10.1016/j.jviromet.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 267.Inoue J, Takahashi M, Yazaki Y, Tsuda F, Okamoto H. Development and validation of an improved RT-PCR assay with nested universal primers for detection of hepatitis E virus strains with significant sequence divergence. J Virol Methods. 2006;137:325–33. doi: 10.1016/j.jviromet.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 268.Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 269.Wang L, Zhuang H. Hepatitis E: an overview and recent advances in vaccine research. World J Gastroenterol. 2004;10:2157–2162. doi: 10.3748/wjg.v10.i15.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Worm HC, Wirnsberger G. Hepatitis E vaccines: progress and prospects. Drugs. 2004;64:1517–31. doi: 10.2165/00003495-200464140-00002. [DOI] [PubMed] [Google Scholar]
- 271.Kamili S, Spelbring J, Carson D, Krawczynski K. Protective efficacy of hepatitis E virus DNA vaccine administered by gene gun in the cynomolgus macaque model of infection. J Infect Dis. 2004;189:258–64. doi: 10.1086/380801. [DOI] [PubMed] [Google Scholar]
- 272.He J, Hayes CG, Binn LN, Seriwatana J, Vaughn DW, Kuschner RA, et al. Hepatitis E virus DNA vaccine elicits immunologic memory in mice. J Biomed Sci. 2001;8:223–6. doi: 10.1007/BF02256416. [DOI] [PubMed] [Google Scholar]
- 273.Meng J, Dai X, Chang JC, Lopareva E, Pillot J, Fields HA, et al. Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology. 2001;288:203–11. doi: 10.1006/viro.2001.1093. [DOI] [PubMed] [Google Scholar]
- 274.Im SW, Zhang JZ, Zhuang H, Che XY, Zhu WF, Xu GM, et al. A bacterially expressed peptide prevents experimental infection of primates by the hepatitis E virus. Vaccine. 2001;19:3726–32. doi: 10.1016/s0264-410x(01)00100-1. [DOI] [PubMed] [Google Scholar]
- 275.Li SW, Zhang J, He ZQ, Ge SX, Gu Y, Lin J, et al. The study of aggregate of the ORF2 peptide of hepatitis E virus expressed in Escherichia coli . Sheng Wu Gong Cheng Xue Bao. 2002;18:463–7. [PubMed] [Google Scholar]
- 276.Robinson RA, Burgess WH, Emerson SU, Leibowitz RS, Sosnovtseva SA, Tsarev S, et al. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr Purif. 1998;12:75–84. doi: 10.1006/prep.1997.0817. [DOI] [PubMed] [Google Scholar]
- 277.Zhang M, Emerson SU, Nguyen H, Engle R, Govindarajan S, Blackwelder WC, et al. Recombinant vaccine against hepatitis E: duration of protective immunity in rhesus macaques. Vaccine. 2002;20:3285–91. doi: 10.1016/s0264-410x(02)00314-6. [DOI] [PubMed] [Google Scholar]
- 278.Zhang M, Emerson SU, Nguyen H, Engle RE, Govindarajan S, Gerin JL, et al. Immunogenicity and protective efficacy of a vaccine prepared from 53 kDa truncated hepatitis E virus capsid protein expressed in insect cells. Vaccine. 2001;20:853–7. doi: 10.1016/s0264-410x(01)00399-1. [DOI] [PubMed] [Google Scholar]
- 279.Purdy MA, McCaustland KA, Krawczynski K, Spelbring J, Reyes GR, Bradley DW. Preliminary evidence that a trpE-HEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV) J Med Virol. 1993;41:90–4. doi: 10.1002/jmv.1890410118. [DOI] [PubMed] [Google Scholar]
- 280.Purcell RH, Nguyen H, Shapiro M, Engle RE, Govindarajan S, Blackwelder WC, et al. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607–15. doi: 10.1016/s0264-410x(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 281.Shrestha MP, Scott RM, Joshi DM, Mammen MP, Jr, Thapa GB, Thapa N, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356:895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 282.Teo CG. The two clinico-epidemiological forms of hepatitis E. J Viral Hepat. 2007;14:295–7. doi: 10.1111/j.1365-2893.2007.00857.x. [DOI] [PubMed] [Google Scholar]