Abstract
The rapidly increasing incidence of multidrug-resistant infections and the alarmingly low rate of discovery of conventional antibiotics create an urgent need for alternative strategies to treat bacterial infections. Host defence peptides are short cationic molecules produced by the immune systems of most multicellular organisms; they are a class of compounds being actively researched. In this review, we provide an overview of the antimicrobial and immunomodulatory activities of natural host defence peptides, and discuss strategies for creating artificial derivatives with improved biological and pharmacological properties, issues of microbial resistance, and challenges associated with their adaptation for clinical use.
Introduction
Host defence peptides (HDPs) are an evolutionarily ancient component of the innate immune system of most multicellular organisms.1 They exhibit a wide range of biological activities from direct killing of invading pathogens to modulation of immunity and other biological responses of the host. In this article, we will refer to the overlapping classes of peptides with these activities as antimicrobial and immunomodulatory, respectively. Despite the enormous diversity in their sequences and structures, most HDPs share the following features: positive charge, high content of hydrophobic residues, and amphipathic fold.2 The structural diversity of natural peptides provides an excellent starting point for the production of artificial peptides and derivatives with more potent and desirable biological activities, for clinical and commercial applications.3
The diversity of host defence peptides
Two major families of naturally occurring HDPs have been distinguished: defensins and cathelicidins (Table 1). Defensins are cationic amphipathic peptides with an average length of 30 residues and a triple-stranded antiparallel b-sheet structure, stabilised by three disulphide bonds.4 Defensins are further subdivided into three subfamilies of α, β, θ defensins, based on the pattern of disulphidebonding (Table 1).
Table 1.
The diversity of mammalian host defence peptides
| Peptide family and structure | Subfamily | Expression and diversity | |||
|---|---|---|---|---|---|
| Human | Mouse | Pig | Cow | ||
| Defensin | α-defensins | Neutrophils and other leukocytes | Paneth cells of the intestines Epithelial cells | No α-defensins | |
| Amphipathic, cationic peptides Triple-stranded antiparallel β-sheet | Three disulphide bonds linking cysteines 1–6, 2–4, 3–5 | Paneth cells of the intestines Mucosal epithelia, skin | |||
| β-defensins | Mucosal epithelia and skin Monocytes, macrophages, dendritic cells | Epithelial cells | Epithelial cells | Epithelial cells Neutrophils and other leukocytes | |
| Three disulphide bonds linking cysteines 1–5, 2–4, 3–6 | |||||
| Cathelicidin | Non-applicable | LL-37 expressed by neutrophils, and other leukocytes mucosal epithelia, skin | CRAMP expressed by neutrophils, and other leukocytes mucosal epithelia, skin | Large variety: protegrins, PR-39, prophenins, etc. Expressed by neutrophils and other leukocytes | Large variety: bactenecin, BMAP, indolicidin, etc. Expressed by neutrophils and other leukocytes |
| Amphipathic, cationic peptides Diverse sequence and structure Cleaved from a cathelin-like precursor protein | |||||
In humans, but not in cattle or mice, α-defensins are found in the secretory granules of neutrophils and other leukocytes.5, 6 In most mammals other than cattle, a set of α-defensins is also produced by Paneth cells in the crypts of the small intestine.7, 8 These α-defensins, also known as cryptidins, are synthesised as inactive precursors and activated by a removal of an N-terminal segment catalysed by metalloprotease matrilysin (MMP7) in mice and by trypsin in humans.9, 10 After microbial stimulation, the concentration of α-defensins within the crypts is estimated to reach 10 mg/ml, which is more than sufficient for strong microbicidal action.11 This, and the association of Crohn's disease with dysregulation in cryptidin production,12 highlights the importance of a-defensins in the maintenance of immune homeostasis in the gut.
β-Defensins are expressed by most epithelial cells, and their expression is often stimulated by proinflammatory stimuli and infection. They are present in the mucosal secretions of respiratory, gastrointestinal, and urogenital tracts, and in inflamed skin.13–16 β-Defensins are also expressed by human monocytes, macrophages, and dendritic cells, and are important components of the azurophilic granules of bovine neutrophils.4
The much rarer θ-defensins are cyclic molecules, produced in neutrophils and monocytes of rhesus macaques through ligation of two α-defensin-like peptides.17 As a result of their cyclic structure, the (moderate) microbicidal activity of θ-defensins is resistant to salt concentration. However, θ-defensins have not been found in humans or other mammals.
Cathelicidins are the other major family of natural HDPs, although they are grouped by their mechanism of production rather than sequence similarity. All cathelicidins are synthesised as inactive precursors, comprising an N-terminal cathelin-like domain followed by the peptide region, and are proteolytically processed to release mature active HDPs.18 Cathelicidins vary in length, sequence, and structure, having extended, α-helical or β-hairpin folds; however, some are short linear molecules (for example, indolicidin, 13 amino acids), and these are ideal starting points for the design of synthetic peptides with optimised biological activity.
Bovine and porcine immune systems produce a large variety of cathelicidins, including bactenecin, indolicidin, PR-39, protegrins, prophenins, and many others.18 In contrast, in humans there is only one known cathelicidin precursor protein hCAP18, which is proteolytically processed to yield mature cathelicidin LL-37 19 and, in the skin, a series of additional proteolytic derivatives with altered activities.20 LL-37 lacks disulphide bonds and is weakly structured in solution, but adopts an a-helical conformation when interacting with lipid bilayers. Mice also have only one cathelicidin precursor, which is processed to produce mature peptide CRAMP, with 67% sequence identity to LL-37.21 LL-37 is found in the secretory granules of neutrophils and other leukocytes,22 and is also produced by mucosal epithelia and keratinocytes.23–25 The expression of LL-37 is modestly upregulated by proinflammatory stimuli, and is also induced by the HIF-1α and the vitamin D receptor pathways.26–28
Many other HDPs that do not belong to the defensin or cathelicidin families have also been characterised in humans and other mammals. Some important examples include: histatins, histidine-rich cationic peptides with antifungal activity present in the saliva;29 dermcidin, an anionic peptide present in human sweat with modest salt-insensitive antimicrobial properties;30 cationic peptide lactoferricin derived from an iron-binding protein lactoferrin and found in mucosal secretions, milk, and in the granules of leukocytes.31
Biological activities and roles of host defence peptides in immunity
The evolutionarily widespread distribution and the extreme diversity of HDPs highlight their prominent role in immune defences. Historically, this has been attributed to their antimicrobial activity but in recent years potent immunomodulatory properties of HDPs have been characterised and suggested to be an important part of their biological function.19, 32 An in-depth understanding of the physiological roles and mechanisms of action of HDPs is crucial for the development of artificial variants with optimised activity for therapeutic applications.
Antimicrobial properties of HDPs are linked to their amphipathic fold, which allows the peptides to interact with lipid bilayers of microorganisms,2 and kill either through membrane disruption or by translocating across the membrane and inhibiting cytosolic targets. Owing to the relatively nonspecific nature of the interactions between HDPs and the anionic lipids of microbial membranes, many peptides have broad antimicrobial properties, targeting Gram-positive and -negative bacteria, fungi, protozoa, and some viruses.1, 33 Furthermore, HDPs are highly effective against multidrug-resistant bacterial strains. Peptides can also interact with mammalian membranes and this is at least partly responsible for cytotoxicity of some peptides at high concentrations.34 However, distinct composition of mammalian membranes, with preferential localisation of anionic phospholipids into the inner leaflet, offers some protection. Furthermore, membrane interactions and antimicrobial properties of most HDPs are strongly dependent on the composition of the media, being inhibited by divalent cations and serum.19, 14, 34 Thus, although HDPs almost certainly function as powerful microbicidal agents in some physiological settings, such as the phagolysosomes of neutrophils, intestinal crypts, and sites of acute inflammation, under other conditions where salt concentrations are high (100 mM monovalent and 2 mM divalent cations) and peptide concentrations are modest, their immunomodulatory activities are almost certainly more physiologically relevant.
A wide range of HDPs from different species have been shown to act as chemoattractants for cells of innate and adaptive immunity. As an example, human cathelicidin LL-37 attracts neutrophils, monocytes, T cells, and mast cells, using formyl peptide receptor-like 1 (FPRL1), and a distinct Gi-coupled receptor.35, 36 Optimal chemotactic activity of LL-37 is observed in a concentration range that can be reached in vivo under inflammatory conditions.35 Furthermore, synthetic peptide IDR-1, which is protective in mouse models of bacterial infections, similarly chemoattracts neutrophils, acting through receptor FPRL1.37, 38
Apart from direct chemotactic effects, HDPs also elicit other complex responses in leukocytes and epithelial cells, altering gene expression and behaviour to facilitate and to modulate immune responses (Figure 1). For example, when LL-37 was used to stimulate primary human monocytes and macrophage cell lines, microarrays demonstrated the induction of a wide range of chemokines, chemokine receptors, and other genes involved in cell adhesion, communication, and motility.40 In particular, induction of chemokines IL8, Gro-α, MCP1, MIP-1β, and MIP-3α, but not proinflammatory cytokines, was consistently seen with a wide range of natural and synthetic peptides.
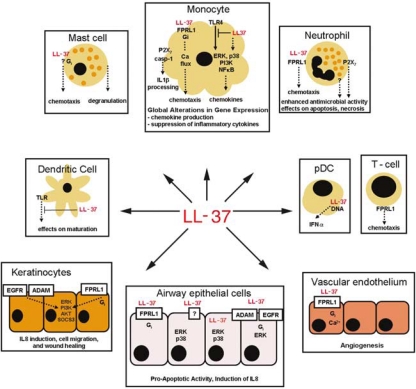
Figure 1.
Immunomodulatory activity of host defence peptides: human cathelicidin LL-37. LL-37 induces global alterations in gene expression in monocytes, signalling through p38, ERK, PI3K, and NF-kB pathways, and promoting expression of chemokines and other genes involved in cell communication and motility.32, (39) LL-37 also acts as a direct chemoattractant for monocytes, neutrophils, T cells, and mast cells through the FPRL1 receptor,35 and an unknown Gi-coupled receptor.36 LL-37 is also strongly anti-endotoxic and inhibits the production of proinflammatory cytokines in monocytes in response to LPS,40 and also the maturation of monocyte-derived dendritic cells by TLR ligands.41 In contrast, LL-37 pretreatment of monocytes modulates the process of dendritic cell differentiation, enhancing their function; 42 and in plasmacytoid dendritic cells (pDCs) LL-37 promotes responses to TLR9 ligands.43 Other activities of LL-37 include the promotion of antimicrobial functions of neutrophils,44 mast cell degranulation,45 and IL-1β processing in LPS-primed monocytes.46 LL-37 also affects neutrophil and epithelial cell apoptosis., 47–49 In keratinocytes, LL-37 induces IL8, and promotes migration and wound healing, and these activities depend on ADAM family metalloproteinase, EGFR and FPRL1.50, (51) In airway epithelial cells, LL-37 activates p38 and ERK pathways and induces chemokine secretion, and this is reported to be mediated by receptors: FPRL1,35, (52) ADAM family metalloproteinase and EGFR,53 and through active peptide internalisation.52 LL-37 also promotes angiogenesis, through FPRL1 receptor on vascular endothelium., 54
HDPs also act as powerful modulators of cellular responses to inflammatory stimuli. Thus, LL-37 can inhibit secretion of inflammatory cytokines in response to lipoploysaccharide,40 and offer some protection in experimental endotoxaemia in mice.32 Direct interaction with lipoploysaccharide accounts for only a fraction of the anti-endotoxic activity of LL-37, and transcriptional profiling of human monocytes stimulated with lipoploysaccharide, with and without LL-37, established a complex pattern of modulation of the lipoploysaccharide response, with a profound inhibition of proinflammatory genes, but retention of expression of chemotactic mediators and negative regulators of TLR signalling.40 On the basis of these studies, we have proposed that at least in some physiological settings LL-37 and possibly other peptides act to promote localised immunity to infection, while preventing systemic hyperinflammatory response.
In contrast to the well-established anti-endotoxic activity of some peptides, the effects of peptides on cellular responses to other proinflammatory stimuli are cell type and stimulus dependent. This is well illustrated by the effects of peptides on dendritic cells. Immature monocyte-derived dendritic cells generated in the presence of LL-37 have increased phagocytic activity and, after maturation, show enhanced capacity to induce Th1-polarised immunity.42 In contrast, simultaneous treatment of immature monocyte-derived dendritic cells with LL-37 and TLR ligands inhibits dendritic cell maturation.41 In plasmacytoid dendritic cells, LL-37 strongly augments responses to host and bacterial DNA, and this activity may contribute to the pathology of psoriasis.43 Furthermore, murine β-defensin 2 was reported to act as an endogenous TLR4 ligand, promoting dendritic cell maturation and Th1 polarisation of immune response.55
The biological importance of HDPs in mammalian immunity has been investigated in several mouse models. The role of cryptidins in the gut is highlighted by the following reports: MMP7-knockout mice lacking mature cryptidins and NOD2-null mice with reduced cryptidin production are more susceptible to oral challenges with Escherichia coli and Listeria,10, 56 whereas transgenic mice with a Paneth cellspecific expression of human cryptidin HD5 are more resistant to oral challenges with Salmonella.57 The role of cathelicidins in the immune defences of epithelial surfaces is addressed by the studies of CRAMP-null mice, which develop necrotic skin infections when challenged with group A Streptococcus,58 and are also more susceptible to infections of the urinary tract.59
Dysregulation of HDP production is also implicated in the pathology of a number of human diseases. In the specific granule deficiency and morbus Kostmann syndromes, deficiencies in neutrophil HDPs (and other proteins of neutrophil granules) are associated with persistent bacterial infections.60, 61 Reduced HDP production in the skin is also associated with human disorders, with reduced LL-37 and β-defensin levels in atopic dermatitis being linked to frequent skin infections.62 In contrast, abnormally high levels and altered proteolytic processing of cathelicidins are implicated in skin inflammatory conditions of rosacea and psoriasis.43, 63 Disruption of HDP production and function at mucosal surfaces is also implicated in human pathologies, in particular in Crohn's disease and cystic fibrosis. It was suggested that the bacterial colonisation of the airways of cystic fibrosis patients, is at least in part, the result of inactivation of antimicrobial peptides by the high salt contents of airway fluids in cystic fibrosis.64, 65 Mutations in NOD2, associated with Crohn's disease, result in impaired production of cryptidins and this was proposed to contribute to the chronic intestinal inflammation by disrupting the interactions between the immune system and the microflora.12, 56
In summary, HDPs with their direct antimicrobial properties and diverse immunomodulatory activities play an important role in mammalian immunity to infections and immune homoeostasis. The potential for these activities for clinical and commercial purposes remains to be fully explored.
Therapeutic potential of host defence peptides
With increasing bacterial resistance to conventional antibiotics,66 there is a growing interest in exploiting the biological activities of HDPs to target multidrug-resistant pathogens.3 However, despite much progress in the laboratory, so far only a few HDP-derived compounds have progressed into the clinic, with most clinical trials focusing on topical rather than systemic applications (Table 2).
Table 2.
Peptides and peptidomimetics in commercial development
| Company | Drug | Stage of development | Medical use |
|---|---|---|---|
| AM-Pharma (Bunnik, The Netherlands) | hLF-1-11 | Phase II | Allogeneic bone marrow stem cell transplantation-associated infections |
| BioLineRx Ltd (Jerusalem, Israel) | BL2060 | Preclinical | Gram-negative pneumonia |
| Ceragenix (Denver, Colorado, USA) | CSA-13 a /CGX313 | Preclinical | Prevention of nasal carriage of Staphylococcus |
| Helix Biomedix (Bothell, Washington, USA) | Lipohexapeptide | Preclinical | Anti-infective |
| Inimex (Burnaby, British Columbia, Canada) | IMX942 | Preclinical | Immunomodulation; treatment of fevers and neutropaenia in chemotherapy patients |
| Migenix Inc. (Vancouver, British Columbia, Canada) | CPI/MX-226 | Phase IIIb | Prevention of catheter-related infections |
| CLS001 | Phase II+ | Inflammation in Rosacea | |
| Novozymes A/S (Bagsvaerd, Denmark) | Plectasin | Preclinical | Systemic anti-Gram positive, especially pneumococcal infections |
| Pacgen (Vancouver, British Columbia, Canada) | PAC113 | Phase IIb | Oral candidiasis |
| Polymedix (Radnor, Philedelphia, USA) | PMX30063 b | Phase I | Systemic anti-infective peptidomimetic |
A cationic steroid antibiotic.
Non-peptidic structural analogue of an antimicrobial peptide.
This is a listing of known antimicrobial and/or immunomodulatory agents in development and/or clinical trials within private companies. Only agents currently in clinical trials or likely to enter the clinic soon are recorded. This information is based on a prior review,3 updated by us primarily from company press releases and public presentations. Several peptides that went through clinical trials but were not approved3 or polymyxins and gramicidin S that are generic anti-infectives that have long been available are not described
The traditional approach to developing HDP-based therapeutics has focused on the antimicrobial properties of natural peptides. Various methodologies for identifying, selecting, or designing highly active artificial antimicrobial peptides have been developed. One approach is to screen extensive libraries of semirandom peptides for bactericidal properties.67, 68 Alternatively, systematic substitutions in the amino-acid sequence of natural antimicrobial HDPs can be used to improve their activity. For example, combining highthroughput peptide synthesis on cellulose sheets with automated screening for antimicrobial properties using a luciferase reporter system allowed the development of bactenecin derivatives with dramatically improved activity.69 Furthermore, several computer-aided approaches to analyse structure–function relationships in natural and artificial peptide libraries permitted the prediction of peptide activity and design of novel peptides with stronger antimicrobial properties.70, 71
One of the limitations that need to be overcome before peptides can be widely used in the clinic is the high cost of peptide production. Costs can be reduced to some extent by focusing on linear peptides of minimal length, and truncated derivatives of bactenecin and indolicidin with lengths of 8–12 amino acids are good candidates.69 However, more complex structures can be effectively generated on a large scale using recombinant technology72 and should certainly be pursued. Lantibiotics are bacterial peptides with antimicrobial properties produced on an industrial scale for use as food preservatives. They make up one class of such compounds.73 Their potential therapeutic value certainly warrants further investigation. Another approach to reducing the costs of peptide therapeutics is to improve peptide stability and pharmacokinetics, thus decreasing the required dose. Currently, this is investigated with protease-resistant D-amino acid peptides and various peptidomimetics.3
Concerns have been raised that a widespread use of HDPs in the clinic would select for pathogens resistant to natural immune defences. Indeed, many bacterial species already possess modestly effective resistance mechanisms, including peptide degradation, sequestration, efflux, and chemical modifications of cell walls and membranes to reduce HDP binding;74, 75 resistance to HDPs can be selected in the laboratory.76 Nevertheless, HDPs are less prone to inducing resistance than conventional antibiotics because they often use several microbicidal mechanisms simultaneously, target ing many microbial systems with low affinity rather than having one specific target.75 Also, because of the diverse mechanisms of peptide action, the use of synthetic peptides that do not occur in nature could partly alleviate the problem of resistance to natural HDPs. Finally, immunomodulatory peptides that act on the host rather than on the pathogen offer a unique opportunity to minimise the direct selective pressures for pathogen resistance.
Recently, such an immunomodulatory peptide, an innate defence regulator IDR-1, was shown to protect mice against bacterial infections, including infections with multidrugresistant pathogens, and this provides an important proof of principle for the immunomodulatory approach.38 The peptide was shown to act as a neutrophil chemoattractant37 and furthermore to induce chemokine production and promote cell recruitment in vitro and in vivo; these activities may account for some of its protective effects. Importantly this peptide, as well as many natural HDPs, exerts anti-inflammatory and anti-endotoxic effects at the same time as promoting local clearance of infection.38, 40 Thus, unlike other treatments aimed at boosting the immune system, such peptides can offer protection without the risk of inducing dangerous hyperinflammatory states. In the immediate future, the field of immunomodulatory peptide therapeutics faces the challenges of developing highthroughput screens for immunomodulatory activity, and also of firmly defining which in vitro activities correlate with the protection against infection and other desirable biological outcomes in vivo. Furthermore, other activities of HDPs, such as their roles in wound healing and angiogenesis, require further investigation to assess their potential therapeutic value.54
Conclusion
Over the course of evolution, nature has created an impressive arsenal of HDPs with extreme diversity in structure and biological activity. These can serve as excellent templates for development of both antimicrobial and immunomodulatory compounds, often combining both activities in the same molecule. The potential therapeutic value of such compounds is just beginning to be fully recognised. These compounds can be used in combination with conventional antibiotics, and also to target resistant pathogens where conventional antibiotics fail. Some peptides may even have potential against diseases of unknown aetiology (emerging infectious diseases), as their spectrum of activity is often very broad and may involve stimulation of the immune response of the host rather than targeting the pathogen. Importantly, immunomodulatory peptides that target the host immune system rather than the pathogen also offer an excellent opportunity to minimise the risks of pathogen resistance to these compounds. The current challenge is to develop new biologically active synthetic peptides or mimetics thereof with improved pharmacokinetics, low toxicity, and low manufacturing costs for both topical and systemic applications.
Acknowledgements
We are financially supported by Genome Canada and Genome BC, the Foundation for the National Institutes of Health, the Gates Foundation, and the Canadian Institutes of Health Research (CIHR). AN is a CIHR and Michael Smith Foundation postdoctoral research fellow. REWH is a Canada Research Chair in Microbiology.
Footnotes
Author contributions
Both authors contributed equally to the development, intellectual concepts, and writing of this paper.
REW Hancock has founded companies for the exploitation of host defence peptides as antimicrobials (Migenix) and immunomodulators (Inimex). In both companies, he is a minor shareholder, whereas in the latter he is a member of the Scientific Advisory Board and consultant.
Provenance and peer review
Commissioned without payment, externally peer-reviewed.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Powers JP, Hancock RE. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–91. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–7. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 4.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 5.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 6.Zeya HI, Spitznagel JK. Antibacterial and enzymic basic proteins from leukocyte lysosomes: separation and identification. Science. 1963;142:1085–7. doi: 10.1126/science.142.3595.1085. [DOI] [PubMed] [Google Scholar]
- 7.Ayabe T, Ashida T, Kohgo Y, Kono T. The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends Microbiol. 2004;12:394–8. doi: 10.1016/j.tim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Wehkamp J, Schauber J, Stange EF. Defensins and cathelicidins in gastrointestinal infections. Curr Opin Gastroenterol. 2007;23:32–8. doi: 10.1097/MOG.0b013e32801182c2. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–90. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 10.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, López-Boado YS, Stratman JL, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–17. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 11.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ, et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–18. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 12.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129–34. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, et al. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998;102:874–80. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM, et al. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–60. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 15.Harder J, Bartels J, Christophers E, Schro der JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 16.Stolzenberg ED, Anderson GM, Ackermann MR, Whitlock RH, Zasloff M. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci USA. 1997;94:8686–90. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 18.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 19.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE, et al. Impact of LL-37 on anti-infective immunity. J Leukoc Biol. 2005;77:451–9. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 20.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure–function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–8. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 21.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–93. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–803. [PubMed] [Google Scholar]
- 23.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL 37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 25.Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Ståhle-Bäckdahl M, et al. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–6. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Tolllike receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 27.Peyssonnaux C, Boutin AT, Zinkernagel AS, Datta V, Nizet V, Johnson RS, et al. Critical role of HIF-1alpha in keratinocyte defense against bacterial infection. J Invest Dermatol. 2008;128:1964–8. doi: 10.1038/jid.2008.27. [DOI] [PubMed] [Google Scholar]
- 28.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–15. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavanagh K, Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol. 2004;56:285–9. doi: 10.1211/0022357022971. [DOI] [PubMed] [Google Scholar]
- 30.Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–7. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 31.Farnaud S, Evans RW. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol Immunol. 2003;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 32.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–91. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 33.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–56. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 34.Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–24. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 35.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, et al. LL-37, the neutrophil granuleand epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–6. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HY, Bae YS. The anti-infective peptide, innate defense-regulator peptide, stimulates neutrophil chemotaxis via a formyl peptide receptor. Biochem Biophys Res Commun. 2008;369:573–8. doi: 10.1016/j.bbrc.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 38.Scott MG, Dullaghan E, Mookherjee N, Glavas N, Waldbrook M, Thompson A, et al. An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechnol. 2007;25:465–72. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
- 39.Bowdish DM, Davidson DJ, Speert DP, Hancock RE. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol. 2004;172:3758–65. doi: 10.4049/jimmunol.172.6.3758. [DOI] [PubMed] [Google Scholar]
- 40.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–64. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 41.Kandler K, Shaykhiev R, Kleemann P, Klescz F, Lohoff M, Vogelmeier C, et al. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol. 2006;18:1729–36. doi: 10.1093/intimm/dxl107. [DOI] [PubMed] [Google Scholar]
- 42.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–56. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 43.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 44.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–31. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 45.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human betadefensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 47.Barlow PG, Li Y, Wilkinson TS, Bowdish DM, Lau YE, Cosseau C, et al. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80:509–20. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol. 2006;176:3044–52. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Cherryholmes G, Shively JE. Neutrophil secondary necrosis is induced by LL-37 derived from cathelicidin. J Leukoc Biol. 2008;84:780–8. doi: 10.1189/jlb.0208086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carretero M, Escamez MJ, Garcia M, Duarte B, Holguín A, Retamosa L, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128:223–36. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 51.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–8. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 52.Lau YE, Rozek A, Scott MG, Goosney DL, Davidson DJ, Hancock RE, et al. Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect Immun. 2005;73:583–91. doi: 10.1128/IAI.73.1.583-591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sørensen OE, Borregaard N, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 54.Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/ hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 57.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–6. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 58.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 59.Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–41. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 60.Ganz T, Metcalf JA, Gallin JI, Boxer LA, Lehrer RI, et al. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak–Higashi syndrome and ‘specific’ granule deficiency. J Clin Invest. 1988;82:552–6. doi: 10.1172/JCI113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Putsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–9. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 62.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 63.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 64.Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Invest. 1999;103:303–7. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–36. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 66.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:122–9. doi: 10.1038/nm1145. (12 Suppl) [DOI] [PubMed] [Google Scholar]
- 67.Houghten RA, Pinilla C, Blondelle SE, Appel JR, Dooley CT, Cuervo JH, et al. Generation and use of synthetic peptide combinatorial libraries for basic research and drug discovery. Nature. 1991;354:84–6. doi: 10.1038/354084a0. [DOI] [PubMed] [Google Scholar]
- 68.Watt PM. Screening for peptide drugs from the natural repertoire of biodiverse protein folds. Nat Biotechnol. 2006;24:177–83. doi: 10.1038/nbt1190. [DOI] [PubMed] [Google Scholar]
- 69.Hilpert K, Volkmer-Engert R, Walter T, Hancock RE. High throughput generation of small antibacterial peptides with improved activity. Nat Biotechnol. 2005;23:1008–12. doi: 10.1038/nbt1113. [DOI] [PubMed] [Google Scholar]
- 70.Jenssen H, Fjell CD, Cherkasov A, Hancock RE. QSAR modeling and computer-aided design of antimicrobial peptides. J Pept Sci. 2008;14:110–14. doi: 10.1002/psc.908. [DOI] [PubMed] [Google Scholar]
- 71.Loose C, Jensen K, Rigoutsos I, Stephanopoulos G. A linguistic model for the rational design of antimicrobial peptides. Nature. 2006;443:867–9. doi: 10.1038/nature05233. [DOI] [PubMed] [Google Scholar]
- 72.Mygind PH, Fischer RL, Schnorr KM, Hansen MT, Sönksen CP, Ludvigsen S, et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–80. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 73.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777–88. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 74.Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8:11–26. [PubMed] [Google Scholar]
- 75.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–36. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 76.Perron GG, Zasloff M, Bell G. Experimental evolution of resistance to an antimicrobial peptide. Proc Biol Sci. 2006;273:251–6. doi: 10.1098/rspb.2005.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]