Abstract
We report the molecular characterization of the spineless (ss) gene of Drosophila, and present evidence that it plays a central role in defining the distal regions of both the antenna and leg. ss encodes the closest known homolog of the mammalian dioxin receptor, a transcription factor of the bHLH–PAS family. Loss-of-function alleles of ss cause three major phenotypes: transformation of distal antenna to leg, deletion of distal leg (tarsal) structures, and reduction in size of most bristles. Consistent with these phenotypes, ss is expressed in the distal portion of the antennal imaginal disc, the tarsal region of each leg disc, and in bristle precursor cells. Ectopic expression of ss causes transformation of the maxillary palp and distal leg to distal antenna, and induces formation of an ectopic antenna in the rostral membrane. These effects indicate that ss plays a primary role in specifying distal antennal identity. In the tarsus, ss is expressed only early, and is required for later expression of the tarsal gene bric à brac (bab). Ectopic expression causes the deletion of medial leg structures, suggesting that ss plays an instructive role in the establishment of the tarsal primordium. In both the antenna and leg, ss expression is shown to depend on Distal-less (Dll), a master regulator of ventral appendage formation. The antennal transformation and tarsal deletions caused by ss loss-of-function mutations are probably atavistic, suggesting that ss played a central role in the evolution of distal structures in arthropod limbs.
Keywords: Homeotic gene, dioxin receptor, aryl hydrocarbon receptor, PAS domain, antennal specification, leg development, spineless-aristapedia
The identities of body segments in the trunk and posterior head of Drosophila are controlled by the homeotic genes of the Antennapedia (ANT-C) and bithorax (BX-C) complexes (for reviews, see Lewis 1978; Duncan 1987; Kaufman et al. 1990). Although the ANT-C and BX-C genes specify most aspects of body segment specialization, at least three appendage structures develop independently of these genes. First, Carroll et al. (1995) have shown that wing formation in the second thoracic segment (T2) requires no input from the ANT-C and BX-C genes. Instead, these genes function to repress wing development in all segments but T2. Second, the antenna receives no input from the ANT-C and BX-C genes, as these are not expressed within the antennal segment (Kaufman et al. 1990; Jürgens and Hartenstein 1993). Third, formation of the distal part (the tarsus) of the T2 leg appears not to require ANT-C or BX-C genes, as normal second leg tarsi are produced by clones homozygous for null alleles of Scr, Antp, or Ubx (Struhl 1981, 1982b; Abbott and Kaufman 1986), the ANT-C and BX-C genes that specify segment identities in the thorax. These genes do, however, play an important role in more proximal regions of the second leg, and in both proximal and distal regions of the first and third legs (Struhl 1982b).
What genes are responsible for specifying development of the wing, antenna, and T2 tarsus? For the wing, a key gene appears to be vestigial (vg). vg encodes a novel nuclear protein that is expressed in the pouch region of the wing imaginal disc (Williams et al. 1991). Ectopic expression of vg causes the development of wing tissue in other discs, indicating that vg is a primary determinant of wing development (Kim et al. 1996). However, no genes have been identified that play similar roles for the antenna or T2 tarsus. In this report, we present evidence that the spineless (ss) gene plays a key role in specifying the development of both structures. Loss-of-function alleles of ss cause transformation of the distal part of the antenna (arista) to distal second leg, and deletion of most of the tarsal region of each of the legs (Lindsley and Zimm 1992). In addition, these alleles cause a severe reduction in size of most bristles. Almost all ss alleles cause the antennal homeotic transformation, and are known as spineless–aristapedia (ssa) alleles. Most alleles affect bristle size to some degree, but only very strong alleles also cause tarsal deletions (Struhl 1982a). Although ss has been known for many years, there has been little interest in the gene because its pleiotropy has been taken to suggest that it plays some general, perhaps metabolic, role (see, e.g., Raff and Kaufman 1983).
In this report we describe the molecular characterization of ss. The results are not consistent with a general metabolic function. We find that ss encodes the closest known homolog of the mammalian dioxin receptor, a bHLH–PAS domain transcription factor. ss is not expressed uniformly, but shows distinct expression patterns that correspond to its three major functions. ss transcripts accumulate in the distal portion of the antennal disc, in the tarsal regions of the leg discs, and in bristle precursor cells. ss is also expressed in the embryo in the antennal segment, the thoracic imaginal primordia, and the peripheral nervous system. Ectopic expression of ss causes transformation of the maxillary palp and distal leg to distal antenna, and induces development of an ectopic antenna in the rostral membrane, which normally has no appendage. These effects argue that ss is a primary determinant of antennal identity. In the tarsus, ss is expressed only early in development and is required for later expression of the tarsal gene bric à brac (bab) (Godt et al. 1993). This suggests that ss functions in the establishment of the tarsal primordium. Ectopic expression of ss causes the deletion of medial leg structures, perhaps because these are incorrectly specified as tarsus. ss expression in both the antenna and leg is shown to be dependent upon Distal-less (Dll), a master regulator of ventral appendage development (Cohen and Jürgens 1989; Gorfinkiel et al. 1997). We argue that ss has played an important role in the evolution of both the antenna and the distal leg.
Results
Cloning of the ss gene
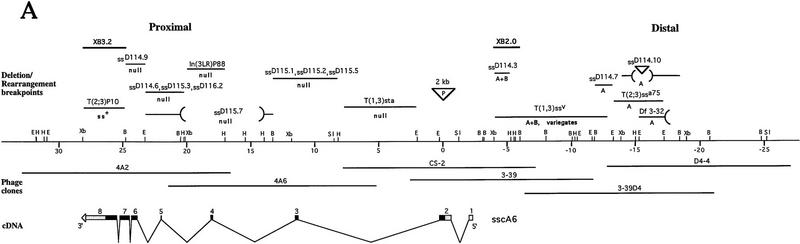
The ss locus was tagged by a P-element insertion. The insertion allele, now lost, caused a weak ssa phenotype when homozygous, carried a P-element at the ss locus (polytene bands 89C1,2; Lewis 1963), and was revertable to ss+ by mobilization of the P element. Following isolation of P-element containing clones from the locus, phage clones covering ∼30 kb to either side of the P-element insertion were recovered by chromosome walking. A map of the cloned DNA is presented in Figure 1A.
Figure 1.
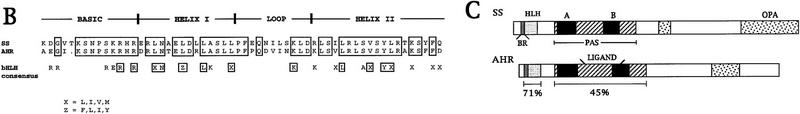
Molecular analysis of the ss locus. (A) Map of the cloned DNA. Coordinates are in kb; position 0 is the insertion site of the P-element tag used to clone the locus. (B) BamHI; (E) EcoRI; (H) HindIII; (Sl) SalI; (Xb) XbaI. Overlapping phage clones are indicated below the restriction map; phage 3-39 is the P-element containing clone that initiated the walk. The positions of 17 breakpoint mutants are noted above the map; breakpoints and their uncertainties are indicated by lines, deletions by parentheses, and insertions by inverted triangles. The phenotypes of these mutants are noted as null, antennal transformations (A) or antennal transformations coupled with bristle defects (A+B). XB3.2 and XB2.0 indicate genomic fragments that detect transcripts when used to probe Northern blots. Coding sequences and untranslated regions within the exons of the sscA6 cDNA are indicated by black and shaded boxes, respectively. (B) Amino acid identity of ss to AHR in the bHLH domain. Boxed amino acids are identical between the two proteins, and identities of both ss and AHR to the bHLH consensus is noted below. (C) Structure of the ss and AHR proteins drawn to scale. Motifs are noted on the ss protein schematic. (BR) Basic region; (HLH) helix–loop–helix; (A, B, PAS) A and B repeats of the PAS domain; (OPA) region of opa or CAX repeats. The position of the ligand binding domain of AHR is also noted.
To clarify the genetic organization of ss, a screen for new X-ray-induced alleles was conducted. The 25 mutants recovered are summarized in Table 1. Eleven of these, as well as six pre-existing rearrangements, were localized by Southern blotting and in situ hybridization to mutant polytene chromosomes. A proximal limit to the locus at +25 to +28 kb (0 being the site of P-element insertion) is provided by the breakpoint of Tp(3;2)P10, which does not affect ss function. All ten of the null mutations mapped are broken within the region from about +28 through +2. As described below, these alleles all interrupt the ss transcribed region. Six of the alleles mapped lie in the upstream region from −4 to −18. These mutants all cause strong homeotic transformation of the distal antenna (arista) to tarsus, but do not cause distal deletions in the legs or transformed antennae. Effects on bristle size vary with position; the most proximal of the mutants in the upstream group (ssD114.3) causes a strong reduction in bristle size, the T(1;3)ssV allele variegates for bristle size, and the four more distal mutants in this group cause a weak reduction in bristle size. The distal limit of ss is not known.
Table 1.
New X-ray-induced ss alleles
| Allele
|
Cytology
|
Hemizygous phenotypea
|
Breakpointsb
|
|---|---|---|---|
| ssD114.1 | Df(3R)89A-B;89E present | — | — |
| ssD114.2 | normal | null | N.D. |
| ssD114.3 | T(1;3)6C;89C1-2 | A, B | −4/−5.5 |
| ssD114.4 | Df(3R)89B;89C | — | — |
| ssD114.5 | T(2,3)55C;80AB, normal at 89C | moderate A, moderate B, variable L | N.D. |
| ssD114.6 | Tp(3;het);89C;90B | null | +21/+23 |
| ssD114.7 | 89C/heterochromatin | moderate A, moderate B | −12/−13 |
| ssD114.8 | normal | null | N.D. |
| ssD114.9 | In(3R)89B9-14;C1-2 | null | +24/+25 |
| ssD114.10 | normal | moderate A | −14/−17, internal deletion of at least 1 kb + insertion |
| ssD114.12 | Complex T(Y;3). Probable new order: Y/84D-89CD/99F-90A/84D-61 + Y/90A-89CD/100A-100F | A, moderate B | N.D. |
| ssD115.1 | In(3R)88CD;89C1-2 | null | +8/+13.5 |
| ssD115.2 | normal | null | +8/+13.5 |
| ssD115.3 | normal | null | +21/+23 |
| ssD115.5 | T(2;3)43A;89C1-2 | null | +8/+13.5 |
| ssD115.6 | normal | null | N.D. |
| ssD115.7 | normal | null | internal deletion; proximal break at +21/+23, distal break at +13/+14 |
| ssD115.8 | Df(3R)89B18-22;89D3-5 | — | — |
| ssD115.12 | Tp(3;3)71A;89E;90B + Df(3R)89B20-22;89E | — | — |
| ssD116.1 | In(3R)81;89C1,2 | null | N.D. |
| ssD116.2 | T(Y;3). New order: Y/89C-84E/89C-100 + 61-84E/Y | null | +21/+23 |
| ssD116.4 | normal | A, B | N.D. |
| ssD116.5 | normal | A, B | N.D. |
| ssD116.7 | normal | null | N.D. |
| ssD116.8 | In(3R)81;99E. 89C normal | null | N.D. |
Phenotype when heterozygous with Df(3R)ssD114.4. (A) Antennal transformation; (B) reduction in bristle size; (L) tarsal deletions.
As determined by Southern blotting. Coordinates indicate altered restriction fragments. (N.D.) No changes detected.
Northern blotting revealed two transcribed regions distal to the Tp(3;2)P10 breakpoint. A probe from the more proximal of these (from +25 to +28 kb) detects a smear of RNAs ranging from 0.7 to 6.5 kb that has several embedded bands, the largest of which is 5.4 kb (not shown). The second transcribed region lies more distally, at −4 to −6 kb. This region hybridizes to a major transcript of 1.2 kb and minor transcripts of 2.2 and 3.3 kb. These are detected from the second larval instar through adulthood (not shown).
To assess which transcripts from ss are most important functionally, genomic regions were used as probes for in situ hybridization to transcripts in imaginal discs. The proximal region of ss gave specific signal in the three regions expected for ss: the antenna, legs, and bristle precursor cells. This, and other evidence described below, indicates that transcripts from this region are the major functional products of ss. In contrast, the distal transcribed region gave no specific signal in imaginal discs or embryos, and cDNAs from this region do not contain appreciable ORFs (data not shown). The significance of these transcripts is unknown. They are transcribed from a probable regulatory region of ss (see below), and may therefore be similar to the bxd and iab region transcripts of the bithorax complex (Lipshitz et al. 1987; Cumberledge et al. 1990).
Isolation of ss cDNAs
To isolate cDNAs for the proximal transcribed region of ss, a genomic fragment from +25 to +28 was used to screen an eye–antennal imaginal disc library (kindly provided by Dr. G. Rubin, University of California, Berkeley). Approximately 1.5 × 106 plaques were screened, and 10 distinct cDNAs obtained. The largest of these, which is 5.2 kb in length, was chosen for sequence analysis. This cDNA, designated sscA6, is probably near full length, as it is only 200 bp shorter than the largest band seen on Northerns. Exons in this cDNA are spread over a 30-kb genomic region that encompasses all the ss null breakpoints mapped, indicating that it represents a functional transcript of the locus.
The ss protein
Sequencing of sscA6 revealed a single ORF that encodes a protein of 884 amino acids (Fig. 2). This ORF has good Drosophila codon bias throughout. Although the sequence surrounding the putative initiating methionine does not match the Drosophila consensus well (Cavener 1987), homology considerations (see below) suggest this codon is the initiator. The 3′ portion of the ORF is rich in opa (CAX) repeats, which encode primarily glutamine or histidine. These repeats extend beyond the ORF for an additional 600–700 nucleotides. Curiously, the 3′ end of sscA6 lies to the left of the Tp(3;2)P10 breakpoint, which is ss+, implying either that the 3′ end of the ss transcribed region is dispensable, or that sequences able to substitute for the ss 3′ end are introduced by this rearrangement. As indicated in Figure 2, an additional cDNA of 2.8 kb (sscA5) was sequenced. The protein it encodes is three residues shorter than that encoded by sscA6. This difference likely results from alternate splicing.
Figure 2.
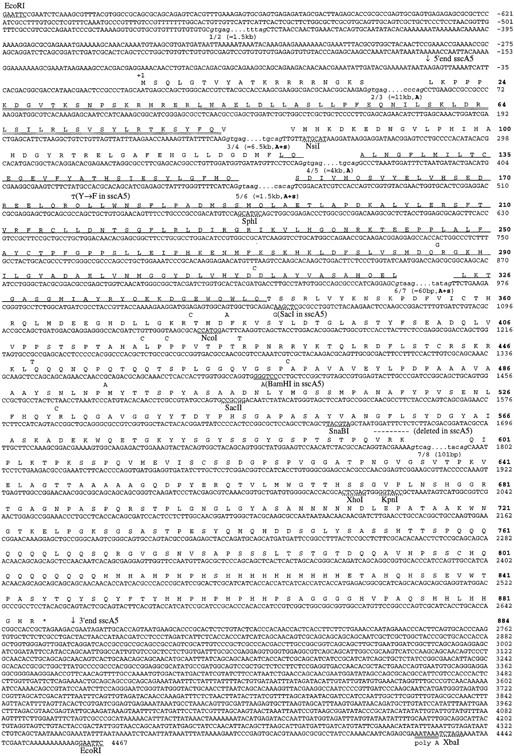
Sequence of the ss cDNAs. The sequence and conceptual translation of the ss cDNA sscA6 is shown, along with the polymorphisms associated with the sscA5 cDNA. The nucleotide sequence is numbered by plain text at the end of each row; the corresponding amino acid sequence numbering is depicted in boldface type. Downward-pointing arrows indicate the ends of the sscA5 cDNA; broken lines above nucleotides +1789 through +1797 indicate the three codons absent in the sscA5 cDNA. The bHLH region is double underlined; the PAS domain is single underlined. The translation stop is noted by an asterisk. The positions of the seven exon splice junctions are in lowercase text; the donor and splice acceptors are italicized; the three flanking intronic nucleotides are in plain text. The approximate length of each intron is in parentheses, and those splice sites conserved in Ahr or sim are noted by a bold A and s, respectively.
The putative ss protein shows extensive similarity to the human and murine aryl hydrocarbon receptor (AHR, also known as the dioxin receptor), as well as lower similarity to the human aryl hydrocarbon nuclear translocator, hypoxia-inducible factor 1, and endothelial PAS-1 (Tian et al. 1997) and the Drosophila proteins single-minded (sim), period, and trachealess (trh) (for review, see Schmidt and Bradfield 1996). These proteins comprise a recently recognized family distinguished by the PAS domain, which can mediate protein–protein interactions (Huang et al. 1993). Most members of the family also contain a bHLH domain. ss is most similar to AHR; as can be seen in Figure 1, there is 71% identity between the two proteins in the bHLH region, 45% identity in the PAS domain, and 41% identity overall. The organization of domains within the two proteins is also closely similar. ss and AHR share almost twice as much sequence identity as either shares with their next closest relative, sim.
Sequencing of exons reveals that all five of the ss splice sites within the bHLH and PAS coding sequences are conserved in Ahr (Fig. 2). These splice sites lie within conserved codons in the two genes, and separate precisely the same nucleotide positions within these codons (Schmidt et al. 1993). Ahr has three additional splice sites in the PAS domain coding sequence that are not shared with ss. Three of the five splice junctions of ss are also shared with sim.
Transcript distributions in imaginal discs and the ss mutant phenotype
In situ hybridization of ss probes to imaginal discs reveals distinct phases of transcript accumulation that correspond to the leg, antennal, and bristle functions of ss.
The leg
In leg discs, ss staining is first seen in the late second instar in a central ring that corresponds to the presumptive tarsal region (Fig. 3E) (all references here to imaginal fate maps are from Bryant 1978). This ring is transient, and fades out by the late third instar. The ss tarsal ring likely corresponds to the tarsal structures deleted in ss− mutants, which include the distal part of the first tarsal segment and the second through fourth tarsal segments (Fig. 4E). After the tarsal ring fades out, ss becomes expressed in a patch in the anterior-proximal portion of the disc in a region that will give rise to structures of the thorax proper (Fig. 3F). ss null alleles show no defects in these structures.
Figure 3.
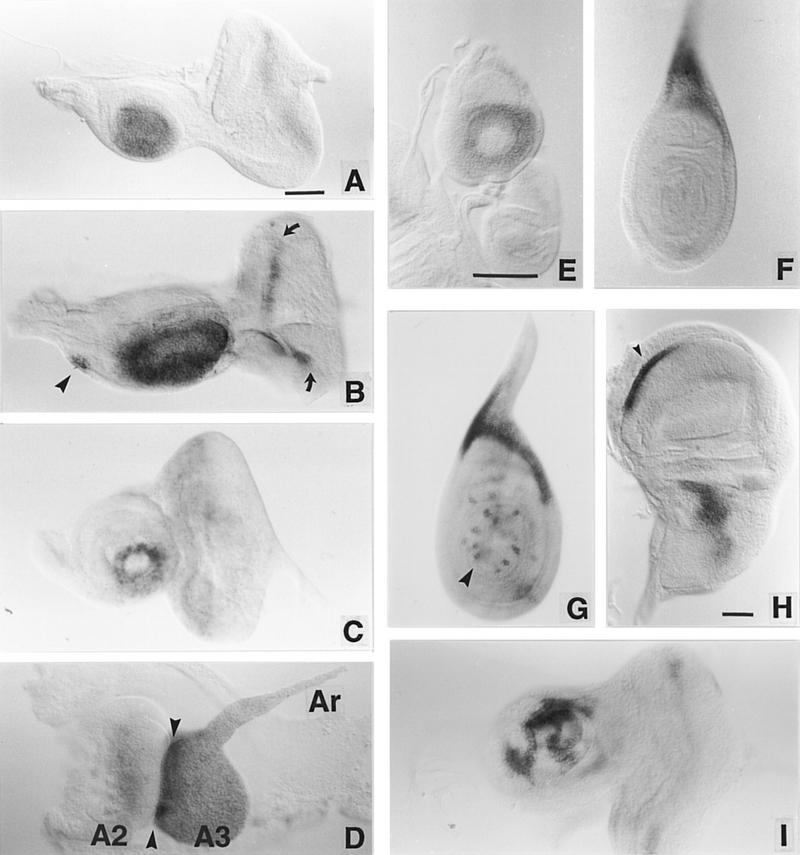
ss expression in imaginal discs. Imaginal discs from third instar larvae and pupae were hybridized with RNA probes transcribed from the XB3.2 genomic clone or the sscA6 cDNA. (A,B) Eye–antennal discs from early and late wild-type third instar larvae. In B the arrowhead indicates the maxillary palp anlage and the arrows indicate the morphogenetic furrow in the eye disc. (C) ss transcript accumulation in ssa mutant (ssD114.3/ssD114.4) third instar eye–antennal discs. The pattern resembles that in the wild-type early third instar leg (cf. E). (D) ss expression in an everted antennal disc. Arrowheads indicate the boundary between the second (A2) and third (A3) segments. (Ar) The arista. (E– G) ss expression pattern in early E and late F third instar leg discs, and in a leg disc just prior to eversion (G). The arrowhead in G indicates bristle precursor cell labeling. (H) ss expression in a mature third instar wing disc. Expression is seen in an anteroventral stripe (arrowhead) and a patch in the presumptive notal region. (I) ss antennal expression in a heterozygote for the Antp gain-of-function allele Antp73b. Variegated reduction in ss expression is seen. Scale bars, 50 μm; the bar in A refers to A– D and I; the bar in E refers to E, F, and G.
Figure 4.
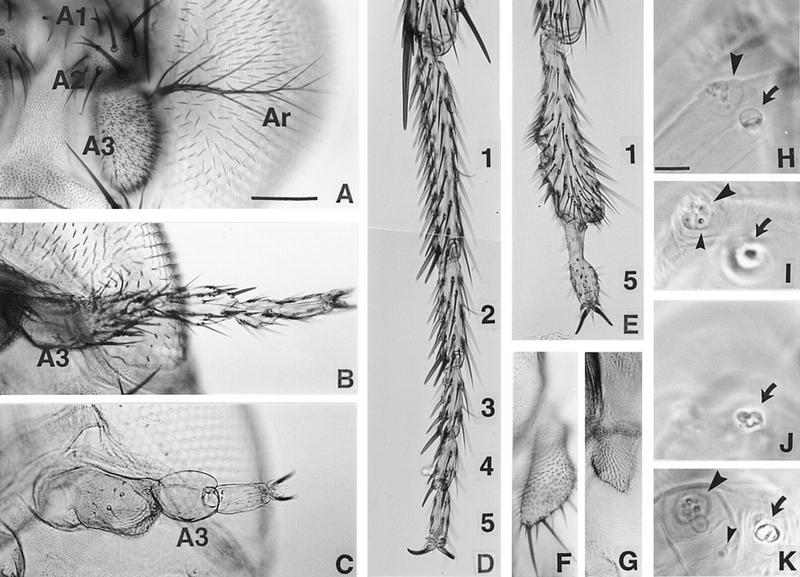
Cuticular phenotypes of ss mutants. The antennal and leg phenotypes of ss mutants have been described by others [see Lindsley and Zimm (1992) and references therein]. (A,B) Antennae from wild-type (A) and ssa (ssD114.10/ssD114.4) (B) adults. The first (A1), second (A2), and third (A3) antennal segments and the arista (Ar) are indicated. The antennal tarsus in ssa mutants is judged to have T2 identity by the following criteria: Paired rows of stout bracted macrochaetae present ventrally (Hollingsworth 1964) are like those on the second leg; the most distal bristle pair in each segment is larger than more proximal pairs; on average, there are three pairs of bristles in the fifth tarsal segment (Lawrence et al. 1979); and there are no posterior transverse bristle rows in the second tarsal segment. (C) Antenna from a ss null mutant (ssD115.7). A3 has no bristles or trichomes, and most of the distal tarsal region is absent. (D–E) Distal second leg of wild-type and a ss null mutant. Tarsal segments 2–4 and part of segment 1 are deleted in E. (F,G) Wild-type and ss null mutant maxillary palps. The mutant palps are truncated. (H– K) Wild-type (H,I) and ss null mutant (J,K) first instar antennal and maxillary sense organs (AnSO and MxSO). The AnSO is indicated with a large arrow, the MxSO with a large arrowhead, and the dorsomedial papilla (DMP) with a small arrowhead. In the mutant the AnSO is cauliflower-shaped and has no clear stalk, and migration of the DMP is impaired. Scale bars, A–G (shown in A), 100 μm; H–K (shown in H), 10 μm.
The antenna
ss staining in the antennal disc is first detected during the late second instar (not shown). At all times after this, intense staining is seen in an oval patch in the central (distal) portion of the antennal disc (Fig. 3A,B). After disc eversion, the limits of intense ss expression coincide precisely with the boundary between the second (AII) and third (AIII) antennal segments (Fig. 3D).
ss alleles broken in the upstream region of the gene have no effect on ss expression in the leg, but alter expression in the antenna to resemble that normally seen in the leg. The effects of a translocation broken at −4 kb to −5 kb (ssD114.3) are shown in Figure 3C. ss is expressed in a transient ring in the antennal and leg discs in this mutant. This indicates that the antennal expression pattern of ss is controlled by the region upstream of −4 kb to −5 kb, whereas the tarsal pattern of expression is likely controlled by a downstream, perhaps intronic, region. Consistent with their effects on ss expression, mutants located in the upstream region cause transformation of the distal antenna to tarsus (Fig. 4B). However, only the distal part of AIII and the arista are affected. Detailed examination of one upstream mutant (ssD114.7) indicates that the antennal tarsus produced has second leg identity (see legend to Fig. 4), as suggested previously for the ssa allele (Morata and Lawrence 1979).
In ss null mutants, the antenna shows both transformation to leg and tarsal deletion (Fig. 4C). In this case, the entire AIII segment and arista are affected. Strikingly, the AIII segment in ss null mutants is unlike any normal appendage segment in that it completely lacks bristles or cuticular hairs. This segment is followed distally by most or all of a fifth tarsal segment terminated by claws.
Other adult structures
In the late third instar, ss becomes expressed in a small patch in the antennal disc that corresponds to the maxillary palp anlage (Fig. 3B). Consistent with this, the maxillary palps of null mutants are truncated (Fig. 4F,G). This suggests a general requirement for ss in the development of distal structures in ventral appendages.
In the wing disc, ss is expressed in the presumptive notum and wing hinge region, and in a ventral stripe (Fig. 3H). Perhaps related to this expression, the wings of null mutants are held perpendicular to the body and curve ventrally. The haltere disc stains in a similar pattern (not shown). Expression is also detected in the genital and labial discs (not shown), and in the morphogenetic furrow of the eye disc (Fig. 3B). Genital, labial, and eye development appear normal in ss mutants.
Bristle precursor cells
At pupariation and disc eversion, stereotyped patterns of single large intensely stained cells are seen in most discs. This is shown only for the leg (Fig. 3G). The pattern of labeled cells is identical to that shown by the neuralized enhancer trap A101 (Huang et al. 1991), indicating that these late ss expressing cells are sensory organ precursors. At later stages, intense staining is seen in developing bristle cells, but not in the associated socket cells.
Embryos
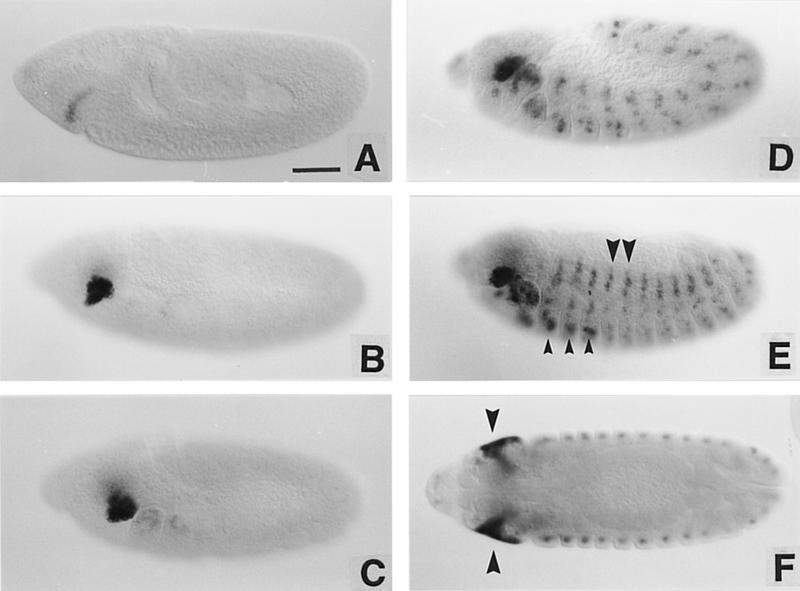
As shown in Figure 5, ss is also expressed in embryos. ss staining first appears at stage 8 (all staging as in Campos-Ortega and Hartenstein 1985) in a crescent just anterior to the cephalic furrow. This staining develops rapidly into an intense patch. As germ-band extension continues, staining develops in the maxillary, labial, and mandibular segments, followed by expression in a ventral patch in all three thoracic segments. These patches were identified as the leg anlage by double-labeling for transcripts from ss and aristaless (Campbell et al. 1993; Schneitz et al. 1993) (not shown). ss staining also appears in cells of the peripheral nervous system in each abdominal segment, as determined by double labeling for the A37 enhancer trap line (Ghysen and O’Kane 1989) (not shown). The ss staining pattern is maintained through germ-band retraction and continues until the deposition of larval cuticle makes it difficult to follow further.
Figure 5.
ss transcript distribution in embryos. (A– E) The pattern of ss transcript distribution beginning at stage 8 and continuing through late embryogenesis. Small arrowheads in E indicate the leg anlage; large arrowheads indicate expression in the peripheral nervous system. In F, an optical section midway through a germ-band retracted embryo shows the extent of expression in the invaginating eye–antennal discs (arrowheads). Scale bar, 50 μm.
To position the ss head patch, embryos were stained for both ss transcript and engrailed (en) protein. As can be seen in Figure 6, the posterior boundary of the ss antennal patch coincides precisely with the posterior edge of the antennal en stripe. There is a one-to-one correspondence between ss- and en-expressing cells for some distance along this stripe, although the en antennal stripe extends ventrally several cells beyond the ss patch. The anterior limit of ss expression lies just posterior to the en head spot, which delimits the posterior border of the ocular segment (Schmidt-Ott and Technau 1992). Thus, ss is expressed throughout most or all of the embryonic antennal segment, and is expressed in a segmental, not parasegmental, register.
Figure 6.
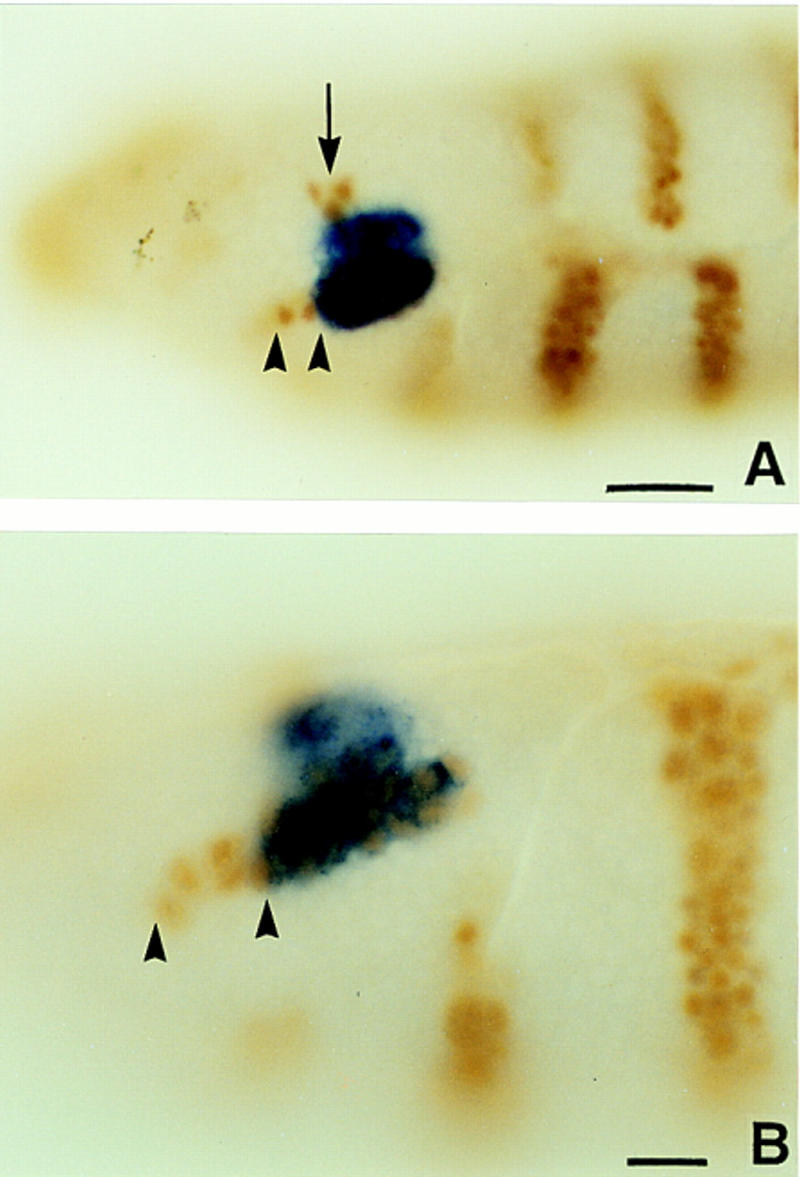
ss expression in the antennal segment of the embryo. (A,B) Wild-type embryos double labeled for ss transcript (blue) and en protein (brown). Note that the limits of ss expression extend from just ventral to the en head spot (arrow) through the antennal en stripe (arrowheads). The most ventral cells of the latter do not express ss. Scale bar in A, 20 μm; in B, 10 μm.
The expression of ss in embryos was unexpected, as no embryonic defects have been described for ss mutants. However, examination of null mutant larvae revealed that the antennal sense organ is misshapen and often sclerotized (Fig. 4J). In addition, the dorso-medial papilla of the maxillary sense organ, which is thought to be derived from the antennal segment (Jürgens et al. 1986), fails to migrate completely (Fig. 4K). Examination of ss− embryos stained with the monoclonal antibody 22C10 (Zipursky et al. 1984) failed to reveal any defects in the peripheral nervous system.
Ectopic expression of ss
To assess the developmental potential of ss, we used the GAL4/UAS system (Brand and Perrimon 1993) to drive ectopic expression of the sscA5 cDNA. Most GAL4 drivers tested proved to be lethal in combination with UAS–ss. Embryos from a cross to the 69B driver (Brand and Perrimon 1993), which is expressed in the embryonic ectoderm and its derivatives (Baylies et al. 1995), were examined in detail. These show a loss of midline structures, as well as tracheal system defects (Fig. 7I,J). These phenotypes are similar to those caused by mutations in the sim and trh genes, which encode PAS domain proteins. Ectopic ss likely interferes with the function of these proteins, perhaps by competition for a common dimerization partner.
Figure 7.
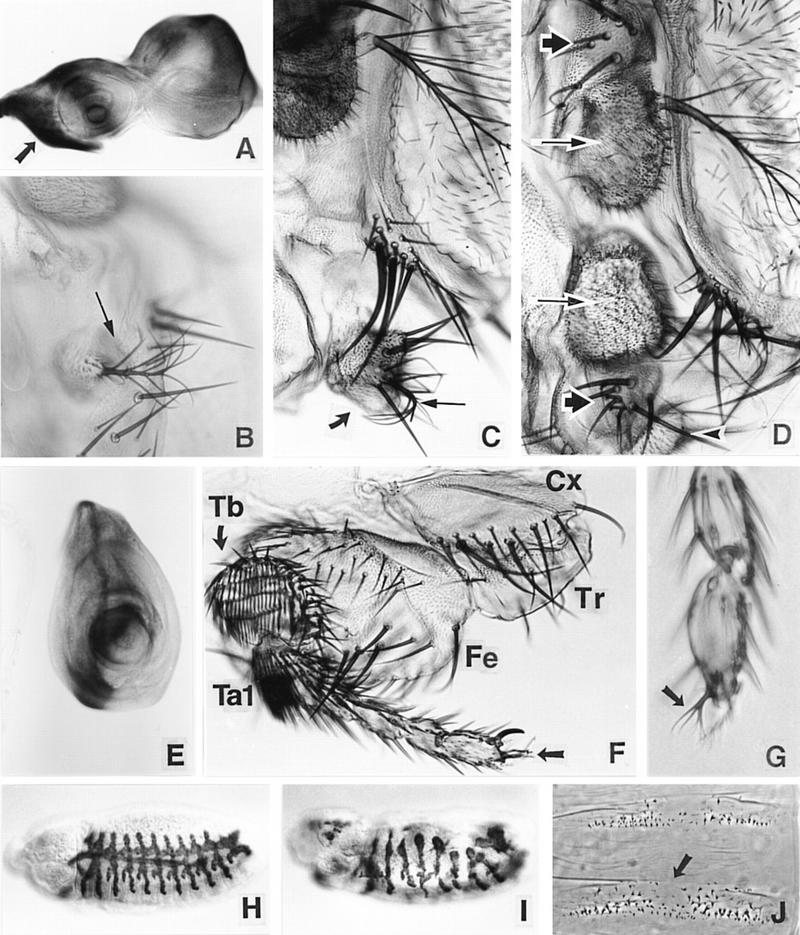
Effects of ss ectopic expression. (A) Pattern of ectopic expression driven by ptc–GAL4 in the eye–antennal disc (β-galactosidase staining). The arrow indicates a zone of high-level ectopic expression that includes the primordia of the palp and rostral membrane. (B–D) The effects of ectopic expression of ss driven by ptc–GAL4 in the head. A partial ectopic antenna (arrow) that has developed in an out pocketing of the rostral membrane is shown in B, and partial transformation of the palp (thick arrow) to arista (thin arrow) and AIII is shown in C. In D, an almost complete ectopic antenna is present in the rostral membrane region. Note that AIII (long arrows) and AII (short arrows) in the normal and ectopic antennae are arranged mirror symmetrically. The palp (arrowhead) is still present. The arista of the ectopic antenna is out of the plane of focus. (E– G) The effects of ectopic ss in the leg. The pattern of ectopic expression driven in the leg disc by ptc–GAL4 is shown in E. Deletion of most of the femur (Fe) and tibia (Tb) is shown in F. The coxa (Cx), trochanter (Tr), and most of the tarsus (Ta) are unaffected. Aristae are present at the distal tips of the legs shown in F and G (see arrows). (H–J) Effects of ectopic ss in embryos. H shows a normal embryo stained for the trh enhancer trap 1-eve-1 (Wilk et al. 1996). Ectopic ss driven by the 69B GAL4 driver causes severe abnormalities in this pattern (I). Ectopic ss also causes the deletion of midline portions of the denticle belts (arrow in J), similar to the effects of sim mutants.
A few driver/UAS–ss combinations survived to the pharate adult stage, allowing us to determine the effects of ectopic ss on adult structures. The results indicate that ss is a primary determinant of distal antennal identity. The ptc–GAL4 driver (Hinz et al. 1994) has been studied in most detail. It is expressed in imaginal discs at high level immediately anterior to the compartment boundary. ss expression driven by ptc–GAL4 causes transformation of the maxillary palp, rostral membrane, and distal leg to antenna. Transformation of the palps varies from essentially no effect to an almost complete transformation to AIII and arista (Fig. 7C). Palps are also often deleted. Surprisingly, ectopic ss induces ectopic antennal structures in the rostral membrane between the palp and the normal antenna. These range from small patches of AIII and arista (Fig. 7B) to entire ectopic antennae (Fig. 7D), and are always arranged in mirror symmetry to the normal antenna. Ectopic ss expression also causes transformation of the distal leg to arista (Fig. 7F,G). In some cases, aristal-claw intermediates are present, indicating that aristae can arise by transformation of claws. More proximal antennal structures are never present in the leg, and the pulvillus and tarsi are unaffected. Curiously, in most cases the basal cylinder of the arista is suppressed by ectopic ss.
The frequency of induction of ectopic antennae in the rostral membrane was 88% (67 half heads scored) in heterozygotes for ptc–GAL4 and our standard UAS–ss insertion (line A1). In this same genotype, transformations of distal leg to arista occurred at a frequency of 66% (100 legs scored). Because palps are usually absent in this genotype, the frequency of palp transformations was determined in flies carrying a weakly responding UAS–ss insertion (line C2). Of 100 palps scored, 27 carried an arista, and 15 showed wavy arista-like bristles.
Ectopic expression of ss also causes the deletion of medial leg structures. This occurs in 100% of ptc–GAL4; UAS–ss legs. Anterior structures from the distal femur and most of the tibia are deleted (Fig. 7F). Much of what appears to be the posterior compartment remains, however, and produces irregular cuticular outgrowths. The distortion these cause makes it difficult to define precisely the limits of the deleted regions. The distal basitarsus is clearly retained, however, because sex combs are present in the first legs of males. More distal tarsal segments are usually unaffected, with the exception of transformation of claw to arista. ss expression driven by ptc–GAL4 also causes deletion of the central part of the wing, and induces a zone of polarity reversal in the abdominal tergites (not shown). A few scattered bristles are also induced in the wing blade. The significance of these effects is not known.
Position of ss in the limb development hierarchy
The Dll gene is required for the development of all leg segments distal to the coxa (Cohen and Jürgens 1989; Gorfinkiel et al. 1997). To test whether ss lies downstream of Dll in limb development, we examined ss expression in a weak Dll loss-of-function mutant, DllPK. This allele survives to the pharate adult stage when heterozygous with Dll null alleles such as DllB, and causes the deletion of distal limb structures. We find that ss expression is almost completely eliminated in the tarsus, antenna, and maxillary palp of DllPK/DllB heterozygotes (not shown). Thus, ss lies downstream of Dll in all three of these appendages. In the antenna, ss expression is reduced in animals that carry only one dose of Dll+ (not shown). This presumably accounts for the weak transformation of distal antenna to leg seen in most Dll mutant heterozygotes (Sunkel and Whittle 1987).
To monitor Dll expression in relation to ss, we (in collaboration with Dr. S. Cohen, EMBL, Heidelberg, Germany) isolated a monoclonal antibody against Dll protein. As shown in Figure 8, Dll is expressed uniformly in the central portions of the leg and antennal imaginal discs. In the early third instar, when ss is first expressed in the leg, the outer edge of the ss tarsal ring coincides precisely with the proximal limit of Dll expression. As the leg disc grows, the boundary of Dll expression expands beyond the ss tarsal ring, so that a proximal zone of cells that express Dll, but not ss, is created. In the antenna, Dll expression extends more proximally than ss at all stages we have examined.
Figure 8.
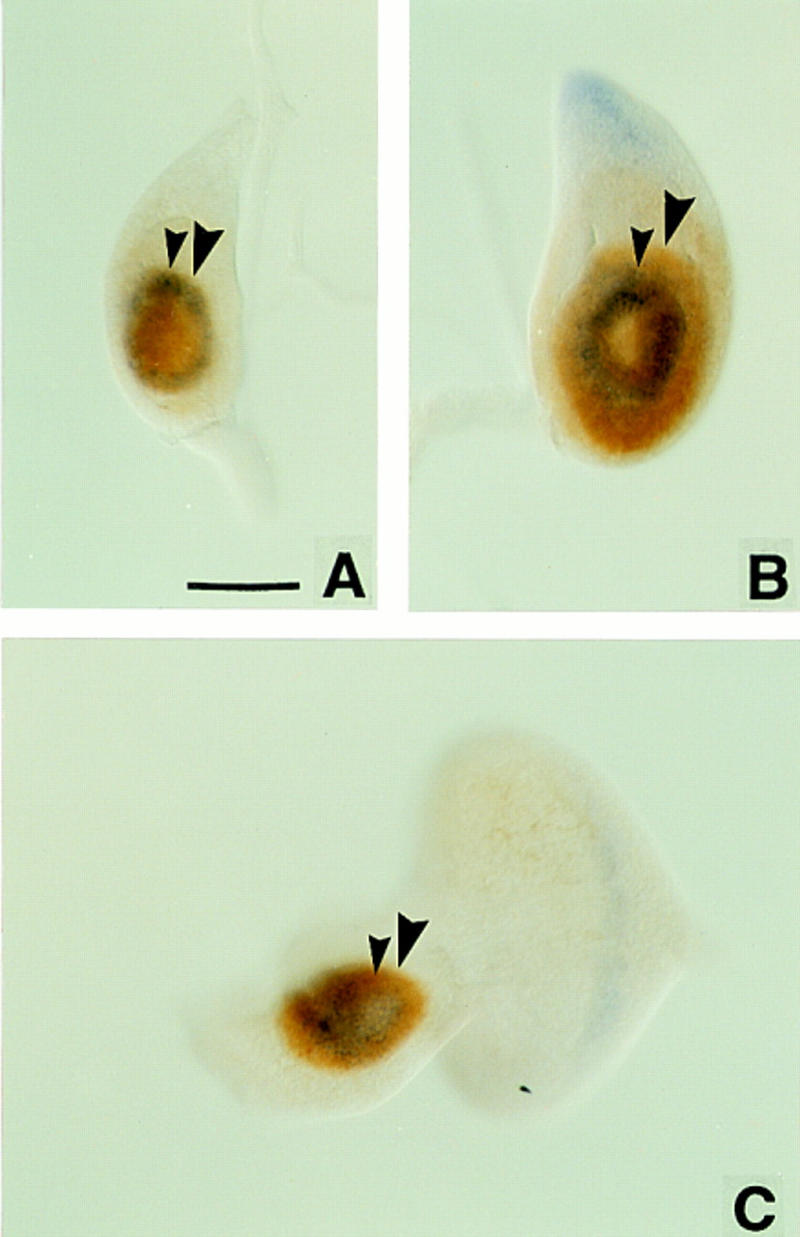
Wild-type imaginal discs double-labeled for ss transcript (blue) and Dll protein (brown). Small arrowheads indicate the proximal extent of ss expression; large arrowheads indicate the proximal extent of Dll expression. In early third instar leg discs (A), the proximal extents of ss and Dll expression coincide; later discs show Dll expression more proximal than ss (B). In antennal discs, Dll expression is always more proximal than ss (C). Scale bar, 50 μm.
The transient early expression of ss in the leg suggests that ss plays a role in the establishment of the tarsal region. Support for such a role is provided by the finding that bab lies downstream of ss. In wild type, bab expression is initiated in the tarsal region in the mid-third instar, and at disc eversion bab expression can be seen to extend from the middle of the first tarsal segment through the fifth segment (Godt et al. 1993). In ss null mutants, bab expression is abolished in the leg (not shown).
Discussion
Homology to the mammalian dioxin receptor
ss is the closest known relative of the mammalian AHR, also known as the dioxin receptor (for review, see Schmidt and Bradfield 1996). All five splice junctions within the coding region for the ss bHLH and PAS domains are conserved precisely in Ahr, indicating that the ss and AHR proteins share common ancestry. The basic regions of the two proteins are identical at 10/13 residues, suggesting they bind similar or identical DNA sequences. AHR functions to activate the transcription of genes that are important in metabolizing polycyclic aromatic hydrocarbons. AHR is normally cytoplasmic, but on binding aryl hydrocarbons, it translocates to the nucleus and forms active heterodimers with another bHLH–PAS protein, the aryl hydrocarbon nuclear translocator. The ligand with the highest affinity known for AHR is dioxin. The similarity of ss to AHR suggests that ss protein might also bind some type of ligand. Despite their close similarity, ss and AHR appear to be unrelated in function. Ahr knockouts in the mouse show no obvious developmental defects (Fernandez-Salguero et al. 1995; Schmidt et al. 1996), although, as expected, they are unresponsive to aryl hydrocarbons.
Control of antennal identity by ss
In previous work, we tested a specific model for how ss might act indirectly to control distal antennal identity (Burgess and Duncan 1990). Because ectopic expression of the Antp gene can cause a distally complete transformation of antenna to second leg (Schneuwly et al. 1987), we suspected that the ssa antennal transformation might result from the ectopic activation of Antp+. To test this, we examined Antp− ssa mitotic recombination clones in the distal antenna. To our surprise, we found these are indistinguishable from Antp+ ssa clones, and are transformed to second leg tarsus. This demonstrated that the ssa antennal transformation is independent of Antp+, and led us to propose that ss+ controls distal antennal identity directly. The molecular characterization of ss provides strong support for this proposal.
In normal development, ss is expressed in the primordia of AIII and the arista in the antennal imaginal disc. This corresponds to the portion of the antenna affected in ss null mutants. When ss is expressed ectopically, we find that it can transform the maxillary palp to AIII and arista, and the distal leg to arista. Ectopic ss also induces formation of an ectopic antenna in the rostral membrane ventral to the normal antenna. These effects argue strongly that ss is an antennal determinant. However, only certain structures are susceptible to transformation by ectopic ss, suggesting that ss must interact with other spatially-restricted regulators to promote antennal identity.
Transformations of distal antenna to leg are caused by ectopic expression of a number of different genes, including Antp, Scr, Ubx, abd-A, and Abd-B (Casares et al. 1996 and references therein) and the mouse genes HoxA5 (Zhao et al. 1993) and HoxB6 (Malicki et al. 1990). Transformation of distal antenna to leg is also seen in Polycomb heterozygotes (Duncan and Lewis 1982) and in animals having only one dose of Dll (Sunkel and Whittle 1987). These effects have led to the view that transformations of the distal antenna to leg are somehow nonspecific. However, in all of these cases (except ectopic expression of Abd-B, which has not yet been tested) we find that distal antennal transformations are correlated with repression of ss in the antenna (Fig. 3I; D.M. Duncan and I.W. Duncan, unpubl.). Thus, rather than indicating any lack of specificity for the ssa transformation, these cases provide strong supporting evidence that ss is a primary determinant of distal antennal identity.
The induction of antennae in the rostral membrane by ectopic ss was quite unexpected. This region normally produces no appendage and does not express Dll, which is thought to be required for ventral appendage formation (Cohen and Jürgens 1989). Moreover, the antennae induced here are often complete, although ss is normally expressed only in AIII and the arista. It would appear that ectopic ss can initiate an entire antennal limb program in the rostral membrane. Consistent with this, we find that ectopic ss induces Dll expression in this location (not shown). Ectopic antennae are always oriented in mirror symmetry to the normal antenna, suggesting that they arise from a cryptic head segment that is organized in mirror symmetry to the antennal segment. The existence of such a segment has also been inferred by Gonzáles-Crespo and Morata (1995).
Establishment of the tarsal primordium
Like the antenna, the second leg tarsus appears to develop without input from the ANT-C and BX-C genes. Mitotic recombination clones homozygous for null alleles of the major homeotic genes expressed in the thorax (Scr, Antp, and Ubx) develop normally in the T2 tarsus (Struhl 1981, 1982b; Abbott and Kaufman 1986), although Antp− clones in more proximal regions of the T2 leg are transformed to antenna. Struhl (1981) suggested that the lack of effect of Antp− clones in the T2 tarsus is caused by nonautonomous rescue by nearby wild-type cells in the adjacent compartment. However, our finding that Antp− ssa clones in the distal antenna produce apparently normal T2 tarsi (Burgess and Duncan 1990) argues that Antp+ simply plays no role in the second leg tarsus.
The results presented in this report suggest that the T2 tarsus is specified by ss. ss is first expressed in the tarsal region in the late second instar. Staining increases in this region through the early third instar, and then gradually declines. As far as we are aware, ss is the only disc-patterning gene known that shows such transient expression. Consistent with this expression pattern, the temperature-sensitive period of ss for tarsal development is in the first half of the third instar (Mglinets 1976). This period probably coincides with when the tarsal primordium is established, as Schubiger (1974) found that when leg discs are forced to undergo premature metamorphosis, the tarsal region first develops competence to differentiate in the early third instar. Significantly, Schubiger found that the tarsal regions differentiated by leg discs of this age are not segmented. Thus, it would appear that tarsal development occurs in two phases: First, a uniform tarsal region is established, and then this region is subdivided into specialized segments. We suggest that ss is responsible for the first of these phases, whereas downstream genes are responsible for the second. The timing of maximal ss expression, the temperature-sensitive period of ss, and the finding that ss expression is not segmentally modulated within the tarsal ring are all consistent with this view. Ectopic expression of ss in the leg causes the deletion of medial structures. Although it is not clear how these pattern deletions arise, one possibility is that ectopic ss specifies medial leg regions as tarsus, causing them to become incorporated into the tarsal primordium.
Our results indicate that ss occupies an intermediate position in the leg developmental hierarchy. Tarsal expression of ss is abolished in a Dll loss-of-function mutant, indicating that Dll is an important upstream regulator of ss. When ss first becomes expressed in the tarsal primordium, its proximal limit of expression coincides precisely with that of Dll. This suggests that Dll might directly activate ss in the leg, thereby defining its proximal limit of expression. Dll also appears to be a positive regulator of ss in the antenna: ss expression here is reduced in animals having only one dose of Dll+, and is almost eliminated in a Dll loss-of-function mutant. In light of these observations, it is surprising that ectopic ss induces Dll expression in the rostral membrane. Whether this reversal of the expected regulatory relationship between Dll and ss has any relevance to normal development is not clear, because Dll expression appears to be unaffected in ss null mutants (not shown). We find that tarsal expression of bab, which is required for proper subdivision of the tarsus into segments (Godt et al. 1993), is abolished in ss null mutants, indicating that bab lies downstream of ss in leg development.
What determines whether ss specifies tarsal or antennal development?
At least in the antenna, the level of ss function appears to determine whether tarsal or distal antennal development is specified. The many ss alleles induced by chemical mutagens, which are likely to be mutations in the coding sequence, can be arranged in a phenotypic series in which weak alleles cause only transformations of arista to tarsus, whereas strong alleles also cause tarsal deletions and a loss of AIII identity (Struhl 1982a). These observations suggest that a high level of ss function is required to specify aristal identity, whereas lower levels are able to specify tarsus and AIII identity. Consistent with this, we find that the intensity of ss staining is much higher in the antennal disc than in the leg disc. The level of function also appears to be critical for the proboscipedia (pb) gene of the ANT-C: pb null alleles cause a transformation of proboscis to tarsus, weak activity of pb+ causes a transformation to arista, and stronger activity allows normal proboscis development (Cribbs et al. 1995). Whether the tarsal and aristal transformations seen in pb mutants result from the activation of different levels of ss in the proboscis has not been determined.
It is perhaps surprising that when ss expression was driven ectopically in the leg, the tarsal segments were never transformed to antenna, although the distal tip was often transformed to arista. However, we find that the ptc–GAL4 driver used does not activate ss in the leg nearly as strongly as it does in the antennal disc. Thus, the level of ectopic expression attained in the tarsal region may not have been sufficient to cause a transformation. Alternatively, ectopic ss may have no effect in tarsi because of the absence or presence of some critical cofactor in the leg.
The ss− antennal appendage may be close to an epigenetic ground state
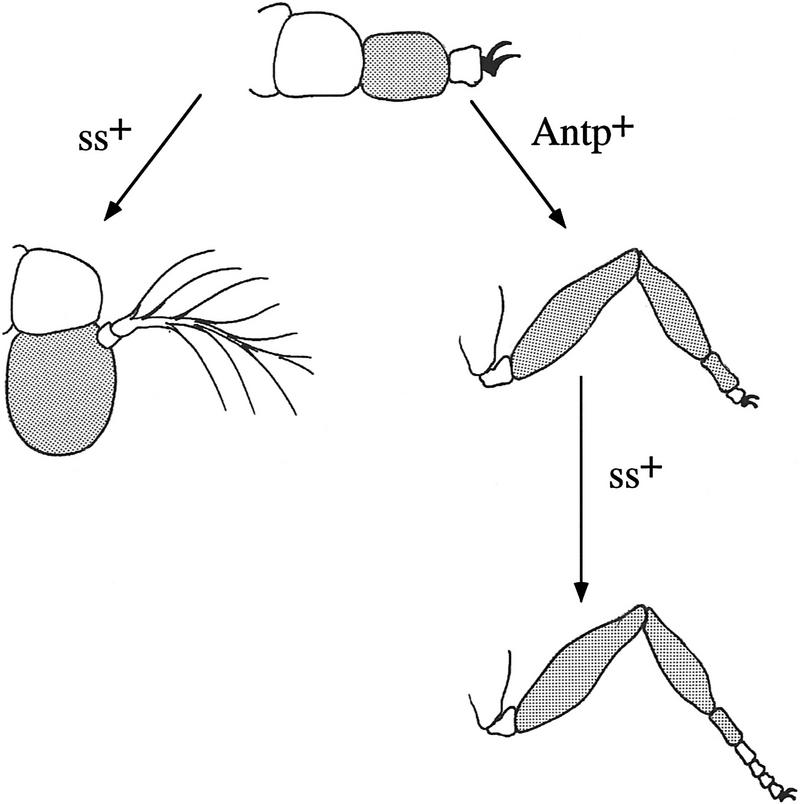
The ss− antennal appendage develops in the absence of ANT-C and BX-C, as well as ss, gene function, suggesting it is close to a developmental ground state. The prevailing view has been that the limb ground state is T2 leg (e.g., see Struhl 1982b and references therein). However, this has been challenged by the finding that Antp− clones in the proximal second leg are transformed to antenna (Struhl 1981, 1982b; Abbott and Kaufman 1986), and the finding that all trunk body segments develop antennae in Tribolium embryos deficient for the HOM-C (Stuart et al. 1991). A possible resolution was suggested by Struhl (1981, 1982b), who proposed that Antp+ functions to preserve the T2 ground state by repressing putative head-determining genes in the second leg. This model predicts that inactivation of these head-determining genes should cause the antenna to develop as second leg. The distal antennal transformation caused by weak ssa mutants would seem to conform to this prediction. However, in ss null mutants, AIII is not transformed to second leg, but develops as an undecorated segment with no apparent identity. We suggest that this is the fate of an appendage segment that has not been specified by any homeotic gene. As described in Figure 9, we propose that the limb ground state is a four-segmented appendage similar to the ss− antenna, and that ss+ and Antp+ function to direct the specialization of this appendage to produce the antenna and second leg.
Figure 9.
The ss− antennal appendage is close to an epigenetic ground state. The antennal appendage in ss− animals is indicated schematically at the top. The third segment of this appendage and its derivatives in the antenna and leg are shaded to indicate homology. As indicated, ss+ functions in the antenna to specialize the third and fourth segments of the ground state appendage to produce AIII and arista. In T2, Antp+ specifies the identities of the proximal two segments of the ground state appendage as coxa and trochanter, and directs the third segment to expand and subsegment to produce the femur, tibia, and first tarsal segment. These functions of Antp are consistent with the phenotype of Antp− clones in the leg (Struhl 1981, 1982b; Abbott and Kaufman 1986), and with the effects of ectopic expression of Antp in the antenna (Postlethwait and Schneiderman 1971). In the distal leg, ss+ directs the expansion and segmentation of the tarsus. The diagram predicts that the Antp− ss− second leg will look like the ss− antennal appendage. Although this has not yet been tested, the predicted phenotype is consistent with the additive effects of ss− and Antp− single mutants. The proximal two segments of the indicated ground state appendage are normal antennal segments; presumably the identities of these are specified by some other, currently unknown, homeotic gene or genes. Whether the evolutionary ground state of the limb is the same as the developmental ground state is not clear. However, it seems reasonable to suggest that it may be, and that an ancestral four-segmented appendage similar to the ss− antenna became specialized in the head to produce the antenna, and in the thorax to produce legs.
Evolutionary speculations
It is generally considered that the arthropod antenna evolved from a leg-like locomotory appendage (e.g., see Callahan 1979), a view that has received support from work on Drosophila homeotic and segmentation genes (Postlethwait and Schneiderman 1971; Cohen and Jürgens 1989; Schmidt-Ott and Technau 1992; this report). It is also generally accepted that unsegmented tarsi are ancestral in the hexapods (Boudreaux 1987), because simple tarsi resembling those present in ss mutant Drosophila occur in crustaceans and primitive hexapods. Thus, both the transformation of antenna to leg and the tarsal deletions caused by ss mutations appear to be atavistic, suggesting that ss played an important role in the evolution of distal limb structures in the arthropods.
Because antennal specialization occurred very early in arthropod evolution, we think it likely that the first function of ss was antennal specification, and that ss was recruited into tarsal development much later, during the evolution of the hexapods. Antennae are often elongated appendages used for probing the environment, and we think it plausible that as part of antennal specification, ss came to have an appendage elongation function. Transient expression of this function could then have served to extend other limbs, including the legs. This evolutionary sequence predicts that ss homologs will prove to be expressed in the antenna, but not the legs, of crustaceans and primitive hexapods, but in both locations in the insects.
Materials and methods
Screen for new ss alleles
Isogenic ss+ males (either from Canton-S or a st pp line) were irradiated (4000 rads of X rays) and crossed to sbd2 ssaM204/TM1 females. Non-TM1 progeny were then screened for the ssa antennal transformation. ssaM204 is a weak ss allele kindly provided by Dr. Ernesto Sánchez-Herrero (Universidad Autónoma de Madrid, Madrid, Spain). It shows a good antennal transformation, but little or no bristle or leg effects, when heterozygous with ss null alleles.
Cloning
Cloning, Southern blotting, and restriction mapping were by standard procedures (Sambrook et al. 1989). In situ hybridization to polytene chromosomes was as described by Cai et al. (1994). Previously described mutations mapped in this report include Tp(3;2)P10, In(3LR)P88, T(1;3)ssv, and T(2;3)ssa75 (Lindsley and Zimm 1992); Tp(1;3)sta (Melnick et al. 1993); and Df(3R)RS3-32 (Hopmann et al. 1995).
In situ hybridizations
Digoxigenin-labeled RNA probes were used in a modification of the method of Tautz and Pfeifle (1989) (R. Blackman, pers. comm.). After staining, samples were dehydrated through an ethanol series and mounted in 80% Permount (Fisher scientific) : 20% methyl salicylate or wet mounted in 50% glycerol in 50 mm Tris at pH 8.8. Double in situ/antibody staining was essentially as described by Manoukian and Krause (1992).
cDNA analysis
ss cDNA clones were isolated from a λgt10 eye/antennal imaginal disc library kindly provided by G. Rubin. cDNAs corresponding to the centrally located 1.2-kb transcript were obtained from an adult λExlox library (Novagen). Clones were sequenced in entirety on both strands from single-stranded DNA by use of the Sequenase kit (U.S. Biochemical).
Antibody staining
With the exception of imaginal discs, tissue staining was essentially as described by Kellerman et al. (1990). Imaginal discs were found to give unacceptable background when streptavidin-based reagents were used, presumably because of the presence of endogenous biotin. Therefore, prior to incubation with biotinylated secondary antibodies, discs were blocked by a 20-min treatment with unlabeled streptavidin (0.5 μg/ml in PBS) followed by a 20-min treatment with biotin (20 μg/ml in PBS). Tissues were dehydrated, mounted, and photographed as for the in situ samples. The anti-en hybridoma 4D9 (Patel et al. 1989) was obtained from the American Type Culture Collection.
Monoclonal anti-Dll antibody
Balb/c mice were immunized with bacterially expressed Dll protein and shipped to us by Dr. Stephen Cohen. Spleen cells were fused to Sp2/0 myeloma cells, and supernates of clones surviving HAT selection were screened by staining of 6- to 18-hr embryos. Clones showing positive responses were expanded and used for the production of ascites stocks. The antibody used in this analysis, MAb DMDll.1, stains imaginal discs well, but stains embryos relatively poorly. The antibody is of isotype IgG2b.
Larval and adult cuticle preparations
Larvae were mounted in a mixture of 38% Shandon Immumount, 52% saturated chloral hydrate in water, and 10% lactic acid syrup. Processing of adult tissues was as described in Duncan (1982).
Sequence analysis
All sequence analysis was performed by use of the Wisconsin Genetics Computer Group sequence analysis software (Devereux et al. 1984) on a Vaxstation 3100 computer. Genbank databases were searched by the BLAST program (Altschul et al. 1990). The sequence of the sscA6 cDNA has been deposited with GenBank under accession number AF050630.
Acknowledgments
We thank Gerard Campbell, Shigeo Hayashi, Frank Laski, Martha O’Brien, Norbert Perrimon, Jim Posakony, and Ernesto Sánchez-Herrero for providing stocks; Konrad Basler, Nick Brown, Tom Kornberg, and Gerry Rubin for cDNA libraries; Ron Blackman for an in situ hybridization protocol; Markus Noll for the al cDNA, and Paul Taghert for the 22C10 monoclonal. Steve Cohen provided Dll-immunized mice, and Mark Sturtevant constructed several of the plasmid subclones used. We are especially grateful to Paula Kiefel and Elaine Round for help in sequencing splice junctions and Richard Emmons for isolating UAS–ss transformants. Finally, we thank Mark Kankel, Richard Emmons, Artyom Kopp, and the anonymous reviewers for comments on the manuscript.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL duncan@biodec.wustl.edu; FAX 314 935-5125.
References
- Abbot MK, Kaufman TC. The relationship between the functional complexity and the molecular organization of the Antennapedia locus of Drosophila melanogaster. Genetics. 1986;114:919–942. doi: 10.1093/genetics/114.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baylies MK, Martinez Arias A, Bate M. wingless is required for the formation of a subset of muscle founder cells during Drosophila embryogenesis. Development. 1995;121:3829–3837. doi: 10.1242/dev.121.11.3829. [DOI] [PubMed] [Google Scholar]
- Boudreaux HB. Arthropod Phylogeny, with special reference to insects. Malabar, FL: Robert E. Krieger Publishing; 1987. [Google Scholar]
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bryant PJ. Pattern formation in imaginal discs. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila. 2c. New York, NY: Academic Press; 1978. pp. 230–335. [Google Scholar]
- Burgess EA, Duncan I. Direct control of antennal identity by the spineless-aristapedia gene of Drosophila. Mol & Gen Genet. 1990;221:347–352. doi: 10.1007/BF00259398. [DOI] [PubMed] [Google Scholar]
- Cai H, Kiefel P, Yee J, Duncan I. A yeast artificial chromosome clone map of the Drosophila genome. Genetics. 1994;136:1385–1401. doi: 10.1093/genetics/136.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan PS. Evolution of antennae, their sensilla and the mechanism of scent detection in Arthropoda. In: Gupta AP, editor. Arthropod phylogeny. New York, NY: Van Nostrand Reinhold Co; 1979. pp. 259–298. [Google Scholar]
- Campbell G, Weaver T, Tomlinson A. Axis specification in the developing Drosophila appendage: the role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell. 1993;74:1113–1123. doi: 10.1016/0092-8674(93)90732-6. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin, Germany: Springer-Verlag; 1985. [Google Scholar]
- Carroll SB, Weatherbee SD, Langeland JA. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375:58–61. doi: 10.1038/375058a0. [DOI] [PubMed] [Google Scholar]
- Casares F, Calleja M, Sánchez-Herrero E. Functional similarity in appendage specification by the Ultrabithorax and abdominal-A Drosophila HOX genes. EMBO J. 1996;15:3934–3942. [PMC free article] [PubMed] [Google Scholar]
- Cavener DR. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987;15:1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Jürgens G. Proximal-distal pattern formation in Drosophila: Cell autonomous requirement for Distal-less gene activity in limb development. EMBO J. 1989;8:2045–2055. doi: 10.1002/j.1460-2075.1989.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DL, Benassayag C, Randazzo FM, Kaufman TC. Levels of homeotic protein function can determine developmental identity: Evidence from low-level expression of the Drosophila homeotic gene proboscipedia under Hsp70 control. EMBO J. 1995;14:767–778. doi: 10.1002/j.1460-2075.1995.tb07055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberledge S, Zaratzian A, Sakonju S. Characterization of two RNAs transcribed from the cis-regulatory region of the abd-A domain within the Drosophila bithorax complex. Proc Natl Acad Sci. 1990;87:3259–3263. doi: 10.1073/pnas.87.9.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan IM. Polycomblike: A gene that appears to be required for the normal expression of the bithorax and Antennapedia gene complexes of Drosophila melanogaster. Genetics. 1982;102:49–70. doi: 10.1093/genetics/102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I. The bithorax complex. Annu Rev Genet. 1987;21:285–319. doi: 10.1146/annurev.ge.21.120187.001441. [DOI] [PubMed] [Google Scholar]
- Duncan IM, Lewis EB. Genetic control of body segment differentiation in Drosophila. In: Subtelny S, Green PB, editors. Developmental order: Its origin and regulation, the 40th symposium of the Society for Developmental Biology. New York, NY: Alan Liss; 1982. pp. 533–554. [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SST, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Ghysen A, O’Kane C. Neural enhancer-like elements as specific cell markers in Drosophila. Development. 1989;105:35–52. doi: 10.1242/dev.105.1.35. [DOI] [PubMed] [Google Scholar]
- Godt D, Couderc J-L, Cramton SE, Laski FA. Pattern formation in the limbs of Drosophila: bric à brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119:799–812. doi: 10.1242/dev.119.3.799. [DOI] [PubMed] [Google Scholar]
- González-Crespo S, Morata G. Control of Drosophila adult pattern by extradenticle. Development. 1995;121:2117–2125. doi: 10.1242/dev.121.7.2117. [DOI] [PubMed] [Google Scholar]
- Gorfinkiel N, Morata G, Guerrero I. The homeobox gene Distal-less induces ventral appendage development in Drosophila. Genes & Dev. 1997;11:2259–2271. doi: 10.1101/gad.11.17.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz U, Giebel B, Campos-Ortega JA. The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell. 1994;76:77–87. doi: 10.1016/0092-8674(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MJ. Sex-combs of intersexes and the arrangement of the chaetae on the legs of Drosophila. J Morphol. 1964;115:35–52. [Google Scholar]
- Hopmann R, Duncan D, Duncan I. Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: Homology independent interactions in trans. Genetics. 1995;139:815–833. doi: 10.1093/genetics/139.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Dambly-Chaudiere C, Ghysen A. The emergence of sense organs in the wing disc of Drosophila. Development. 1991;111:1087–1095. doi: 10.1242/dev.111.4.1087. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Edery I, Rosbash M. PAS is a dimerization domain common to Drosophila Period and several transcription factors. Nature. 1993;364:259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- Jürgens G, Hartenstein V. The terminal regions of the body pattern. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 687–746. [Google Scholar]
- Jürgens G, Lehmann R, Schardin M, Nüsslein-Volhard C. Segmental organisation of the head in the embryo of Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1986;195:359–377. doi: 10.1007/BF00402870. [DOI] [PubMed] [Google Scholar]
- Kaufman TC, Seeger MA, Olsen G. Molecular and genetic organization of the Antennapedia Gene Complex of Drosophila melanogaster. Adv Genet. 1990;27:309–362. doi: 10.1016/s0065-2660(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Kellerman KA, Mattson DM, Duncan I. Mutations affecting the stability of the fushi tarazu protein of Drosophila. Genes & Dev. 1990;4:1936–1950. doi: 10.1101/gad.4.11.1936. [DOI] [PubMed] [Google Scholar]
- Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, Morata G. Bristle patterns and compartment boundaries in the tarsi of Drosophila. J Embryol Exp Morphol. 1979;51:195–208. [PubMed] [Google Scholar]
- Lewis EB. Genes and developmental pathways. Am Zool. 1963;3:33–56. [Google Scholar]
- ————— A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The Genome of Drosophila melanogaster. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Lipshitz HD, Peattie DA, Hogness DS. Novel transcripts from the Ultrabithorax domain of the bithorax complex. Genes & Dev. 1987;1:307–322. doi: 10.1101/gad.1.3.307. [DOI] [PubMed] [Google Scholar]
- Malicki J, Schugart K, McGinnis W. Mouse Hox-2.2 specifies thoracic segmental identity in Drosophila embryos and larvae. Cell. 1990;63:961–967. doi: 10.1016/0092-8674(90)90499-5. [DOI] [PubMed] [Google Scholar]
- Manoukian AS, Krause HM. Concentration-dependent activities of the even-skipped protein in Drosophila embryos. Genes & Dev. 1992;6:1740–1751. doi: 10.1101/gad.6.9.1740. [DOI] [PubMed] [Google Scholar]
- Melnick MB, Noll E, Perrimon N. The Drosophila stubarista phenotype is associated with a dosage effect of the putative ribosome-associated protein D-p40 on spineless. Genetics. 1993;135:553–564. doi: 10.1093/genetics/135.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mglinets VA. Time of gene action in the ontogenesis of Drosophila. Temperature-sensitive period of the ssa40a mutation in Drosophila melanogaster. Ontogenez. 1976;7:192–196. [PubMed] [Google Scholar]
- Morata G, Lawrence PA. Development of the eye-antenna imaginal disc of Drosophila. Dev Biol. 1979;70:355–371. doi: 10.1016/0012-1606(79)90033-2. [DOI] [PubMed] [Google Scholar]
- Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Postlethwait JH, Schneiderman HA. Pattern formation and determination in the antenna of the homoeotic mutant Antennapedia of Drosophila melanogaster. Dev Biol. 1971;25:606–640. doi: 10.1016/0012-1606(71)90008-x. [DOI] [PubMed] [Google Scholar]
- Raff RA, Kaufman TC. Embryos, genes, and evolution. New York, NY: Macmillan Publishing; 1983. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Carver LA, Bradfield CA. Molecular characterization of the murine Ahr gene. J Biol Chem. 1993;268:22203–22209. [PubMed] [Google Scholar]
- Schmidt JV, Su GH-T, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr allele: Animal model for the toxicity of halogenated dioxins and biphenyls. Proc Natl Acad Sci. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott U, Technau GM. Expression of en and wg in the embryonic head and brain of Drosophila indicates a refolded band of seven segment remnants. Development. 1992;116:111–125. doi: 10.1242/dev.116.1.111. [DOI] [PubMed] [Google Scholar]
- Schneitz K, Spielmann P, Noll M. Molecular genetics of aristaless, a prd-type homeo box gene involved in the morphogenesis of proximal and distal pattern elements in a subset of appendages in Drosophila. Genes & Dev. 1993;7:114–129. doi: 10.1101/gad.7.1.114. [DOI] [PubMed] [Google Scholar]
- Schneuwly S, Klemenz R, Gehring WJ. Redesigning the body plan of Drosophila by ectopic expression of the homeotic gene Antennapedia. Nature. 1987;325:816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Schubiger G. Acquisition of differentiative competence in the imaginal leg discs of Drosophila. Wilhelm Roux’ Arch. 1974;174:303–311. doi: 10.1007/BF00579118. [DOI] [PubMed] [Google Scholar]
- Struhl G. A homoeotic mutation transforming leg to antenna in Drosophila. Nature. 1981;292:635–638. doi: 10.1038/292635a0. [DOI] [PubMed] [Google Scholar]
- ————— spineless-aristapedia: A homeotic gene that does not control the development of specific compartments in Drosophila. Genetics. 1982a;102:737–749. doi: 10.1093/genetics/102.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci. 1982b;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JJ, Brown SJ, Beeman RW, Denell RE. A deficiency of the homeotic complex of the beetle Tribolium. Nature. 1991;350:72–74. doi: 10.1038/350072a0. [DOI] [PubMed] [Google Scholar]
- Sunkel CE, Whittle JRS. Brista: A gene involved in the specification and differentiation of distal cephalic and thoracic structures in Drosophila melanogaster. Wilhelm Roux’s Arch Dev Biol. 1987;196:124–132. doi: 10.1007/BF00402034. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes & Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Wilk R, Weizman I, Shilo B-Z. trachealess encodes a bHLH-PAS protein that is an inducer of tracheal cell fates in Drosophila. Genes & Dev. 1996;10:93–102. doi: 10.1101/gad.10.1.93. [DOI] [PubMed] [Google Scholar]
- Williams JA, Bell JB, Carroll SB. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes & Dev. 1991;5:2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- Zhao JJ, Lazzarini RA, Pick L. The mouse Hox-1.3 gene is functionally equivalent to the Drosophila Sex combs reduced gene. Genes & Dev. 1993;7:343–354. doi: 10.1101/gad.7.3.343. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Venkatesh TR, Teplow DB, Benzer S. Neuronal development in the Drosophila retina: Monoclonal antibodies as molecular probes. Cell. 1984;36:15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]