Abstract
Context:
Leptin is involved in the hormonal regulation of the reproductive, somatotropic, thyroid, and autonomic axes and ultimately in the regulation of energy balance. In parallel to the metabolic adaptation observed in response to caloric restriction (CR), plasma leptin concentrations are substantially decreased, suggesting a role for this hormone in the drop in energy expenditure beyond that predicted by the changes in body composition (metabolic adaptation).
Aim:
The aim of the study was to explore the changes in 24-h leptin circadian rhythm in response to CR and to investigate the relationship between these changes and metabolic adaptation.
Design:
In a randomized, controlled trial (Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy), 48 subjects were assigned to a control group or one of three CR groups for 6 months. Leptin concentration was assessed every 30 min for 24 h, and leptin circadian variations were fitted by Cosinor analysis. Sedentary energy expenditure and urinary catecholamine excretion were measured for 24 h in a metabolic chamber.
Results:
Six months of CR decreased body weight by −11.4 ± 0.6% (mean ± sem; P < 0.001). Mean 24-h circulating leptin concentration decreased by −44 ± 3% (P < 0.001), whereas leptin diurnal amplitude slightly increased over the 6 months of CR. CR caused a metabolic adaptation of −126 ± 25 kcal/d (P <0.001) and a significant decrease in urinary norepinephrine (−13 ± 3%) and T3 concentrations (10 ± 2%). The metabolic adaptation was significantly and independently related to the changes in 24-h leptin (r2 = 0 .22, P < 0.01) but not to the changes in leptin amplitude.
Conclusion:
Our results confirm an important role for leptin as an independent determinant of the metabolic adaptation in response to CR.
Significant weight loss induced by caloric restriction (CR) is characterized by reduced thyroid hormones, catecholamine, and leptin concentrations associated with a hypometabolic state, i.e. a drop in energy expenditure (EE) beyond that expected on the basis of changes in fat-free mass (FFM) and fat mass (FM) (1–4). This hypometabolic state referred to as metabolic adaptation persists with reduced body weight over the long term (4), opposes further decreases in body weight (2), and predisposes some individuals to weight regain (2).
Leptin, an adipokine secreted in proportion to body fat stores (5), is involved in the control of energy balance and body composition (6) by regulating both energy intake and expenditure (7). Leptin concentrations have been shown to be predictive of body weight changes (8, 9), and changes in leptin have been shown to be related to the decrease in EE associated with weight loss (10–12). In calorie-restricted animals and humans, exogenous leptin administration has been shown to reverse the metabolic adaptation induced by CR, restoring not only EE (3, 13, 14) but also catecholamine and thyroid hormone concentrations and skeletal muscle efficiency to baseline values (14, 15). This suggests a role for leptin in the regulation of EE during energy deprivation, possibly to protect against excessive loss of body fat stores (6).
The aim of this study was to characterize for the first time the changes in leptin circadian variations in response to 6 months of CR and to explore its relationships with CR-induced metabolic adaptation (16–18). We therefore hypothesized that the metabolic adaptation occurring with weight loss induced by CR was associated with the decrease in plasma leptin. Plasma leptin concentrations are known to follow a diurnal excursion (19, 20). The amplitude of the circadian variation potentially influences the amount of leptin tissues are exposed to and consequently the biological effect of the hormone (21). Because acute changes in the leptin variation amplitude have been reported to be associated with changes in energy intake (21–23) and with changes in body weight gain (23, 24), we further hypothesized that the metabolic adaptation would be associated with a decrease in leptin diurnal amplitude.
Subjects and Methods
Subjects and study design
The Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE) study was approved by the Pennington Biomedical Research Center Institutional Review Board. As previously reported (16, 18), from a total of 599 screened potential overweight (25 kg/m2 ≤ body mass index < 30 kg/m2) participants, 48 subjects provided informed consent to participate in this study and 46 (26 females and 20 males) completed the study.
Participants were randomized to one of four experimental groups for 6 months: 1) control (weight maintenance diet based on an American Heart Association Step 1 diet), 2) CR (25% calorie restriction from baseline EE), 3) CR+EX = 12.5% CR with exercise (12.5% increase in EE by structured exercise), and 4) LCD (low calorie diet, i.e. 890 kcal/d until a 15% reduction in body weight followed by a weight maintenance diet). The group assignment was stratified to ensure even distributions of sex and body mass index in the four groups. Physiological testing was performed over a 5-d inpatient stay in the institutional clinic at baseline as well as during week 24 (month 6) of the study. Participants were provided with all food during baseline and while undergoing physiological testing based on individual energy requirements and treatment group assignment (16, 17). Body weight was measured in the morning in an overnight fasting state, after voiding. Whole-body fat content was measured using dual-energy x-ray absorptiometry (Hologics QDR 4500 A; Bedford, MA). FM and FFM were calculated from percent body fat assessed by dual-energy x-ray absorptiometry and the measured body weight.
Energy expenditure
Sedentary EE was assessed over 24 h in a metabolic chamber (16, 17). Sleeping EE was calculated between 0200 and 0500 h when no spontaneous physical activity was detected by microwave motion sensors. All urine was collected and acidified (10 ml HCl) for subsequent determination of urinary excretion of nitrogen (ANTEK, Houston, TX) and epinephrine and norepinephrine (Bio-Rad, Philadelphia, PA). Metabolic adaptation was defined as measured minus predicted 24-hr EE (MetAd24 h) and sleeping EE (MetAdsleep) at month 6. Predicted values of 24-h and sleeping EE were calculated on the basis of baseline values by a stepwise multivariate regression with FM, FFM, age, and sex as independent variables as previously reported (16).
Twenty-four-hour leptin profiles
After exiting the metabolic chamber, a 24-h blood sampling protocol was performed. Beginning at 0800 h, blood samples were taken every 30 min to assess leptin circadian rhythms. Similarly to the chamber stay, meals were consumed at 0900, 1130, and 1700 h. Lights were turned off from 2030 to 0700 h, and physical activity was limited. Plasma leptin concentration was measured by RIA (Linco, St. Charles, MO). Circadian variations of plasma leptin concentration were determined by cosinor analysis, and the following descriptors were derived algebraically: the mesor (mean 24 h leptin concentration), the amplitude (mean deviation between the peak and nadir and the mesor), and the acrophase (time to peak). Amplitude was then expressed as relative to the leptin mesor.
Statistical analysis
All data are expressed as mean ± sem. Statistical analysis were performed using the R software (25). Differences between sexes were assessed by unpaired Student t tests. To account for the large interindividual variability in plasma leptin, an analysis of covariance with repeated-measures over time, and baseline values as covariates was used to test for the effects of treatment (CR groups vs. control), time (months 0 and 6), and their interactions. The effect of sex was assumed to be accounted for by including baseline values as covariates. Post hoc multiple comparisons were performed using the Bonferonni adjustment. Measures of association between single variables were obtained by Pearson's product-moment correlations. Significance was considered at a P < 0.05.
Results
At baseline, in controls and CR groups (CR, CR+EX, and LCD), body weight was 81.5 ± 1.8 vs. 82.3 ± 2.9 kg and percent body fat 31.3 ± 1.8 and 32.2 ± 1.3%. Details about the changes in body composition have already been published (16). Briefly, body weight (−11.4 ± 0.6%), FM (−26.8 ± 1.7%), and FFM (−4.5 ± 0.3%) were significantly (P < 0.001) decreased in all three intervention groups at month 6, whereas they remained stable in the control group.
Twenty-four-hour leptin profiles
The overall effect of the different treatments on leptin circadian variations is displayed in Table 1. Leptin mesor and fasting leptin were significantly decreased in response to CR (P < 0.001) and over time (P < 0.001) but independently of the type of CR and not differently between females and males (Table 1). The percent change in leptin mesor was strongly and negatively associated to the baseline leptin mesor at month 6 (r2 = 0.61, P < 0.001, respectively). Controlling for baseline leptin mesor, the changes in leptin mesor, were positively and significantly related to the changes in fat mass (P < 0.04). Leptin amplitude was slightly but significantly (P < 0.01) increased by CR (Table 1) but not differently between males and females. The changes in leptin amplitude were inversely related to the changes in leptin mesor (r2 = 0.10, P = 0.07) and to the absolute change in fat mass (r2 = 0.11, P = 0.05). The leptin acrophase remained unchanged (Table 1). In controls, leptin circadian variations remained unchanged.
Table 1.
Changes in leptin circadian rhythm variations, urinary catecholamine excretion, and thyroid hormones after 6 months of caloric restriction
| Baseline | Month 6 | |
|---|---|---|
| Control | ||
| Mesor (ng/ml) | 23.6 ± 4.8 | 21.2 ± 4.3 |
| Amplitude (% mesor) | 17.4 ± 2.0 | 15.8 ± 1.7 |
| Acrophase (h) | 14.8 ± 0.3 | 14.6 ± 0.5 |
| r2 (fitting of data) | 0.66 ± 0.07 | 0.68 ± 0.06 |
| Fasting leptin (ng/ml) | 20.8 ± 4.1 | 17.3 ± 3.3 |
| Epinephrine (nmol/d) | 79 ± 19 | 57 ± 9 |
| Norepinephrine (nmol/d) | 260 ± 35 | 286 ± 47 |
| T3 (ng/dl) | 144 ± 8 | 149 ± 7 |
| T4 (μg/dl) | 7.2 ± 0.4 | 7.3 ± 0.4 |
| Calorie restricted | ||
| Mesor (ng/ml) | 17.8 ± 3.8 | 11.3 ± 2.4a |
| Amplitude (% mesor) | 20.2 ± 1.9 | 22.8 ± 1.7 |
| Acrophase (h) | 14.8 ± 0.5 | 14.4 ± 0.6 |
| r2 (fitting of data) | 0.73 ± 0.05 | 0.74 ± 0.03 |
| Fasting leptin (ng/ml) | 15.6 ± 3.1 | 8.2 ± 1.8a |
| Epinephrine (nmol/d) | 66 ± 14 | 73 ± 13 |
| Norepinephrine (nmol/d) | 264 ± 25 | 228 ± 20 |
| T3 (ng/dl) | 139 ± 7 | 130 ± 7 |
| T4 (μg/dl) | 7.4 ± 0.4 | 7.5 ± 0.5 |
| Calorie restricted + exercise | ||
| Mesor (ng/ml) | 21.3 ± 3.9 | 14.1 ± 3.6a |
| Amplitude (% mesor) | 17.4 ± 1.6 | 17.9 ± 1.7 |
| Acrophase (h) | 14.1 ± 0.4 | 13.6 ± 0.5 |
| r2 (fitting of data) | 0.62 ± 0.07 | 0.58 ± 0.05 |
| Fasting leptin (ng/ml) | 18.8 ± 3.4 | 8.7 ± 2.1b |
| Epinephrine (nmol/d) | 77 ± 17 | 67 ± 5 |
| Norepinephrine (nmol/d) | 332 ± 29 | 275 ± 22 |
| T3 (ng/dl) | 135 ± 6 | 120 ± 6 |
| T4 (μg/dl) | 7.4 ± 0.4 | 7.0 ± 0.4 |
| Low-calorie diet | ||
| Mesor (ng/ml) | 19.1 ± 3.0 | 10.1 ± 1.9a |
| Amplitude (% mesor) | 18.6 ± 1.8 | 22.3 ± 2.5 |
| Acrophase (h) | 14.4 ± 0.5 | 13.8 ± 0.5 |
| r2 (fitting of data) | 0.70 ± 0.03 | 0.68 ± 0.05 |
| Fasting leptin (ng/ml) | 14.3 ± 2.1 | 6.6 ± 1.0b |
| Epinephrine (nmol/d) | 58 ± 5 | 60 ± 9 |
| Norepinephrine (nmol/d) | 274 ± 32 | 216 ± 25 |
| T3 (ng/dl) | 156 ± 9 | 133 ± 6 |
| T4 (μg/dl) | 8.1 ± 0.5 | 6.9 ± 0.1 |
Leptin, T3, and T4 data can be converted to SI units by multiplying by 1, 0.0154, and 12.87, respectively. Values are shown as means ± sem.
P < 0.001 vs. baseline.
P < 0.01 vs. baseline.
Urinary catecholamines and thyroid hormones
Twenty-four-hour urinary epinephrine excretion was unchanged by CR (P = NS, Table 1). Urinary norepinephrine excretion was significantly decreased by CR (P < 0.01) over time (P < 0.05), with no difference between females and males (Table 1). No difference was found between calorie-restricted groups with regard to norepinephrine excretion. Changes in urinary catecholamine excretion were not correlated to the changes in leptin variations. As reported previously, there was a significant effect of CR on plasma T3 and T4 (Table 1) (16). The change in plasma T3 was significantly related to the change in leptin (P = 0.01), controlling for baseline leptin.
EE and metabolic adaptation
Twenty-four-hour and sleeping EE were significantly decreased in all three caloric-restricted groups (not shown, P < 0.001), whereas it remained unchanged in controls. At month 6, a metabolic adaptation was observed in calorie-restricted groups only (16) when measured over 24 h and during sleep (MetAd24 h −126 ± 25 kcal/d, MetAdsleep −83 ± 17, kcal/d, P < 0.001) .
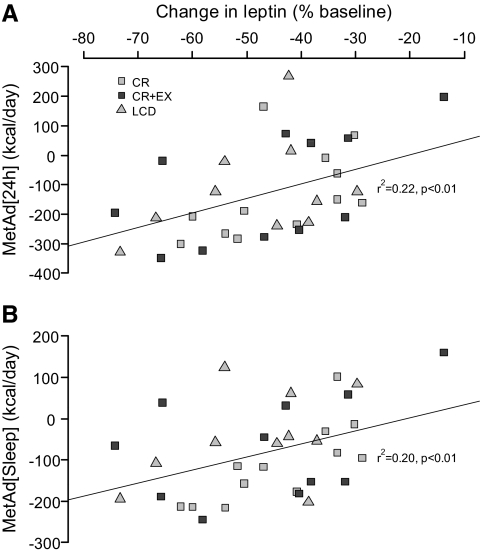
At month 6, MetAd24 h was positively and significantly related to the percent change in leptin mesor (r2 = 0.22, P < 0.01) (Fig. 1A) and to the absolute change in mesor controlling for baseline leptin concentration (r2 = 0.20, P = 0.03). Similarly, MetAdsleep was related to the percent decrease in leptin mesor (r2 = 0.20, P < 0.01) (Fig. 1B) and to the absolute change in mesor controlling for baseline leptin concentration (r2 = 0.31, P < 0.01). At month 6, the metabolic adaptation was also related to the relative decrease in norepinephrine excretion (MetAd24 h: r2 = 0.1, P = 0.06 and MetAdsleep: r2 = 0.20, P < 0.01). In contrast, the metabolic adaptation was unrelated to the changes in leptin amplitude.
Fig. 1.
Relationship between the changes in leptin mesor and the metabolic adaptation as calculated from 24-h EE (MetAd[24 h], A) and sleep EE (MetAd[sleep], B) measures. Gray squares, Calorie-restriction group; black squares, calorie restriction + exercise; gray triangle, low-calorie diet.
To further delineate the determinants of the metabolic adaptation in response to CR, we performed a multivariate regression analysis between the metabolic adaptation at month 6 and changes in leptin mesor, urinary norepinephrine, and thyroid hormone concentrations. The change in leptin mesor was the only significant and independent determinant of MetAd24 h (P = 0.03). Changes in leptin mesor (P = 0.04) as well as change in norepinephrine excretion (P = 0.03) were additional significant predictors of the MetAdsleep.
Discussion
The main finding of this study is that the decline in 24-h leptin in response to CR is a significant and independent determinant of metabolic adaptation induced by CR. These results indicate that the drop in plasma leptin concentration is probably underlying the additional reduction in energy expenditure observed in some individuals following diet-induced weight loss.
A role for blunted leptin levels, along with organochlorine compounds, in the metabolic adaptation has been previously demonstrated in obese individuals undergoing weight loss (26). Indeed, the reversal of the hypometabolic phenotype induced by calorie restriction with recombinant leptin treatment in subjects undergoing weight loss (3, 14) and those with leptin deficiency (13) supports a role for leptin in the mechanisms related to the metabolic adaptation. Moreover, changes in leptin have also been associated with changes in EE in caloric restricted subjects (10, 11). The fact that leptin administration restores thyroid hormone and catecholamine levels suggests a central effect of leptin (3). Low circulating leptin blunts the leptin signaling cascade at the level of the hypothalamus and triggers an exaggerated drop in energy expenditure beyond that expected for the changes in body composition (6). Yet the exact mechanism by which a sustained decrease in leptin induces the metabolic adaptation is unknown. Because the changes in leptin and T3 are related and the changes in leptin are independently predictive of the metabolic adaptation, we speculate leptin may also exert peripheral effects in skeletal muscle. In support of this hypothesis, a decrease in the energy cost of physical activity beyond that predicted by the changes in body composition was associated with lower plasma leptin concentrations after weight loss (1), and this improved energy efficiency was reversed by exogenous leptin administration (14). Taken together, these observations indicate that a potential mechanism by which CR induces a metabolic adaptation is through leptin-mediated changes in skeletal muscle efficiency, known to be improved during CR (27).
In conclusion, this study reports that the metabolic adaptation occurring during CR is related to the fall in mean 24-h leptin concentration, independently of changes in leptin diurnal excursion amplitude. The hypothalamus and the skeletal muscle seemingly respond to the fall in leptin by decreasing the thyroid and sympathetic axes and by increasing the mitochondrial efficiency, respectively.
Acknowledgments
V.L. is supported by Grant PBLAP3–133026 from the Swiss National Science Foundation. L.M.R. is funded by National Institutes of Health Grant K99-HD060762. The CALERIE study was supported by Grant U01AG20478 (principal investigator: E.R.) and this analysis in part by a Nutrition Obesity Research Center Grant 2P30-DK072476-06. The CALERIE clinical trial registration number is NCT00099151 (www.clinicaltrials.gov).
Disclosure Summary: E.R. has been a consultant with Amylin Pharmaceuticals. The other authors have nothing to disclose.
Footnotes
- CALERIE
- Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy
- CR
- caloric restriction
- EE
- energy expenditure
- FFM
- fat-free mass
- FM
- fat mass
- MetAd24 h
- metabolic adaptation defined as measured minus predicted 24-hr EE
- MetAdsleep
- metabolic adaptation defined as measured minus predicted sleeping EE.
References
- 1. Doucet E, Imbeault P, St. Pierre S, Alméras N, Mauriège P, Després JP, Bouchard C, Tremblay A. 2003. Greater than predicted decrease in energy expenditure during exercise after body weight loss in obese men. Clin Sci (Lond) 105:89–95 [DOI] [PubMed] [Google Scholar]
- 2. Leibel RL, Rosenbaum M, Hirsch J. 1995. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332:621–628 [DOI] [PubMed] [Google Scholar]
- 3. Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. 2002. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 87:2391–2394 [DOI] [PubMed] [Google Scholar]
- 4. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. 2008. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr 88:906–912 [DOI] [PubMed] [Google Scholar]
- 5. Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. 1996. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 81:3424–3427 [DOI] [PubMed] [Google Scholar]
- 6. Leibel RL. 2002. The role of leptin in the control of body weight. Nutr Rev 60:S15–S19; discussion S68–S84, S85–S87 [DOI] [PubMed] [Google Scholar]
- 7. Rosenbaum M, Leibel RL. 2010. Adaptive thermogenesis in humans. Int J Obes (Lond) 34(Suppl 1):S47–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martínez JA, Casanueva FF. 2010. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab 95:5037–5044 [DOI] [PubMed] [Google Scholar]
- 9. Ravussin E, Pratley RE, Maffei M, Wang H, Friedman JM, Bennett PH, Bogardus C. 1997. Relatively low plasma leptin concentrations precede weight gain in Pima Indians. Nat Med 3:238–240 [DOI] [PubMed] [Google Scholar]
- 10. Doucet E, St. Pierre S, Alméras N, Mauriège P, Richard D, Tremblay A. 2000. Changes in energy expenditure and substrate oxidation resulting from weight loss in obese men and women: is there an important contribution of leptin? J Clin Endocrinol Metab 85:1550–1556 [DOI] [PubMed] [Google Scholar]
- 11. Labayen I, Ortega FB, Ruiz JR, Lasa A, Simon E, Margareto J. 2011. Role of baseline leptin and ghrelin levels on body weight and fat mass changes after an energy-restricted diet intervention in obese women: effects on energy metabolism. J Clin Endocrinol Metab 96:E996–E1000 [DOI] [PubMed] [Google Scholar]
- 12. Wadden TA, Considine RV, Foster GD, Anderson DA, Sarwer DB, Caro JS. 1998. Short- and long-term changes in serum leptin dieting obese women: effects of caloric restriction and weight loss. J Clin Endocrinol Metab 83:214–218 [DOI] [PubMed] [Google Scholar]
- 13. Galgani JE, Greenway FL, Caglayan S, Wong ML, Licinio J, Ravussin E. 2010. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J Clin Endocrinol Metab 95:851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. 2005. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 115:3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. 1996. Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252 [DOI] [PubMed] [Google Scholar]
- 16. Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. 2006. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295:1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. 2007. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 15:2964–2973 [DOI] [PubMed] [Google Scholar]
- 18. Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. 2009. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 4:e4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Licinio J, Negrão AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Negro PP, Mulla A, Veldhuis JD, Cearnal L, Flier JS, Gold PW. 1998. Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab 83:4140–4147 [DOI] [PubMed] [Google Scholar]
- 20. Saad MF, Riad-Gabriel MG, Khan A, Sharma A, Michael R, Jinagouda SD, Boyadjian R, Steil GM. 1998. Diurnal and ultradian rhythmicity of plasma leptin: effects of gender and adiposity. J Clin Endocrinol Metab 83:453–459 [DOI] [PubMed] [Google Scholar]
- 21. Havel PJ, Townsend R, Chaump L, Teff K. 1999. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes 48:334–341 [DOI] [PubMed] [Google Scholar]
- 22. Kasa-Vubu JZ, Barkan A, Olton P, Meckmongkol T, Carlson NE, Foster CM. 2002. Incomplete modified fast in obese early pubertal girls leads to an increase in 24-hour growth hormone concentration and a lessening of the circadian pattern in leptin. J Clin Endocrinol Metab 87:1885–1893 [DOI] [PubMed] [Google Scholar]
- 23. Weigle DS, Cummings DE, Newby PD, Breen PA, Frayo RS, Matthys CC, Callahan HS, Purnell JQ. 2003. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab 88:1577–1586 [DOI] [PubMed] [Google Scholar]
- 24. Matkovic V, Ilich JZ, Badenhop NE, Skugor M, Clairmont A, Klisovic D, Landoll JD. 1997. Gain in body fat is inversely related to the nocturnal rise in serum leptin level in young females. J Clin Endocrinol Metab 82:1368–1372 [DOI] [PubMed] [Google Scholar]
- 25. R Development Core Team 2010. R: a language and environment for statistical computing. Version 2.11.1 ed. Vienna: R Foundation for Statistical Computing, http://www.R-project.org [Google Scholar]
- 26. Tremblay A, Pelletier C, Doucet E, Imbeault P. 2004. Thermogenesis and weight loss in obese individuals: a primary association with organochlorine pollution. Int J Obes Relat Metab Disord 28:936–939 [DOI] [PubMed] [Google Scholar]
- 27. Esterbauer H, Oberkofler H, Dallinger G, Breban D, Hell E, Krempler F, Patsch W. 1999. Uncoupling protein-3 gene expression: reduced skeletal muscle mRNA in obese humans during pronounced weight loss. Diabetologia 42:302–309 [DOI] [PubMed] [Google Scholar]