Abstract
Context:
Stem cells are undifferentiated cells with the property of self-renewal and give rise to highly specialized cells under appropriate local conditions. The use of stem cells in regenerative medicine holds great promise for the treatment of many diseases, including those of the thyroid gland.
Evidence Acquisition:
This review focuses on the progress that has been made in thyroid stem cell research including an overview of cellular and molecular events (most of which were drawn from the period 1990–2011) and discusses the remaining problems encountered in their differentiation.
Evidence Synthesis:
Protocols for the in vitro differentiation of embryonic stem cells, based on normal developmental processes, have generated thyroid-like cells but without full thyrocyte function. However, agents have been identified, including activin A, insulin, and IGF-I, which are able to stimulate the generation of thyroid-like cells in vitro. In addition, thyroid stem/progenitor cells have been identified within the normal thyroid gland and within thyroid cancers.
Conclusions:
Advances in thyroid stem cell biology are providing not only insight into thyroid development but may offer therapeutic potential in thyroid cancer and future thyroid cell replacement therapy.
Similar to all other organs of the body, the thyroid gland is no exception in exhibiting a variety of developmental and acquired diseases. In many of these cases, either the disease itself or the current therapeutic modalities result in irreversible hypothyroidism requiring lifelong thyroid hormone replacement (1). How to provide physiological thyroid replacement remains controversial and has resulted in multiple commercial thyroid preparations becoming available, varying from desiccated thyroid extract to gelatin-coated levothyroxine. For this simple reason, the study of thyroid stem cells may offer the possibility of not just revealing the cellular and molecular events leading to some of these thyroid abnormalities but may also offer the potential for simple thyroid cell therapy resulting in a more physiological approach to thyroid hormone replacement. Adult thyroid stem cells have now been identified in normal thyroid tissue, but their role in normal thyroid renewal has yet to be firmly established. Similarly, stem cells have been identified within thyroid tumors, but their role in the development of thyroid cancer remains to be determined. In addition, embryonic stem (ES) cell populations have demonstrated their ability to express a thyroid-like phenotype under defined in vitro growth conditions (2–5).
Here we review the current state of research on thyroid stem cells including an overview of cellular and molecular events that are observed in embryonic thyroid development. We update how ES cells can be driven toward thyroid progenitor cells and potentially functional thyroid cells and discuss the problems to be overcome in the laboratory to obtain reliable functional thyrocytes.
Overview of Thyroid Gland Development
The thyroid gland, located anterior and inferior to the thyroid cartilage, consists of thyroid follicular cells (TFC) and parafollicular C cells. Thyroid follicles are spherical structures serving as thyroglobulin (Tg) storage sites and allowing controlled release of thyroid hormones (6). The parafollicular cells secrete calcitonin and are scattered among the interfollicular spaces in a parafollicular position. These two cell types have distinct embryonic origins. The TFC derive from the endodermal epithelium in the pharyngeal floor, whereas parafollicular cells arise from within the ultimobranchial body derived from the fourth pharyngeal pouch (7) (Fig. 1). Upon invagination into the pharyngeal floor, the thyroid diverticulum migrates caudally and eventually bifurcates, giving rise to the two thyroid lobes. The ultimobranchial bodies fuse to the thyroid lobes, giving rise to the intermixed population of cell types.
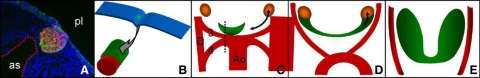
Fig. 1.
Thyroid gland development. Dissociation of the mouse thyroid bud from the endoderm and formation of a bilobed gland. A, The thyroid bud (evidenced by red Titf-1 staining) at E10.5. The bud maintains association with the aortic sac (dotted line) that has started to retract (as, aortic sac; pl, pharyngeal lumen). B, After dissociation from the endoderm (blue), the thyroid bud transiently loses contact with the caudally retracting aorta. The primordium (green) comes to rest as a cap-like structure on the cranial surface of the aortic sac (red) at E11.5. C, The thyroid primordium (green) extends bilaterally along the course of the third pharyngeal arch arteries. The lateral ends of the thyroid and the ultimobranchial bodies (brown) are approaching each other (arrow) (Ao, aorta; D, dorsal aorta; 3, 3rd pharyngeal arch arteries; 4, 4th pharyngeal arch arteries). D, At E13.5, the ultimobranchial bodies merge (arrow) with the lateral ends of the thyroid primordium. The pharyngeal arch arteries and dorsal aorta remodel into the carotid arteries and the final vascular anatomy as well as the bilobed shape of the thyroid can be discerned. E, At E18.5, the final shape of the thyroid is established with bilateral lobes close to the carotid arteries. [Adapted with permission from H. Fagman and M. Nilsson: J Mol Endocrinol 46:R33, 2011 (108). © Society for Endocrinology.]
The first evidence of thyroid hormone secretion occurs at approximately embryonic d 16.5 (E16.5) in mice (∼E75 in man) with the terminal differentiation into TFC and the expression of thyroid-specific genes necessary for thyroid hormone biosynthesis seen by approximately E16 in mice (∼E70 in man). The thyroid-specific genes appear during development according to a specific temporal pattern: Tg, thyroperoxidase (TPO), and the TSH receptor (Tshr) genes are expressed by E14.5 (8, 9) and the sodium iodide symporter (NIS) by E16 (10) (Fig. 2). Full expression of these genes is a requisite for synthesis of thyroid hormones. TSH is the principal regulator of thyroid hormone biosynthesis and secretion and is first synthesized at E12.5 (11) (human 10–12 wk). After the binding of TSH to its G protein-coupled receptor, it activates multiple signaling pathways that regulate both differentiation and proliferation of thyroid cells as well as the formation of thyroid hormones (12–14).
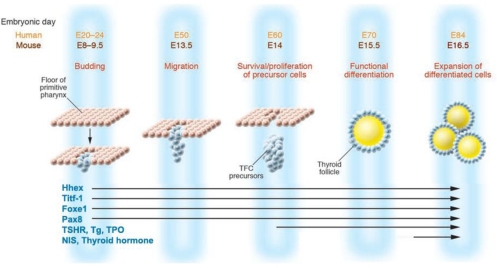
Fig. 2.
Timing of thyroid gene expression in development: schematic representation of the stages of development of the TFC and the expression of relevant genes. At mouse E8 (E20 in human), the thyroid bud appears as a thickening in the floor of the pharynx and expresses a combination of transcription factors such as PAX8, the transcription factor essential for the thyrocyte promoter activation of TPO, Tg and NIS, and Titf-1 and Foxe1, responsible for morphogenesis of the thyroid gland and maintenance of the thyrocyte cell type. At about E13.5 in mouse and E50 in human, the thyroid diverticulum starts its migration from the pharyngeal floor and reaches its definitive pretracheal position. By E14 (E60 in human), the TFC precursors express the TSHR. By E15.5, the thyroid follicular organization appears with the expression of a series of proteins that are essential for thyroid hormone biosynthesis, including TPO, Tg, and NIS. [Adapted with permission from T. F. Davies, et al.: J Clin Invest 115:1972, 2005 (109). © American Society for Clinical Investigation.]
Control of thyroid development
The embryonic progenitors of the TFC derive from the definitive endoderm (15, 16). The definitive endoderm, one of the three germ layers formed during gastrulation, gives rise to the inner lining of the gut and its associated organs such as the entire gastrointestinal tract, glandular structures of the pharynx, the respiratory tract, the liver, the pancreas, and thyroid (17). Key factors involved in endoderm specification are regulated by the nodal family of signaling molecules and their complex glycosylation patterns (18, 19). A higher residual nodal activity initiates the formation of definitive endoderm and favors the development of its associated organs (such as thyroid) (20). The disruption of nodal-signaling components affects definitive endoderm formation and thyroid morphogenesis. Thus, induction of endoderm by nodal signaling and its downstream effectors is a prerequisite for TFC development (21).
Cell type-specific transcription factors control cell differentiation (22, 23). We now know that survival and growth of thyroid progenitors depends on a thyroid-specific signature of such transcription factors. This signature includes paired box gene 8 (Pax8), thyroid transcription factor (TTF) 1 (also known as Titf-1/Nkx2.1), and TTF2 (also known as Foxe1), in combination with a variety of additional molecules including hematopoietically expressed homeobox (Hhex) factor, and products of the homeobox gene A3 (Hoxa3), the eyes absent homolog 1 gene (Eya1), and the fibroblast growth factor receptor 2 (Fgfr2) (9, 24–29). The combined expression of Titf-1, Pax8, Foxe1, and Hhex is unique to the developing thyroid (30), and gene inactivation studies in mice have demonstrated that each of these factors is essential for the proper development and differentiation of the thyroid gland (9, 24, 26–29, 31–35). They may not be individually required for thyroid specification or early bud formation because experimental data suggest that progenitor cell recruitment and survival leading to enlargement of the thyroid bud depends on their concerted action (30). It is also important to note that each of these transcription factors exerts distinct roles in a variety of embryonic tissues, but it is only in thyroid progenitor cells that their cooperation is essential to drive organogenesis. This implies that a regulatory network among many transcription factors controls the various aspects of thyroid development.
Evidence in favor of this control mechanism has been developed. For example, Hhex has an essential role in maintenance of Titf-1, Pax8, and Foxe1 expression, whereas Titf-1and Pax8 together regulate proliferation, survival, and differentiation of TFC, and Foxe1, their migration. Thus, coordinated action is required for TFC differentiation and regulation of the transcriptional activity of thyroid-specific gene expression (36–39).
Although TSH is the main growth stimulus to the thyroid gland, the early growth and development of the fetal thyroid appears to be generally independent of TSH (40, 41) because its secretion cannot be shown before E12.5 (11) (10–12 wk in humans). In addition, the expression of TSHR mRNA is not detected in murine thyroid until E13.5–E14 and then greatly increases by E17. Hence, TSHR mRNA is detected in the developing thyroid only after the final migration of the precursor cells, before follicular organization in the gland. Furthermore, the analysis of thyroid development (10) in mice carrying spontaneous (43) or induced (44) mutations in the TSHR gene has shown that TSHR-null mice display severe hypothyroidism, associated with thyroid hypoplasia, but in both these mutants, although the size of the thyroid may be greatly reduced, the gland displays only minor alterations in its structure (44–46). A detailed analysis performed at the end of organogenesis, at E17, has revealed that in the absence of a functional TSHR, the follicular structure of the thyroid is not affected, and Tg is produced, whereas the expression of both TPO and NIS is markedly down-regulated (10). These data indicate that, during embryonic life, TSH/TSHR signaling is required to complete the differentiation program of the TFC but is not relevant in controlling the development of the gland.
Types of Stem Cells: Definition and Lineage Potentials
Stem cells are defined as undifferentiated cells with the ability to indefinitely self-renew while also maintaining pluripotency. Stem cells possess varying degrees of potential (Table 1). The totipotency of the zygote can give rise to all somatic cell types as well as the cells that make up the extraembryonic tissues, such as the amnion, the chorion, and the placenta. The pluripotency of ES cells can give rise to all of the cell types in the body except the extraembryonic tissues, but there are also multipotent stem cells that are adult stem cells that have the ability to develop into more than one cell type but not all cell types and are more often referred to as progenitor cells.
Table 1.
Stem cell characteristics
| ES cells (perinatal) | Adult stem cells | Bioengineered cells | |
|---|---|---|---|
| Origin | Isolated from the inner cell mass of the blastocyst | Found in peripolesis with differentiated cells within a tissue | Reprogrammed somatic cells |
| Hard to access & purify | Efficiency low (∼1%) | ||
| Pluripotent | Multipotent | Totipotent | |
| Advantages | Self-renewal and highly replicative | Autologous | Customized |
| Typically lineage committed | Autologous | ||
| Clinical safety and efficacy data | Large reservoir of cells | ||
| No ethical issues | No ethical issues | ||
| No teratoma risk | |||
| Disadvantages | Immunological concerns | Limited number | Potential for teratoma and teratocarcinoma |
| Potential for teratoma and teratocarcinoma | Limited replicative capacity | No clinical data yet | |
| Serious ethical issues | Lineage restricted |
ES cells can give rise to multiple lineages of determined progenitor cells that can differentiate into functional cell types. These features allow stem cells to induce organogenesis and to control tissue homeostasis by ensuring regeneration and repair. Therefore, during the course of development, each organ in the body most likely harbors multipotent quiescent populations of progenitors. The microenvironment of the tissue provides a niche for their survival and governs the extrinsic and intrinsic signals that must determine the fate of these cells. The characterization of these rare cells and the determination of the various checkpoints that cause them to leave their quiescent state remains a major challenge in many tissues including the thyroid gland. The identification of stem-like cells within differentiated thyroid tissues has been definitive, but their isolation and characterization from adult tissues has been more difficult. Therefore, much recent work has been based on either ES cells or induced pluripotent stem cells. This latter approach of dedifferentiating cells by inducing critical transcriptional down-regulation has been a major advance in contemporary stem cell research (47).
ES cell differentiation into thyroid-like cells
Pluripotent ES cells are derived from the inner cell mass of the early developing embryo in vitro. They are unique in their ability to undergo unlimited self-renewal by proliferation without senescence and can differentiate into almost all adult cell types if the appropriate extrinsic and intrinsic stimuli are provided in the culture. In 1981, ES cells were isolated for the first time in mice (48, 49), and it was not until 17 yr later, in 1998, that the first human ES cells were derived from human blastocysts (50). These cells provided the first models for studying molecular mechanisms occurring during cell commitment and differentiation in early development. These properties also made ES cells an attractive candidate for cell-based replacement therapies, although the problem of immune compatibility would remain.
Using these approaches over the past few years, researchers have made significant progress in understanding thyroid stem cell biology (51–53) and the developmental stimuli that derive thyroid progenitors from ES cells in vitro. Currently, there are two main strategies for the differentiation of ES cells into thyroid cells: 1) TSH-dependent induction (2, 4, 54) and 2) TSH-independent induction (5).
TSH-dependent induction of thyroid stem cells
The TSHR is a constitutively active receptor, which means that it signals, to a low extent, in the absence of ligand (55). Nevertheless, binding of TSH to the TSHR transmits the major trophic stimulus to the thyroid gland as well as relaying the additional signals necessary for the production and release of thyroid hormones. TSH stimulation of the TSHR transcriptionally regulates the genes necessary for thyroid hormone synthesis, namely those of the NIS (56), TPO (56, 57), and Tg (58). Based on this logic, thyrocyte-like cells derived from murine ES cell lines were first reported using a multistep/multifactorial process (2–4, 59). Briefly, ES cells were permitted to aggregate and form embryoid bodies (EB) that were then allowed to differentiate by withdrawing them from leukemia inhibitory factor, which, in vitro, normally maintains ES cells in an undifferentiated state. The EB were then treated with TSH, insulin, and/or insulin-like growth factor I and allowed to grow and differentiate into thyroid progenitors in a similar time period to their differentiation in vivo. The ES cell-derived EB treated in this manner expressed genes traditionally associated with TFC: Pax8, NIS, Tg, TPO, and TSHR. The EB also exhibited thyroid-specific function, such as cAMP generation and iodide uptake in the presence of TSH (2, 4). These attributes suggested that thyrocyte development was at least partly recapitulated in the ES cell model system and that it should be possible to study the precise effects of various growth factor or signaling pathways on ES cell differentiation. However, thyroid hormone synthesis in such cells has not been demonstrated.
The action of TSH through its receptor (TSHR) is important for the proliferation and maintenance of the differentiated function of TFC, and TSH/TSHR signaling is required for the expression of NIS and TPO (10, 44) but not Tg. The TSHR is, therefore, a useful but not specific marker for cell sorting to enhance the isolation of thyroid-specific lineages during the differentiation process. To achieve this aim, we developed an ES cell line carrying a GFP (green fluorescent protein-neomycin resistant) fusion gene under the control of the TSHR promoter (44) causing the cell to appear green when the TSHR was expressed (44). Based on the use of this GFP-TSHR fusion protein, a modified induction method was developed to enrich for TSHR-expressing thyroid cells obtained during ES cell differentiation in vitro. This novel approach consisted of incubating ES cells, engineered to express the fusion protein, with TSH followed by enrichment of TSHR-expressing cells in early EB by GFP-based cell sorting. Finally, the enriched cells were matured in the presence of TSH. We found that these ES cell-derived GFP-positive cells formed thyroid follicle-like cell clusters (spheroids) when cultured in a supportive extracellular matrix (Fig. 3, A and B). Immunofluorescent studies confirmed the colocalization of TSHR with NIS in the clusters. Furthermore, active iodide uptake by these cells implied that the NIS expressed was functional (2). The findings indicated that the generation of a TSHR-based selectable marker was a useful strategy that enabled enrichment and reliable isolation of thyroid lineage progenitors from ES cell cultures.
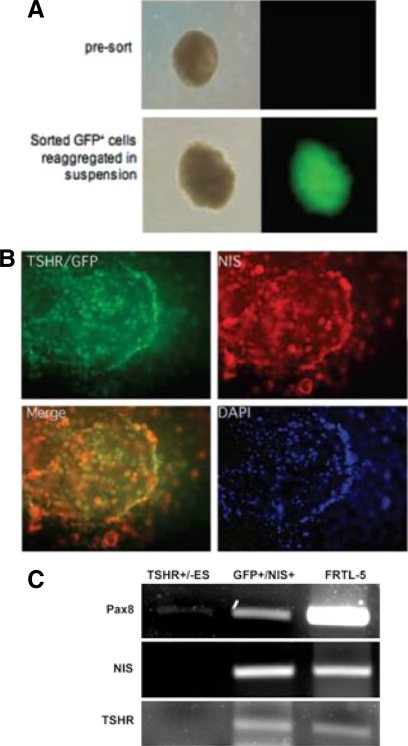
Fig. 3.
Differentiating ES cells. A, Spheroids differentiated from murine ES cells expressing the TSHR reported as green fluorescence. TSHR+/− ES cells carried a GFP fusion gene under the control of the TSHR promoter. Therefore, when the TSHR was expressed, the cell appeared green. Shown is a fluorescent view of EB in suspension formed from GPF-positive cells. Note that presorted EB do not show detectable levels of fluorescence. Left panels, Phase-contrast images; right panels, fluorescent microscopic images. [Adapted with permission from M. C. Arufe, et al.: Endocrinology 147:3007, 2006 (2). © The Endocrine Society.] B, Thyroid potential of GFP+NIS+ cells. An example of extensive anti-NIS staining in large GFP+ cellular aggregates. Several clusters of GFP+NIS+ cells derived from TSHR−/+ ES cells after staining with an antibody to NIS (red). In merging, some areas showed overlay (yellow). Blue indicates nuclear 4′,6-diamidino-2-phenylindole staining. [Adapted with permission from M. C. Arufe, et al.: Endocrinology 147:3007, 2006 (2). © The Endocrine Society.] C, Activin A induced thyroid gene expression. Pax8, NIS, and TSHR expression was detected by RT-PCR, after 5 d activin A exposure, using NIS and TSHR double-positive cells selected by fluorescence-activated cell sorting. All three were present whereas non-activin A-exposed EB cells served as negative controls. Rat (FRTL-5) thyroid cells served as positive controls. [Modified with permission from R. Ma, et al.: Endocrinology 150:1970, 2009 (5). © The Endocrine Society.]
TSH-independent induction of thyroid stem cells
The TSHR knockout (KO) mouse, which is congenitally hypothyroid (44), still develops a thyroid gland in the correct position. However, we found that when normalized to body weight, the TSHR-KO thyroids were 50% of the size of control normal thyroids, yet TSHR-KO follicles appeared mostly normal, showing epithelial cells covered with microvilli (44). Past studies from our laboratory using a model of newly formed neo-follicles had also determined that TSH was not essential for thyroid follicle formation (60). As discussed earlier, these findings, and other data, indicated that the thyroid follicles were partly TSH independent and that factors other than TSH must also be involved in thyroid follicle development.
Given the fact that the TFC arise from the endoderm during vertebrate development, defining the regulation of ES cell-derived endoderm should lead to further understanding the development and function of the thyroid gland. One of the known major influences on transformation to endodermal cells has been shown to be activin A, together with nodal, which belongs to the TGF-β superfamily and which activates Smad2-mediated intracellular signaling (61). Activin A enhances endoderm formation from ES cells and favors further differentiation into more mature cells of endodermal organs (62–65). To identify the induction signals for the differentiation of ES cells into thyroid-specific endodermal stem cells, EB derived from undifferentiated murine ES cells were treated with activin A to induce endoderm differentiation (7). The resulting endodermal cells showed appropriate down-regulation of Oct-4 and REX1 gene expression, gene transcriptional activity normally required to sustain stem cell self-renewal and pluripotency. In contrast, the DNA binding protein GATA-4 and α-fetoprotein, both endodermal-specific markers, increased in response to activin A. After 5 d of culture, TSHR and NIS gene and protein expression were markedly induced. Cells isolated by the fluorescence-activated cell sorter simultaneously expressed not only TSHR and NIS proteins but also PAX8 mRNA, the expression pattern known to be unique to thyroid cells and expected in committed thyroid progenitor cells (Fig. 3C). Such expression continued until d 21 of culture with no influence seen by the addition of TSH or IGF-I. Hence, the sequence of gene expression changes observed in these experiments demonstrated the emergence of definitive thyroid endoderm. The activin A induction of thyroid-specific markers NIS and TSHR occurred in the absence of TSH stimulation, and therefore, the emergence of thyroid endoderm in vitro paralleled the emergence of thyroid cells in TSHR-KO mice and confirmed activin A as a major regulator of thyroid endoderm (5).
Thyroid stem/progenitor cells in the mature thyroid gland
The EB formation approach, used in both TSH-dependent and TSH-independent induction models described above to generate thyrocytes from mouse ES cells produced a heterogeneous cell population, and the proportion of thyroid-like cells was low (∼2–5%). Furthermore, thyroid hormone synthesis has not been induced. Hence, the isolation of stem cells from mature thyroid tissue is an attractive alternative source for thyroid stem/progenitor cells. Once differentiation of the thyroid is completed, the gland enlarges in parallel with body weight and then remains stable throughout adult life (66). The factors that inherently limit the number of thyroid cells are not known, and the thyroid has a low cell turnover rate of approximately five times over the course of a lifetime (66, 67). Most thyrocytes appear to be able to respond with only a few divisions to a proliferation stimulus in vivo and in vitro, and therefore, the mechanism of thyroid gland regrowth after surgery remains to be explained.
The existence of stem cells that are able to replenish the pool of fully differentiated thyrocytes has, therefore, long been postulated (66). Indeed, the expression of Oct-4 can be detected in mouse thyroid gland, suggesting that the adult mouse thyroid contains stem cells. In experimental autoimmune thyroiditis, a widely used autoimmune model, a remarkable regenerative capacity of the thyroid along with the expression of Oct-4 has been observed (51). Oct-4 expression decreased after immunization with Tg and the development of thyroiditis causing thyroid cell apoptosis. This is in keeping with the notion that Oct-4 expression decreases when stem cells begin to differentiate. The results supported the presence of stem cells in the mature mouse thyroid that could differentiate after a severe insult (51).
In murine thyroid, it has also been shown that side population cells exist. Side population cells are selected by their expression of the ATP-binding cassette subfamily G member 2 (ABCG2) multidrug resistance transporter (68) and have been demonstrated to express many of the characteristics of stemness (68). For example, Oct-4+ side population cells were weakly positive for the TSHR and Titf-1, suggesting the presence of a thyroid progenitor lineage (68).
The finding that p63 (a p53-homolog nuclear transcription factor) was strongly expressed in solid cell nests of normal and abnormal human thyroids and that such cells displayed a basal/stem cell phenotype (53, 69) first suggested the presence of thyroid stem cells in the mature human thyroid gland. Recently, the presence of Oct-4+ thyroid stem cells in human tissue and the expression of Oct-4, Gata-4, HNF4α, and Pax8 in cultured cells derived from adult human goiters has also confirmed the presence of precursor cells of endodermal origin in human thyroid (Fig. 4, A and B). Thyroid stem cells have also been identified using side population cells as seen in mice (68, 70). In humans, the side population cells (0.1% of total cells) with a high nuclear/cytoplasmic ratio and Oct-4+ phenotype were isolated from primary thyroid cultures of goitrous thyroids rather than normal glands. However, such human side population cells failed to express the endoderm markers Gata-4, HNF4α, and Pax8 or the typical thyroid differentiation markers TSHR, TPO, NIS, and Tg, which were all detected in the tissue. But these cells were able to be grown as monolayers or embedded in collagen and formed follicles in the presence of epidermal growth factor and fibroblast growth factor. These follicles were composed of up to 5% of ABCG2+, Oct-4+ (side population) or Gata-4+, HNF4α+ (non-side population) thyroid stem/progenitor cells. In response to TSH and serum, these cells expressed Pax8, TSHR, TPO, NIS, and Tg and lost their stem and endoderm markers. Hence, these cells showed the hallmarks of differentiated thyrocytes by being capable of generating thyroid follicle-like structures and showing TSH-dependent iodine uptake (70). Similar studies have confirmed these observations in human thyroid and thyroid adenoma specimens (52). The isolated population contained a subset of CD34+CD45− cells (a marker for progenitor cells) that were able, under differentiation conditions, to generate TFC with thyroid hormone production. It was concluded that a predominant functional type of progenitor cell existed within the thyroid, with an intrinsic ability to generate thyroidal cells.
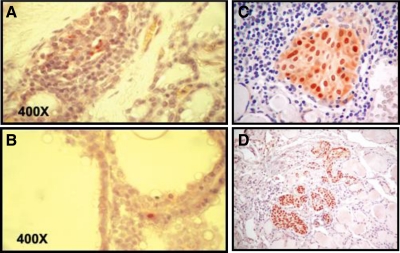
Fig. 4.
Immunostaining for stem cell marker expression in a section of human goiter tissue. A, Oct-4 staining is shown by positive nuclear staining of three cells (red) surrounded by a group of negative (blue) thyrocytes. B, GATA-4 staining is shown by positive nuclear staining of a single cell, whereas all thyrocytes in the vicinity are GATA-4 negative. Magnification, ×400. [Modified with permission from T. Thomas, et al.: Thyroid 16:537, 2006 (53). © Mary Ann Liebert Publishers.] C, Solid cell nest in a patient with Hashimoto's thyroiditis. Solid cell nests are shown as compartmentalized p63 immunostaining surrounded by a lymphocytic infiltrate. D, Solid cell nests in normal thyroid tissue. Within normal thyroid tissue, solid cell nests are shown as compartmentalized p63 immunostaining. [Adapted with permission from D. E. Burstein, et al.: Hum Pathol 35:465, 2004 (110). © W. B. Saunders.]
These studies demonstrate how the mature thyroid is not a terminally differentiated organ and explain how it has the potential for intrinsic regeneration, something well known for many years. Unfortunately, to date, the progenitor cells present in the mature thyroid have not survived continuous culture and, therefore, remain poorly characterized. This may be caused by their natural state of overdifferentiation (52) secondary to intrinsic factors that keep them in a quiescent state. It will be necessary to identify the checkpoints that maintain such a state and develop targets that will allow us to overcome their terminal differentiation.
Thyroid cancer stem cells (CSC)
In recent years, CSC have been recognized as important components in carcinogenesis and may form the basis of many tumor types (71). However, there remains uncertainty over the origin of such cells. Cancer cells may derive from intrinsic immature progenitors or stem cells because they share several properties that set them apart from normal cells (72). However, they may also be induced by the phenomenon of epithelial-to-mesenchymal transition (EMT) (73). Primary carcinomas recruit a variety of cell types into their surroundings such as fibroblasts and macrophages, and this microenvironment may result in the release of EMT-inducing signals. However, the presence of stem cells within normal tissues would strongly suggest that most CSC arise intrinsically.
CSC have been isolated from several types of malignancy, including leukemia (74), glioblastoma (75), breast (76), prostate (77), gastric (78), lung (79), and colon (80, 81) cancers. These cells, which represent only a small population within the bulk of the tumor, possess the ability to self-renew and differentiate into other cell types (82). Of great importance has been the observation that the cells with this stem-like phenotype are capable of resisting conventional chemotherapy, thus leading to disease relapse even when the primary lesion has been eradicated (83, 84). Hence, the existence of CSC might help explain recurrences after successful radiotherapy or chemotherapy because many current cancer therapeutics have been developed based on killing differentiated cancer cells.
This logic suggests that the characterization of thyroid CSC may provide not only insight into thyroid oncogenesis but also new specific targets for chemotherapy (Fig. 5). In fact, studies have already shown that tumorigenic capacity, in both undifferentiated and differentiated thyroid cancers, is confined to stem-like cells with high aldehyde dehydrogenase (ALDH) activity. High ALDH activity has been previously described in primitive cells from cancers of other tissues (85, 86). Cells isolated from thyroid cancers expressing high ALDH activity were able to be expanded indefinitely in vitro as spheroids that retained tumorigenic potential when transplanted into immune-compromised mice (87). Furthermore, the rapid growth pattern of anaplastic thyroid cancer (ATC) could be interpreted as resembling the nature of stem cells, and some ATC gene expression profiles may resemble such undifferentiated cells (88, 89). In one study of a human anaplastic thyroid cell line, a drug-resistant side population was isolated that expressed ABCG2 multidrug resistance transporters and was enriched with Oct-4-positive cells. This implied that an effective treatment of ATC has to destroy CSC that may drive tumor progression (90). However, much of the other research to date on ATC cell lines has focused on the ARO line (91–93) which is now known to have been contaminated with the human colon cancer cell line HT-29 (94). In medullary thyroid cancer cell lines, subpopulations of CD133+ cells (a stem cell marker) have been isolated and expanded as spheroids and passaged multiple times. Such cells still expressed neural progenitor markers, supporting the existence of CSC in medullary thyroid cancer (95).
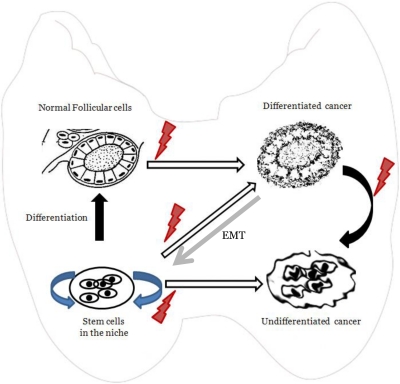
Fig. 5.
The CSC hypothesis of thyroid cancer development. This diagram shows the various stochastic events that lead to differentiated and undifferentiated thyroid cancers. Normal follicular cells that develop errors can give rise to differentiated cancers, which in turn could lead to undifferentiated cancer as a result of dedifferentiation and the development of stem-like cells by the phenomenon of EMT. It is also possible that thyroid cancers could arise from CSC that are derived from intrinsic adult stem cells that reside within a thyroid niche and have developed the genetic errors that can drive them to develop into thyroid cancer.
Microchimerism as a source of thyroid stem and/or progenitor cells
The presence within one individual of a small population of cells from another genetically distinct individual is referred to as microchimerism. Women are microchimeric from their children, and this can be detected for many years after childbirth (96). The types of cells that cross the placenta into the mother include both immune cells and cytokeratin-positive epithelial cells (97). Such naturally acquired microchimerism has been extensively documented and has received particular attention in autoimmune diseases, including autoimmune thyroid disease, as a potential source of autoimmune initiation (98). Male fetal cells frequently populate thyroid tissues from patients with autoimmune Graves' disease and Hashimoto's thyroiditis (98–102), but fetal cells can also be detected in normal thyroid glands and colloid nodules of mothers who have had male babies (98–100). However, the developmental potential of cytokeratin-positive fetal cells in the thyroid gland remains unclear.
Embryological remnant-derived stem cells
Solid cell nests of the thyroid are embryonic remnants of endodermal origin (103). These embryonic structures are composed of main cells and C cells, and cystic structures and mixed follicles are sometimes observed intermingled with solid cell nests (Fig. 4, C and D) (104, 105). The main cells of solid cell nests express p63, a p53-homolog nuclear transcription factor, consistently expressed in epithelial stem cells (106). Moreover, main cells expressed carcinoembryonic antigen and cytokeratins but lacked TTF-1, Tg, and calcitonin. Hence, the main cells of solid cell nests appear to display a stem cell phenotype (69). It is possible that such cell nests are a niche for thyroid stem cell survival, but more characterization is required.
The existence of several degrees of cellular differentiation within thyroid tumors has suggested that a pool of stem cells, at different stages of differentiation, may be responsible for thyroid cancer initiation and progression. One novel hypothesis of thyroid carcinogenesis posits that thyroid cancer cells are derived from the remnants of fetal cells that persist in the mature thyroid gland as thyroid cell nests. According to such a theory, thyroid cancer cells could be generated from the transformation of three types of fetal thyroid cells, thyroid stem cells, thyroblasts, and prothyrocytes, which would result in ATC, papillary thyroid cancer, and follicular thyroid cancer, respectively (107).
Summary and Conclusions
Thyroid stem cells have now been well identified in mature thyroid glands from mice and humans and have begun to be characterized in vitro. However, their origin remains unclear, and it is also uncertain whether the well-known interthyroidal solid cell nests may function as the thyroid stem cell niche. In addition, the identification of thyroid CSC has opened up the possibility of determining their involvement in thyroid neoplasia with potential therapeutic consequences. Studies have also begun to show successful differentiation of ES cells into thyroid-like cells, but thyroid hormone synthesis has not yet been realized by this approach, which may have to await the application of induced pluripotent stem cell methodology.
Acknowledgments
These studies were supported in part by DK69713 and DK 52464 from the National Institutes of Health, the Veterans Affairs Merit Award program, and the David Owen Segal Endowment.
Disclosure Summary: T.F.D. is a member of the Board of Kronus Inc. (Star, ID), which distributes thyroid antibody diagnostic kits. R.M., N.C.M., and R.L. have nothing to declare.
Footnotes
- ABCG2
- ATP-binding cassette subfamily G member 2
- ALDH
- aldehyde dehydrogenase
- ATC
- anaplastic thyroid cancer
- CSC
- cancer stem cells
- E16.5
- embryonic d 16.5
- EB
- embryoid bodies
- EMT
- epithelial-to-mesenchymal transition
- ES
- embryonic stem
- GFP
- green fluorescent protein
- Hhex
- hematopoietically expressed homeobox
- KO
- knockout
- NIS
- sodium iodide symporter
- Pax8
- paired box gene 8
- TFC
- thyroid follicular cells
- Tg
- thyroglobulin
- TPO
- thyroperoxidase
- TSHR
- TSH receptor
- TTF
- thyroid transcription factor.
References
- 1. Setian NS. 2007. Hypothyroidism in children: diagnosis and treatment. J Pediatr (Rio J) 83(5 Suppl):S209–S216 [DOI] [PubMed] [Google Scholar]
- 2. Arufe MC, Lu M, Kubo A, Keller G, Davies TF, Lin RY. 2006. Directed differentiation of mouse embryonic stem cells into thyroid follicular cells. Endocrinology 147:3007–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arufe MC, Lu M, Lin RY. 2009. Differentiation of murine embryonic stem cells to thyrocytes requires insulin and insulin-like growth factor-1. Biochem Biophys Res Commun 381:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin RY, Kubo A, Keller GM, Davies TF. 2003. Committing embryonic stem cells to differentiate into thyrocyte-like cells in vitro. Endocrinology 144:2644–2649 [DOI] [PubMed] [Google Scholar]
- 5. Ma R, Latif R, Davies TF. 2009. Thyrotropin-independent induction of thyroid endoderm from embryonic stem cells by activin A. Endocrinology 150:1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mauchamp J, Mirrione A, Alquier C, André F. 1998. Follicle-like structure and polarized monolayer: role of the extracellular matrix on thyroid cell organization in primary culture. Biol Cell 90:369–380 [PubMed] [Google Scholar]
- 7. Hoyes AD, Kershaw DR. 1985. Anatomy and development of the thyroid gland. Ear Nose Throat J 64:318–333 [PubMed] [Google Scholar]
- 8. De Felice M, Postiglione MP, Di Lauro R. 2004. Thyrotropin receptor signaling in development and differentiation of the thyroid gland: insights from mouse models and human diseases. Endocrinology 145:4062–4067 [DOI] [PubMed] [Google Scholar]
- 9. Lazzaro D, Price M, de Felice M, Di Lauro R. 1991. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113:1093–1104 [DOI] [PubMed] [Google Scholar]
- 10. Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, Marians RC, Davies TF, Zannini MS, De Felice M, Di Lauro R. 2002. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci USA 99:15462–15467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin SC, Li S, Drolet DW, Rosenfeld MG. 1994. Pituitary ontogeny of the Snell dwarf mouse reveals Pit-1-independent and Pit-1-dependent origins of the thyrotrope. Development 120:515–522 [DOI] [PubMed] [Google Scholar]
- 12. Civitareale D, Lonigro R, Sinclair AJ, Di Lauro R. 1989. A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. EMBO J 8:2537–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. 2001. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev 22:631–656 [DOI] [PubMed] [Google Scholar]
- 14. Medina DL, Santisteban P. 2000. Thyrotropin-dependent proliferation of in vitro rat thyroid cell systems. Eur J Endocrinol 143:161–178 [DOI] [PubMed] [Google Scholar]
- 15. Alt B, Reibe S, Feitosa NM, Elsalini OA, Wendl T, Rohr KB. 2006. Analysis of origin and growth of the thyroid gland in zebrafish. Dev Dyn 235:1872–1883 [DOI] [PubMed] [Google Scholar]
- 16. Macchia PE. 2000. Recent advances in understanding the molecular basis of primary congenital hypothyroidism. Mol Med Today 6:36–42 [DOI] [PubMed] [Google Scholar]
- 17. Wells JM, Melton DA. 1999. Vertebrate endoderm development. Annu Rev Cell Dev Biol 15:393–410 [DOI] [PubMed] [Google Scholar]
- 18. Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. 1998. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395:181–185 [DOI] [PubMed] [Google Scholar]
- 19. Tam PP, Kanai-Azuma M, Kanai Y. 2003. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr Opin Genet Dev 13:393–400 [DOI] [PubMed] [Google Scholar]
- 20. Lowe LA, Yamada S, Kuehn MR. 2001. Genetic dissection of nodal function in patterning the mouse embryo. Development 128:1831–1843 [DOI] [PubMed] [Google Scholar]
- 21. Elsalini OA, von Gartzen J, Cramer M, Rohr KB. 2003. Zebrafish hhex, nk2.1a, and pax2.1 regulate thyroid growth and differentiation downstream of Nodal-dependent transcription factors. Dev Biol 263:67–80 [DOI] [PubMed] [Google Scholar]
- 22. Dasen JS, Rosenfeld MG. 2001. Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci 24:327–355 [DOI] [PubMed] [Google Scholar]
- 23. Struhl K. 1999. Fundamentally different logic of gene regulation in eukaryotes and prokaryotes. Cell 98:1–4 [DOI] [PubMed] [Google Scholar]
- 24. Dathan N, Parlato R, Rosica A, De Felice M, Di Lauro R. 2002. Distribution of the titf2/foxe1 gene product is consistent with an important role in the development of foregut endoderm, palate, and hair. Dev Dyn 224:450–456 [DOI] [PubMed] [Google Scholar]
- 25. Manley NR, Capecchi MR. 1998. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol 195:1–15 [DOI] [PubMed] [Google Scholar]
- 26. Pellizzari L, D'Elia A, Rustighi A, Manfioletti G, Tell G, Damante G. 2000. Expression and function of the homeodomain-containing protein Hex in thyroid cells. Nucleic Acids Res 28:2503–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. 1990. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 110:643–651 [DOI] [PubMed] [Google Scholar]
- 28. Xu PX, Zheng W, Laclef C, Maire P, Maas RL, Peters H, Xu X. 2002. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development 129:3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zannini M, Avantaggiato V, Biffali E, Arnone MI, Sato K, Pischetola M, Taylor BA, Phillips SJ, Simeone A, Di Lauro R. 1997. TTF-2, a new forkhead protein, shows a temporal expression in the developing thyroid which is consistent with a role in controlling the onset of differentiation. EMBO J 16:3185–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Felice M, Di Lauro R. 2004. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev 25:722–746 [DOI] [PubMed] [Google Scholar]
- 31. Christophe D. 2004. The control of thyroid-specific gene expression: what exactly have we learned as yet? Mol Cell Endocrinol 223:1–4 [DOI] [PubMed] [Google Scholar]
- 32. Damante G, Tell G, Di Lauro R. 2001. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucleic Acid Res Mol Biol 66:307–356 [DOI] [PubMed] [Google Scholar]
- 33. Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS. 2000. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development 127:2433–2445 [DOI] [PubMed] [Google Scholar]
- 34. Puppin C, D'Elia AV, Pellizzari L, Russo D, Arturi F, Presta I, Filetti S, Bogue CW, Denson LA, Damante G. 2003. Thyroid-specific transcription factors control Hex promoter activity. Nucleic Acids Res 31:1845–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Puppin C, Presta I, D'Elia AV, Tell G, Arturi F, Russo D, Filetti S, Damante G. 2004. Functional interaction among thyroid-specific transcription factors: Pax8 regulates the activity of Hex promoter. Mol Cell Endocrinol 214:117–125 [DOI] [PubMed] [Google Scholar]
- 36. De Felice M, Ovitt C, Biffali E, Rodriguez-Mallon A, Arra C, Anastassiadis K, Macchia PE, Mattei MG, Mariano A, Schöler H, Macchia V, Di Lauro R. 1998. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet 19:395–398 [DOI] [PubMed] [Google Scholar]
- 37. Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ. 1996. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 10:60–69 [DOI] [PubMed] [Google Scholar]
- 38. Manley NR, Capecchi MR. 1995. The role of Hoxa-3 in mouse thymus and thyroid development. Development 121:1989–2003 [DOI] [PubMed] [Google Scholar]
- 39. Mansouri A, Hallonet M, Gruss P. 1996. Pax genes and their roles in cell differentiation and development. Curr Opin Cell Biol 8:851–857 [DOI] [PubMed] [Google Scholar]
- 40. Calvo RM, Jauniaux E, Gulbis B, Asunción M, Gervy C, Contempré B, Morreale de Escobar G. 2002. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab 87:1768–1777 [DOI] [PubMed] [Google Scholar]
- 41. Greenberg AH, Czernichow P, Reba RC, Tyson J, Blizzard RM. 1970. Observations on the maturation of thyroid function in early fetal life. J Clin Invest 49:1790–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burstein DE, Nagi C, Wang BY, Unger P. 2004. Immunohistochemical detection of p53 homolog p63 in solid cell nests, papillary thyroid carcinoma, and hashimoto's thyroiditis: A stem cell hypothesis of papillary carcinoma oncogenesis. Hum Pathol 35:465–473 [DOI] [PubMed] [Google Scholar]
- 43. Beamer WJ, Eicher EM, Maltais LJ, Southard JL. 1981. Inherited primary hypothyroidism in mice. Science 212:61–63 [DOI] [PubMed] [Google Scholar]
- 44. Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. 2002. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA 99:15776–15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beamer WG, Cresswell LA. 1982. Defective thyroid ontogenesis in fetal hypothyroid (hyt/hyt) mice. Anat Rec 202:387–393 [DOI] [PubMed] [Google Scholar]
- 46. Stein SA, Shanklin DR, Krulich L, Roth MG, Chubb CM, Adams PM. 1989. Evaluation and characterization of the hyt/hyt hypothyroid mouse. II. Abnormalities of TSH and the thyroid gland. Neuroendocrinology 49:509–519 [DOI] [PubMed] [Google Scholar]
- 47. Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- 48. Evans MJ, Kaufman MH. 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156 [DOI] [PubMed] [Google Scholar]
- 49. Martin GR. 1981. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78:7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 51. Chen CY, Kimura H, Landek-Salgado MA, Hagedorn J, Kimura M, Suzuki K, Westra W, Rose NR, Caturegli P. 2009. Regenerative potentials of the murine thyroid in experimental autoimmune thyroiditis: role of CD24. Endocrinology 150:492–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fierabracci A, Puglisi MA, Giuliani L, Mattarocci S, Gallinella-Muzi M. 2008. Identification of an adult stem/progenitor cell-like population in the human thyroid. J Endocrinol 198:471–487 [DOI] [PubMed] [Google Scholar]
- 53. Thomas T, Nowka K, Lan L, Derwahl M. 2006. Expression of endoderm stem cell markers: evidence for the presence of adult stem cells in human thyroid glands. Thyroid 16:537–544 [DOI] [PubMed] [Google Scholar]
- 54. Lin RY, Davies TF. 2006. Derivation and characterization of thyrocyte-like cells from embryonic stem cells in vitro. Methods Mol Biol 330:249–261 [DOI] [PubMed] [Google Scholar]
- 55. Latif R, Morshed SA, Zaidi M, Davies TF. 2009. The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. Endocrinol Metab Clin North Am 38:319–341, viii [DOI] [PubMed] [Google Scholar]
- 56. Riedel C, Levy O, Carrasco N. 2001. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem 276:21458–21463 [DOI] [PubMed] [Google Scholar]
- 57. Gerard CM, Lefort A, Libert F, Christophe D, Dumont JE, Vassart G. 1988. Transcriptional regulation of the thyroperoxydase gene by thyrotropin and forskolin. Mol Cell Endocrinol 60:239–242 [DOI] [PubMed] [Google Scholar]
- 58. Van Heuverswyn B, Streydio C, Brocas H, Refetoff S, Dumont J, Vassart G. 1984. Thyrotropin controls transcription of the thyroglobulin gene. Proc Natl Acad Sci USA 81:5941–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang N, Hu Y, Liu X, Wu Y, Zhang H, Chen G, Liang J, Lu X, Liu S. 2010. Differentiation of E14 mouse embryonic stem cells into thyrocytes in vitro. Thyroid 20:77–84 [DOI] [PubMed] [Google Scholar]
- 60. Valentine M, Martin A, Unger P, Katz N, Shultz LD, Davies TF. 1994. Preservation of functioning human thyroid “organoids” in the severe combined immunodeficient mouse. III. Thyrotropin independence of thyroid follicle formation. Endocrinology 134:1225–1230 [DOI] [PubMed] [Google Scholar]
- 61. Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. 2004. Development of definitive endoderm from embryonic stem cells in culture. Development 131:1651–1662 [DOI] [PubMed] [Google Scholar]
- 62. D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. 2005. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541 [DOI] [PubMed] [Google Scholar]
- 63. Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M, Li D, Deng H. 2007. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res 17:333–344 [DOI] [PubMed] [Google Scholar]
- 64. McKiernan E, O'Driscoll L, Kasper M, Barron N, O'Sullivan F, Clynes M. 2007. Directed differentiation of mouse embryonic stem cells into pancreatic-like or neuronal- and glial-like phenotypes. Tissue Eng 13:2419–2430 [DOI] [PubMed] [Google Scholar]
- 65. Rippon HJ, Polak JM, Qin M, Bishop AE. 2006. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells 24:1389–1398 [DOI] [PubMed] [Google Scholar]
- 66. Dumont JE, Lamy F, Roger P, Maenhaut C. 1992. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev 72:667–697 [DOI] [PubMed] [Google Scholar]
- 67. Coclet J, Foureau F, Ketelbant P, Galand P, Dumont JE. 1989. Cell population kinetics in dog and human adult thyroid. Clin Endocrinol (Oxf) 31:655–665 [DOI] [PubMed] [Google Scholar]
- 68. Hoshi N, Kusakabe T, Taylor BJ, Kimura S. 2007. Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology 148:4251–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reis-Filho JS, Preto A, Soares P, Ricardo S, Cameselle-Teijeiro J, Sobrinho-Simões M. 2003. p63 expression in solid cell nests of the thyroid: further evidence for a stem cell origin. Mod Pathol 16:43–48 [DOI] [PubMed] [Google Scholar]
- 70. Lan L, Cui D, Nowka K, Derwahl M. 2007. Stem cells derived from goiters in adults form spheres in response to intense growth stimulation and require thyrotropin for differentiation into thyrocytes. J Clin Endocrinol Metab 92:3681–3688 [DOI] [PubMed] [Google Scholar]
- 71. Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. 2008. Cancer stem cell markers in common cancers: therapeutic implications. Trends Mol Med 14:450–460 [DOI] [PubMed] [Google Scholar]
- 72. Reya T, Morrison SJ, Clarke MF, Weissman IL. 2001. Stem cells, cancer, and cancer stem cells. Nature 414:105–111 [DOI] [PubMed] [Google Scholar]
- 73. Chaffer CL, Weinberg RA. 2011. A perspective on cancer cell metastasis. Science 331:1559–1564 [DOI] [PubMed] [Google Scholar]
- 74. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. 1994. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367:645–648 [DOI] [PubMed] [Google Scholar]
- 75. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. 2004. Identification of human brain tumour initiating cells. Nature 432:396–401 [DOI] [PubMed] [Google Scholar]
- 76. Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. 2005. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 65:5506–5511 [DOI] [PubMed] [Google Scholar]
- 77. Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. 2006. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 25:1696–1708 [DOI] [PubMed] [Google Scholar]
- 78. Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. 2004. Gastric cancer originating from bone marrow-derived cells. Science 306:1568–1571 [DOI] [PubMed] [Google Scholar]
- 79. Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. 2005. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121:823–835 [DOI] [PubMed] [Google Scholar]
- 80. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. 2007. Identification and expansion of human colon-cancer-initiating cells. Nature 445:111–115 [DOI] [PubMed] [Google Scholar]
- 81. O'Brien CA, Pollett A, Gallinger S, Dick JE. 2007. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445:106–110 [DOI] [PubMed] [Google Scholar]
- 82. Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. 2005. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer 5:899–904 [DOI] [PubMed] [Google Scholar]
- 83. Baguley BC. 2006. Tumor stem cell niches: a new functional framework for the action of anticancer drugs. Recent Pat Anticancer Drug Discov 1:121–127 [DOI] [PubMed] [Google Scholar]
- 84. Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. 2008. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 27:1749–1758 [DOI] [PubMed] [Google Scholar]
- 85. Jelski W, Chrostek L, Szmitkowski M. 2007. The activity of class I, III, and IV of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in gastric cancer. Dig Dis Sci 52:531–535 [DOI] [PubMed] [Google Scholar]
- 86. Pearce DJ, Taussig D, Simpson C, Allen K, Rohatiner AZ, Lister TA, Bonnet D. 2005. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells 23:752–760 [DOI] [PubMed] [Google Scholar]
- 87. Todaro M, Iovino F, Eterno V, Cammareri P, Gambara G, Espina V, Gulotta G, Dieli F, Giordano S, De Maria R, Stassi G. 2010. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res 70:8874–8885 [DOI] [PubMed] [Google Scholar]
- 88. Takano T, Amino N. 2005. Fetal cell carcinogenesis: a new hypothesis for better understanding of thyroid carcinoma. Thyroid 15:432–438 [DOI] [PubMed] [Google Scholar]
- 89. Takano T, Ito Y, Matsuzuka F, Miya A, Kobayashi K, Yoshida H, Miyauchi A. 2007. Expression of oncofetal fibronectin mRNA in thyroid anaplastic carcinoma. Jpn J Clin Oncol 37:647–651 [DOI] [PubMed] [Google Scholar]
- 90. Zheng X, Cui D, Xu S, Brabant G, Derwahl M. 2010. Doxorubicin fails to eradicate cancer stem cells derived from anaplastic thyroid carcinoma cells: characterization of resistant cells. Int J Oncol 37:307–315 [DOI] [PubMed] [Google Scholar]
- 91. Friedman S, Lu M, Schultz A, Thomas D, Lin RY. 2009. CD133+ anaplastic thyroid cancer cells initiate tumors in immunodeficient mice and are regulated by thyrotropin. PLoS One 4:e5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V, Yamashita S. 2007. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology 148:1797–1803 [DOI] [PubMed] [Google Scholar]
- 93. Zito G, Richiusa P, Bommarito A, Carissimi E, Russo L, Coppola A, Zerilli M, Rodolico V, Criscimanna A, Amato M, Pizzolanti G, Galluzzo A, Giordano C. 2008. In vitro identification and characterization of CD133(pos) cancer stem-like cells in anaplastic thyroid carcinoma cell lines. PLoS One 3:e3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR. 2008. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab 93:4331–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhu W, Hai T, Ye L, Cote GJ. 2010. Medullary thyroid carcinoma cell lines contain a self-renewing CD133+ population that is dependent on ret proto-oncogene activity. J Clin Endocrinol Metab 95:439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. 1996. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA 93:705–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Klonisch T, Drouin R. 2009. Fetal-maternal exchange of multipotent stem/progenitor cells: microchimerism in diagnosis and disease. Trends Mol Med 15:510–518 [DOI] [PubMed] [Google Scholar]
- 98. Renné C, Ramos Lopez E, Steimle-Grauer SA, Ziolkowski P, Pani MA, Luther C, Holzer K, Encke A, Wahl RA, Bechstein WO, Usadel KH, Hansmann ML, Badenhoop K. 2004. Thyroid fetal male microchimerisms in mothers with thyroid disorders: presence of Y-chromosomal immunofluorescence in thyroid-infiltrating lymphocytes is more prevalent in Hashimoto's thyroiditis and Graves' disease than in follicular adenomas. J Clin Endocrinol Metab 89:5810–5814 [DOI] [PubMed] [Google Scholar]
- 99. Ando T, Imaizumi M, Graves PN, Unger P, Davies TF. 2002. Intrathyroidal fetal microchimerism in Graves' disease. J Clin Endocrinol Metab 87:3315–3320 [DOI] [PubMed] [Google Scholar]
- 100. Imaizumi M, Pritsker A, Unger P, Davies TF. 2002. Intrathyroidal fetal microchimerism in pregnancy and postpartum. Endocrinology 143:247–253 [DOI] [PubMed] [Google Scholar]
- 101. Klintschar M, Schwaiger P, Mannweiler S, Regauer S, Kleiber M. 2001. Evidence of fetal microchimerism in Hashimoto's thyroiditis. J Clin Endocrinol Metab 86:2494–2498 [DOI] [PubMed] [Google Scholar]
- 102. Koopmans M, Kremer Hovinga IC, Baelde HJ, Harvey MS, de Heer E, Bruijn JA, Bajema IM. 2008. Chimerism occurs in thyroid, lung, skin and lymph nodes of women with sons. J Reprod Immunol 78:68–75 [DOI] [PubMed] [Google Scholar]
- 103. Cameselle-Teijeiro J, Varela-Durán J, Sambade C, Villanueva JP, Varela-Núñez R, Sobrinho-Simoes M. 1994. Solid cell nests of the thyroid: light microscopy and immunohistochemical profile. Hum Pathol 25:684–693 [DOI] [PubMed] [Google Scholar]
- 104. Harach HR. 1988. Solid cell nests of the thyroid. J Pathol 155:191–200 [DOI] [PubMed] [Google Scholar]
- 105. Martin V, Martin L, Viennet G, Challier B, Carbillet J, Fellmann D. 2000. [Solid cell nests and thyroid pathologies. Retrospective study of 1,390 thyroids]. Ann Pathol 20:196–201 (French) [PubMed] [Google Scholar]
- 106. Preto A, Cameselle-Teijeiro J, Moldes-Boullosa J, Soares P, Cameselle-Teijeiro JF, Silva P, Reis-Filho JS, Reyes-Santías RM, Alfonsín-Barreiro N, Forteza J, Sobrinho-Simões M. 2004. Telomerase expression and proliferative activity suggest a stem cell role for thyroid solid cell nests. Mod Pathol 17:819–826 [DOI] [PubMed] [Google Scholar]
- 107. Takano T. 2007. Fetal cell carcinogenesis of the thyroid: theory and practice. Semin Cancer Biol 17:233–240 [DOI] [PubMed] [Google Scholar]
- 108. Fagman H, Nilsson M. 2011. Morphogenetics of early thyroid development. J Mol Endocrinol 46:R33–R42 [DOI] [PubMed] [Google Scholar]
- 109. Davies TF, Ando T, Lin RY, Tomer Y, Latif R. 2005. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest 115:1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]