Abstract
Introduction:
Antibodies to thyroglobulin (Tg), thyroperoxidase (TPO), and TSH receptor (TSH-R) are prevalent in autoimmune thyroid diseases. We aimed to assess whether females with Graves disease or Hashimoto thyroiditis are more likely than age-matched controls to have thyroid antibodies before clinical diagnosis and to measure the timing of antibody seroconversion.
Methods:
This was a nested case-control study using the Department of Defense Serum Repository and the Defense Medical Surveillance System, 1998–2007. We assessed thyroid antibodies in the serum of 522 female, active-duty, military personnel including: 87 Graves disease cases, 87 Hashimoto thyroiditis cases, and 348 age matched controls. One serum sample was available at the time of the clinical diagnosis (±6 months); three additional samples were retrieved from the repository up to 7 yr before the clinical diagnosis, for a total of 2088 samples.
Results:
In Hashimoto thyroiditis, TPO antibodies were found in about 66% of the cases at all time points. Tg antibodies showed a similar stationary trend, at a lower prevalence of about 53%at all time points. No TSH-R antibodies were found. In Graves disease, TPO antibodies gradually increased from 31% at 5–7 yr prior to diagnosis to 57% at diagnosis and Tg antibodies from 18 to 47%. TSH-R antibodies were present before diagnosis and showed an increasing prevalence from 2, 7, 20, to 55%.
Conclusions:
Antibodies to Tg, TPO, and TSH-R precede by years the development of the diagnostic autoimmune thyroid diseases phenotype. Overall, the presence of thyroid antibodies in apparently healthy individuals should not be neglected.
Autoimmune thyroid diseases (ATD) comprise Graves disease and Hashimoto thyroiditis. ATD affect predominantly women and are the most common autoimmune diseases in the United States, with female population prevalence around 0.5% (1, 2). Although managed relatively well in most patients with current treatments, ATD are associated with decreased quality of life and significant morbidity from ophthalmological manifestations (3), osteoporosis (4), and cardiovascular diseases (5). ATD are characterized immunologically by the presence of serum antibodies directed against thyroid-specific or thyroid-restricted antigens like the TSH receptor (TSH-R), thyroperoxidase (TPO), and thyroglobulin (Tg).
The last decade has seen a resurgent interest in using antibodies as a clinical tool due to the discovery that antibodies can predict the development of overt clinical disease (6). For example, antibodies to glutamic acid decarboxylase-65 and insulin autoantibody-2 predict the development of type 1 diabetes in asymptomatic first-degree relatives (7), and antibodies to Ro and La (8) and to cyclic citrullinated protein (9) precede by several years the diagnosis of systemic lupus erythmatosus (SLE) and rheumatoid arthritis. Thyroid antibodies have been associated with subsequent hypothyroidism and ATD in those with a family history of ATD (10, 11).
Using the Department of Defense Serum Repository (DoDSR) and the Defense Medical Surveillance System, we designed a nested case-control study to examine whether females with ATD are more likely than age-matched controls to have thyroid antibodies before diagnosis.
Subjects and Methods
Study population
The DoDSR stores sera of active-duty military personnel collected biannually and before and after each deployment for human immunodeficiency virus testing. Approximately 50 million samples have been collected and stored since 1985. The Defense Medical Surveillance System maintains records of inpatient and outpatient medical diagnoses among all active-duty military personnel at the Military Healthcare System treatment facilities and purchased civilian care sites coded by International Classification of Disease, Clinical Modification 9 (ICD-9-CM) codes (10).
The eligible study population was all female, active-duty, U.S. military personnel between January 1, 1998, and December 31, 2007, who utilized the Defense Medical Surveillance System and who had at least four serum specimens available for retrieval in the DoDSR during the specified time intervals. Men were excluded because Graves disease and Hashimoto thyroiditis affect predominantly women, and an oversampling of male cases would have been required to have a sample size sufficient to make meaningful inferences.
Autoimmune thyroiditis cases (n = 174)
Cases were selected from the eligible study population with a specific diagnosis of either Graves disease (ICD-9-CM 242.0) or Hashimoto thyroiditis (ICD-9-CM 245.2). Cases were defined as individuals in the Defense Medical Surveillance System with either one inpatient ICD-9-CM diagnosis code in any diagnosis position (DX1-DX8) or two or more primary (i.e. first diagnosis position) outpatient ICD-9-CM diagnosis codes separated by at least 7 d between diagnosis visits reported at rheumatology, endocrinology, obstetrics and gynecology, family medicine, or internal medicine clinics within the Military Healthcare System or from a civilian care setting. The first inpatient or outpatient diagnosis code was considered the date of clinical onset (the index date). One thousand six hundred eighty-four cases were identified among 543,097 female active military duty personnel during the study period. One hundred seventy-four cases meeting the inclusion criteria were randomly chosen as part of this study: 87 with Graves disease and 87 with Hashimoto thyroiditis.
Healthy controls (n = 348)
For each case we selected two age-matched (±1 yr) apparently healthy controls. All controls were required to be on active duty on the case's index date, with identical demographic, serum, and medical data obtained in relation to this date.
Serum time points.
Each case and control, a total of 522 subjects, were studied at four serum time points for a total of 2088 sera: at the time of the clinical diagnosis ± 6 months (Dx), between 0.5 and 2 yr before the clinical diagnosis (Pre1), between 2 and 5 yr before (Pre2), and between 5 and 7 yr before (Pre3).
Tg, TPO, and TSH-R antibody tests
Tg and TPO antibodies were measured in all 2088 serum samples using commercial ELISA kits (QUANTA Lite Thyroid T and TPO; INOVA Diagnostics, Inc., San Diego, CA), according to the manufacturer's recommendations. The results were expressed as World Health Organization units and considered positive when 100 or greater.
TSH-R antibodies were measured using a commercial ELISA kit (Kronus, Star, ID) and considered positive when greater than 3 IU/liter. TSH-R antibody was measured in all 87 Graves disease cases at all four time points (348 samples) and in a 10% subset of Hashimoto cases (17 patients, all at Pre3) and healthy controls (17 individuals at both Pre3 and Dx).
Potential confounders
The presence of thyroid antibodies as well as clinical Graves disease and Hashimoto thyroiditis are known to differ by age and race. We accounted for differences in age by matching on age in the control selection process. Self-identified race-ethnicity information was recorded in the demographic record as white, black, Hispanic, American Indian/Alaskan Native, Asian/Pacific Islander, other, or unknown. In the analysis, race-ethnicity was coded as white, African-American, Hispanic, or other due to sample size limitations.
Data analysis
Baseline characteristics between cases and controls were compared using Student's t test for continuous variables and χ2 test for categorical variables. Conditional logistic regression was performed to assess whether the odds of having thyroid antibodies before diagnosis and at diagnosis differed between cases and matched controls, adjusting for race-ethnicity. A value of P < 0.05 was considered statistically significant for all analyses, which were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC). A validation study of the index date as the diagnosis date; the sensitivity, specificity, and predictive value of each antibody at each time point; and a model to assess the rate of change in antibody level over time are provided in the Supplemental Tables 1–5, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Results
Cases and controls were similar with respect to the matching factor age but differed for race-ethnicity (P < 0.001, Table 1). Graves disease cases were more likely to be African-American (59.8%) than Hashimoto thyroiditis cases (12.7%) or controls (34.2%). Hashimoto thyroiditis cases were more likely to be white (65.5%) than Graves disease cases (26.4%) or controls (48.3%). Hashimoto thyroiditis cases were also more likely to be Hispanic (14.9%) than Graves disease cases (5.8%) or controls (9.2%).
Table 1.
Demographic comparison of Graves disease cases, Hashimoto thyroidits cases, and controls, 1998–2007
| Graves disease cases | Hashimoto thyroiditis cases | Controls | P value | |
|---|---|---|---|---|
| n | 87 | 87 | 348 | |
| Calendar year of index datea,b | 2004 | 2003 | 2004 | |
| Median | 1.0 | |||
| Age at index (yr)b | 32 | 32 | 32 | |
| Median | 1.0 | |||
| (Minimun-maximum) | (23–50) | (23–50) | (23–50) | |
| Race-ethnicity (%) | <0.001 | |||
| White | 26 | 66 | 48 | |
| African-American | 60 | 13 | 34 | |
| Hispanic | 6 | 15 | 9 | |
| Other | 8 (4 A, 1 I, 0 O, 2 Z) | 7 (1 A, 2 I, 1 O, 2 Z) | 8 (12 A, 4 I, 2 O, 11 Z) | |
| Clinical thyroid testing prior to index date (%) | 7 | 8 | 2 | <0.01 |
A, Asian; I, American Indian/Pacific Islander; O, other; Z, unknown.
Index date is the case's diagnosis date; all information for cases and controls was collected relative to this date.
Matching factor.
Tg and TPO antibodies
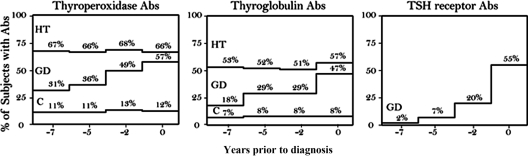
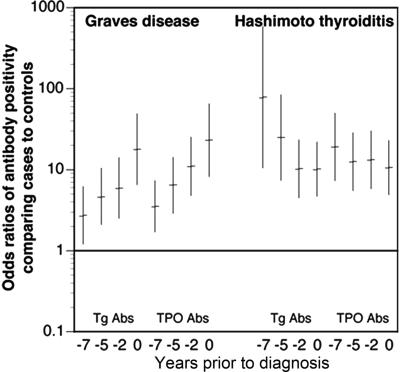
The percentage of Hashimoto thyroiditis cases with positive antibodies remained similar for Tg and TPO antibodies at all time points, whereas the percentage of Graves disease cases increased for both Tg and TPO antibodies as time approached clinical diagnosis (Fig. 1). This increase was statistically significant in the repeated-measures analysis reported in the Supplemental Data. The odds of having Tg or TPO antibodies were significantly greater in Graves disease, and Hashimoto cases than in matched controls at all time points (Fig. 2). In Graves disease, the odds significantly increased approaching diagnosis, whereas in Hashimoto thyroiditis the increase remained constant. Similarly, sensitivity of Tg or TPO antibody positivity was similar up to 7 yr before diagnosis as at diagnosis in Hashimoto thyroiditis (5–7 yr prior vs. diagnostic: sensitivity 72 vs. 74; specificity 86 vs. 86), but sensitivity increased in Graves' disease (5–7 yr prior vs. diagnostic: sensitivity 38 vs. 64; specificity 86 vs. 86; Supplemental Tables 3 and 4).
Fig. 1.
Antibody positivity at each serum collection time point. Abs, Antibodies; HT, Hashimoto thyroiditis; GD, Graves disease; C, control. No control or Hashimoto thyroiditis case was TSH-R antibody positive.
Fig. 2.
Odds ratio comparing antibody positivity in Graves disease or Hashimoto thyroiditis cases and matched controls at each serum collection time point. Odds rations calculated using conditional logistic regression accounting for matching factors and race/ethnicity. Abs, Antibodies.
Table 2.
Odds ratio comparing antibody positivity between cases and controls
| Time of serum collection (since index date) | Tg antibodies |
Thyroid peroxidase antibodies |
||
|---|---|---|---|---|
| Graves disease OR (95% CI)a | Hashimoto thyroiditis OR (95% CI)a | Graves disease OR (95% CI)a | Hashimoto thyroiditis OR (95% CI)a | |
| Pre3 (5–7 yr) | 2.7 (1.2–6.2) | 78.0 (10.5–581.8) | 3.5 (1.7–7.3) | 19.1 (7.3–49.7) |
| Pre2 (2–5 yr) | 4.6 (2.1–10.4) | 25.0 (7.4–83.9) | 6.5 (2.9–14.2) | 12.5 (5.5–28.4) |
| Pre1 (6 months to 2 yr) | 5.9 (2.5–14.0) | 10.2 (4.5–23.3) | 11.0 (4.8–25.3) | 13.2 (5.8–30.2) |
| Diagnostic (±6 months) | 17.8 (6.5–48.7) | 10.1 (4.7–21.9) | 23.1 (8.2–64.9) | 10.6 (4.9–22.9) |
OR, Odds ratio; CI, confidence interval.
Odds ratio was calculated using conditional logistic regression accounting for matching factors and race-ethnicity.
TSH-R antibodies
In Graves disease, the prevalence of TSH-R antibodies at the Pre3 time point (two of 87, 2%, Fig. 1C) and Pre2 time point (six of 87, 7%, Fig. 1C) was overall lower than the prevalence of TPO antibodies (31 and 36%, Fig. 1A) and Tg antibodies (18 and 29%, Fig. 1B). The TSH-R antibody positivity prevalence then rose steeply, approaching that of the TPO and Tg antibodies at the Pre1 time point (17 of 87, 20%, Figure 1C) and were similar to both Tg (47%) and TPO (57%) antibody positivity at diagnosis (48 of 87, 55%, Fig. 1C). No TSH-R antibodies were found in the subset of Hashimoto thyroiditis or healthy controls studied (Fig. 1C). The sensitivity of TSH-R antibodies for Graves disease at pre3 was 2% (specificity 100%) compared with 55% at diagnosis (specificity 100%; Supplemental Table 3). Because no TSH-R antibodies were found in controls, no odds ratios for Graves disease could be calculated.
Combining positivity for Tg, TPO, or TSH-R antibodies at the diagnostic sample yielded a higher sensitivity but similar specificity than the combination of Tg or TPO antibodies (sensitivity 75 vs. 64%; specificity 88 vs. 86%). However, the sensitivity and specificity at the pre3 time point was similar for antibody positivity to Tg, TPO, or TSH-R and Tg or TPO (sensitivity 38 vs. 38; specificity 88 vs. 86).
Discussion
Thyroid antibody positivity precedes a clinical diagnosis of Graves disease or Hashimoto thyroiditis by several years. This anticipation was longer for the TPO and Tg antibodies, which are more commonly associated with Hashimoto thyroiditis. This findings is consistent with the notion that Hashimoto thyroiditis, due to the regenerative capacity of the thyroid gland under the influence of TSH, can exist for several years before clinical hypothyroidism (11). The anticipation was shorter for TSH-R antibodies, which is not surprising as TSH-R antibodies are involved in the causative pathway of Graves disease (11).
The prevalence of Tg or TPO antibody positivity of 14% in our study controls was similar to other estimates from the United States including those from the National Health and Nutrition Examination Survey (NHANES). In NHANES III covering 1988–1994, among women aged 30–39 yr who were free of thyroid disease, 13.6% were Tg antibody positive and 12.6% were TPO antibody positive (1).
Our findings are consistent with studies of the relationship between antibodies and type 1 diabetes (7), SLE (8), and rheumatoid arthritis (9). Using the same DoDSR source, Arbuckle et al. conducted a study of antibodies preceding diagnosis of SLE (8). They found 88% of 130 patients had at least one SLE antibody present before diagnosis. On average, a SLE antibody was detectable 3.3 yr before diagnosis, with antibody positivity in one patient 9.3 yr before diagnosis. In our study, the mean Tg and TPO antibody levels were above the World Health Organization threshold of positivity on the first serum sample tested 5–7 yr before diagnosis, preventing us from estimating the exact timing of conversion to antibody positivity (Supplemental Table 5). The timing of antibody detection may differ between ATD and SLE because the time between antibody seroconversion and diagnosis is shorter in SLE or because there are actual differences in seroconversion timing.
Strengths of the current study include the well-characterized population with equal access to care and the collection of serum samples without regard to health status. The availability of demographic and health information on all women minimized the amount of missing data that is common in retrospective studies. The DoDSR collects serum routinely, independent of health status; thus, findings are unlikely to be preferentially measured when cases and controls may be ill.
A weakness of our study was the inability to adjust for medications and smoking status because this information was not available in the data source. Smoking is known to be more prevalent in military than civilian women in the United States (12) and to have profound and complex effects on the immune system (13). Smoking can be both detrimental and beneficial for ATD. In Graves disease, smoking has been consistently shown to be detrimental, especially in patients with Graves ophthalmopathy. A 2002 metaanalysis of 25 studies reported a summary odds ratio of 3.3 for smoking and Graves disease and also showed a dose-effect because odds increased with greater number of cigarettes smoked (14). In Hashimoto thyroiditis, however, smoking has been associated with a decreased risk of disease (11). Smoking has also been associated with fewer thyroid antibodies. Belin et al. (15) analyzed data from NHANES III and noted that smokers had 11% fewer TPO and/or Tg antibodies than nonsmokers. Studies of female ATD cases have also reported fewer Tg or TPO antibodies in smokers or while cases were smoking (17–19). In our study, taking into account the role of smoking in decreasing thyroid antibodies, increasing the risk of Graves disease and decreasing the risk of Hashimoto thyroiditis, we may have underestimated the relationship between antibodies and Graves disease and overestimated the relationship between antibodies and Hashimoto thyroiditis. Given the strength of the relationship between antibodies and Hashimoto thyroiditis, it is unlikely that accounting for smoking would have nullified or reversed the direction of the relationships.
In conclusion, antibodies to Tg and TPO are present in Graves disease and Hashimoto thyroiditis up to 7 yr before clinical diagnosis and are elevated compared with controls. The antibody levels increased over time before clinical diagnosis of Graves disease but were elevated at all time points for Hashimoto thyroiditis. These findings suggest that thyroid antibodies in apparently healthy individuals should not be neglected and may serve as a useful tool to screen for ATD before clinical diagnosis.
Supplementary Material
Acknowledgments
The authors thank Dr. Angie Eick (Armed Forces Health Surveillance Center), LTC (P) Steven Cersovsky, M.D., M.P.H., Dr. Remington Nevin, and Dr. C. Lynne Burek for their input throughout this study. The authors acknowledge INOVA Diagnostics, Inc. (San Diego, CA) for the generous donation of all thyroglobulin and thyroid peroxidase ELISA kits. INOVA did not play a role in the design, analysis, or interpretation of the data.
This work was supported by a grant from the American Autoimmune Related Disease Association (to S.H.). P.C. was supported by National Institutes of Health Grant DK 55670. This work was also supported by the Johns Hopkins Research Center for Autoimmune Disease.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ATD
- Autoimmune thyroid diseases
- DoDSR
- Department of Defense Serum Repository
- ICD-9-CM
- International Classification of Disease, Clinical Modification 9
- NHANES
- National Health and Nutrition Examination Survey
- SLE
- systemic lupus erythmatosus
- Tg
- thyroglobulin
- TPO
- thyroperoxidase
- TSH-R
- TSH receptor.
References
- 1. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 2. Jacobson DL, Gange SJ, Rose NR, Graham NM. 1997. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 84:223–243 [DOI] [PubMed] [Google Scholar]
- 3. Yeatts RP. 2005. Quality of life in patients with Graves ophthalmopathy. Trans Am Ophthalmol Soc 103:368–411 [PMC free article] [PubMed] [Google Scholar]
- 4. Burman KD. 1997. Thyroid disease and osteoporosis. Hosp Pract (Minneap) 32:71–73, 78–85; discussion 85–86 [DOI] [PubMed] [Google Scholar]
- 5. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J. 2010. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rose NR. 2007. Prediction and prevention of autoimmune disease: a personal perspective. Ann NY Acad Sci 1109:117–128 [DOI] [PubMed] [Google Scholar]
- 7. Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. 1996. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 45:926–933 [DOI] [PubMed] [Google Scholar]
- 8. Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. 2003. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349:1526–1533 [DOI] [PubMed] [Google Scholar]
- 9. Raptopoulou A, Sidiropoulos P, Katsouraki M, Boumpas DT. 2007. Anti-citrulline antibodies in the diagnosis and prognosis of rheumatoid arthritis: evolving concepts. Crit Rev Clin Lab Sci 44:339–363 [DOI] [PubMed] [Google Scholar]
- 10. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, Young ET. 1995. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43:55–68 [DOI] [PubMed] [Google Scholar]
- 11. Effraimidis G, Strieder TG, Tijssen JG, Wiersinga WM. 2011. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. Eur J Endocrinol 164:107–113 [DOI] [PubMed] [Google Scholar]
- 12.Moore M, Eiseman E, Fisher G, Olmsted SS, Sama PR, Zambrano JA. Harnessing Full Value from the DoD Serum Repository and the Defense Medical Surveillance System: RAND Corporation monograph series. 2010 [PMC free article] [PubMed] [Google Scholar]
- 13. Jahnke SA, Haddock CK, Poston WS, Hoffman KM, Hughey J, Lando HA. 2010. A qualitative analysis of the tobacco control climate in the U.S. military. Nicotine Tob Res 12:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arnson Y, Shoenfeld Y, Amital H. 2010. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 34:J258–J265 [DOI] [PubMed] [Google Scholar]
- 15. Vestergaard P. 2002. Smoking and thyroid disorders–a meta-analysis. Eur J Endocrinol 146:153–161 [DOI] [PubMed] [Google Scholar]
- 16. Belin RM, Astor BC, Powe NR, Ladenson PW. 2004. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 89:6077–6086 [DOI] [PubMed] [Google Scholar]
- 17. Strieder TG, Prummel MF, Tijssen JG, Endert E, Wiersinga WM. 2003. Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin Endocrinol (Oxf) 59:396–401 [DOI] [PubMed] [Google Scholar]
- 18. Pedersen IB, Laurberg P, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB. 2008. Smoking is negatively associated with the presence of thyroglobulin autoantibody and to a lesser degree with thyroid peroxidase autoantibody in serum: a population study. Eur J Endocrinol 158:367–373 [DOI] [PubMed] [Google Scholar]
- 19. Effraimidis G, Tijssen JG, Wiersinga WM. 2009. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J Clin Endocrinol Metab 94:1324–1328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.