Abstract
Background:
Recent analyses in small cohorts suggest that pituitary hormones exert time-varying (viz., initial and delayed) dynamic dose-responsive effects on target glands, wherein down-regulating dynamics are inferable on a time scale of single pulses.
Hypothesis:
Age, body mass index (BMI), and sex modulate the rapid potency-down-regulating dynamics of pulsatile pituitary ACTH-adrenal cortisol coupling overnight.
Location:
The study was conducted at a clinical translational research unit.
Subjects:
Subjects included healthy adults (48 women, 63 men; aged 18–77 yr; BMI 18–42 kg/m2).
Outcomes:
Outcomes included analytical dose-response estimates of endogenous ACTH efficacy, dynamic ACTH potency, and adrenal sensitivity from overnight 10-min ACTH-cortisol profiles.
Results:
Stepwise backward-elimination, multivariate-regression analysis revealed that in the combined cohorts (n = 111), age was associated with enhanced initial ACTH potency (R = 0.265, P = 0.005). Moreover, age and BMI jointly attenuated adrenal sensitivity (R = 0.334, P = 0.0017) and augmented down-regulated ACTH potency (R = 0.321 and P = 0.0028). Exploratory gender-segmented analyses showed that these outcomes might be explained by: (1) a negative effect of age in men on adrenal sensitivity (R = 0.270, P = 0.034) and (2) positive effects of age in men (R = 0.332, P = 0.0019) and BMI in women (R = 0.331, P = 0.024) on initial ACTH potency.
Conclusions:
In healthy adults, adrenal sensitivity to endogenous ACTH pulses, ACTH efficacy, and ACTH potency is associated with age, BMI, and gender. These findings may explain conflicting data in earlier literature and introduce the need to control all three of age, BMI, and sex in future studies of the stress-adaptive axis.
Concepts of homeostatic regulation have progressed through successive echelons of complexity (1). Neurohormone measurements were first made by bioassay and subsequently by immunoassay, fluorometry, and chemiluminescence (2). Single blood samples were obtained initially and multiple sequential measurements more recently (3). Concomitantly, analytical methods have advanced to allow not only quantification of burst-like (pulsatile) hormone secretion (4) but also noninvasive estimation of nonlinear dose-response interactions between functionally paired hormones (5, 6). Dose-response interfaces, such as between LH and testosterone or between ACTH and cortisol, represent critical points of regulatory convergence, which mediate strongly nonlinear relationships between hormonal effectors and glandular responses (7).
Despite continuing technical advances, little is known about the pathophysiology of dose-response coupling in the whole animal or person. In part, this knowledge deficit reflects the following: 1) limitations inherent in conventional experimental approaches that require disrupting neuroendocrine connections to infer regulation (8); 2) the need to measure relevantly paired hormone-concentration time series, albeit made less costly and time consuming recently; and 3) the previous lack of analytical tools to reconstruct dynamic dose-response properties in vivo. In the rare circumstances in which all three issues have been addressed, few subjects have been studied (9, 10). An unexpected insight in such studies was that dose-response processes appear to be highly dynamic, i.e. hormone-response adaptations occur not only between successively paired pulses but also within the time span of individual pulse pairs. In particular, the rapid intrapulse response-desensitization (down-regulation) has been quantified in the case of pulsatile the LH-testosterone drive in 26 men (10) and pulsatile ACTH-cortisol stimulation in 28 other adults (9). Whether and how such rapid dose-response dynamics are regulated by physiological variables like age, body mass index (BMI), and sex, and ultimately also in pathophysiology, have not been established. The importance of ACTH agonist-response down-regulation is suggested by the presentation of ACTH-independent Cushing's syndrome in a patient harboring an ACTH (melanocortin-2) receptor mutation associated with genetically impaired desensitization (11).
Materials and Methods
Overview
The 111 healthy subjects (63 men, 48 women) studied previously underwent a 10-min overnight sampling for 8 h or longer (12–15). Measurements of ACTH and cortisol were made at the time of the initial study. For uniformity only the segment midnight to 0800 h was used, making all hormone time series comparable in length and data density. No physical activity was allowed during sampling, except lavatory use. Lights were put out at 2200 h. Subjects took only water by mouth. No electroencephalographic or physical fitness data were available in these subjects. Each reported regular (>6.5 h/night) sleep habits and recreational level exercise. None of the 8-h data has been published or presented previously or analyzed in the present manner.
Volunteer selection
Criteria for inclusion were healthy community-dwelling adults aged 18–80 yr who provided written informed consent
Criteria for exclusion included recent use of psychotropic or neuroactive drugs (within five biological half-lives); BMI less than 18 or greater than 42 kg/m2; drug or alcohol abuse, psychosis, depression, mania, anorexia/bulimia, or severe anxiety; endocrinopathy, other than primary thyroidal failure receiving replacement; nightshift work or recent transmeridian travel (exceeding three time zones within 7 d of admission); acute weight change (loss or gain of > 2 kg in 6 wk); abnormal hepatorenal function; glucocorticoid, anabolic steroid, or reproductive hormone therapy; and/or an unwillingness to provide written informed consent.
Assays
Plasma ACTH was measured using a two-site sandwich assay designed to detect intact ACTH(1–39) molecules. The immunoradiometric assay consisted of a soluble 125I-labeled (indicator) monoclonal antibody directed to the N terminus of ACTH as well as a second polyclonal ACTH antibody directed to the C terminus. The second antibody was covalently conjugated to biotin to react with avidin-coated plastic beads. All incubation reagents including antibodies, human ACTH(1–30) standard, and avidin-coated beads were from Nichols Institute (Allegro immunoradiometric assay; San Juan Capistrano, CA). Each sample was assayed in duplicate, and all samples from any one subject were assayed in the same run. Sensitivity of the immunoradiometric assay was 1.0 pg/ml or 0.22 pmol/liter, and intraassay precision was 3.2–5.8% (range of median intrasample coefficients of variation in all individuals). Cross-reactivity with β-endorphin, TSH, LH, FSH, GH, or prolactin was less than 0.1%.
Cortisol was assayed using antibody-coated tubes and reagents provided by clinical assays (Dade, Baxter-Travenol Diagnostics, Cambridge, MA). Each sample was assayed in duplicate, and all samples from any one subject were assayed together. Sensitivity was 0.5 μg/dl (14 nmol/liter), and intraassay precision 2.5–4.7% (range of median values for all individuals). Interassay coefficients of variation were 3.4 and 5.6%.
Analytical methods
Details of the dynamic dose-response methodology were described in two earlier more technical papers (9, 10). The goal was to relate time-varying ACTH concentrations (input, effector) to time-varying cortisol secretion rates (output, response) via both a classic four-parameter logistic dose-response function and a six-parameter logistic dose-response function that contains two potencies and a lag time (inflection point) to accommodate allowable (possible but not obligatory) down-regulation of ACTH drive. Simultaneous estimation of all six parameters allows for a possible right-shifted inhibitory (negative) adaptation of the dose-response process within each cortisol pulse at the optimized time lag (9, 10).
ACTH (49 samples) and cortisol (49 samples) time series in each subject were first deconvolved by automated analysis (16). Deconvolution reconstructs hormone concentrations as a train of variable-amplitude secretory bursts superimposed upon basal (time invariant) nonpulsatile secretion with hormone elimination proceeding via a biexponential function (4). Secretion and elimination were deconvolved via an integral-equation model rather than the usual difference-based model as discussed fully in (10). The Akaike information criterion was employed in model selection (9, 10). Next, fitted (reconvolved) ACTH concentrations were related to (deconvolved) cortisol secretion rates via a four-parameter (basal, potency, sensitivity, and efficacy) logistic dose-response model modified to include a potency-down-regulated parameter and matching inflection time (thus, a total of six parameters). The optimal inflection point for each paired series is the simultaneously estimated time delay to ACTH dose-response down-regulation within cortisol secretory bursts, as described (9, 10). Down-regulation is defined here as an allowable decrease in potency of ACTH action after the inflection point. The EC50 is calculated algebraically as the ratio of a (negative) exponential potency term to sensitivity. In the two-potency model, there are two potencies and thus two EC50, one for the onset (initial) and the other for the recovery (down-regulated) time windows within paired ACTH-cortisol pulses. Random effects on cortisol secretory-burst mass and the sd of residual model error were estimated concurrently, as described (9, 10). The significance of potency shifts from onset to recovery was tested by signed-ranks comparison of onset and recovery potency values (before and after putative down-regulation). Overall model choice (potency down-regulation vs. no down-regulation) was evaluated by generalized likelihood ratio testing using a χ2 statistic of twice the (negative) log-likelihood function differences. At present there is no model available for testing simultaneous down-regulation of potency and sensitivity parameters or potency and efficacy parameters. In the potency down-regulation model presented here, neither sensitivity nor efficacy down-regulation is allowed concurrently.
Statistics
Data are presented in tables as median (range) and in figures as box-and-whisker plots containing the median, interquartile range, 90% confidence interval, and extreme values. Gender comparisons were made via an unpaired two-tailed t test after logarithmic transformation and confirmed by the nonparametric rank-sum test.
Stepwise backward-elimination multivariate regression analysis was applied to identify significant associations between [the natural logarithm (ln) of] ACTH-cortisol dose-response parameters and age, BMI, or gender. All three variables were tested first. The α to remove nonsignificant variables one at a time beginning with the least significant was 0.05. R2 and P values were estimated at each stage against the null hypothesis of no correlation (Systat Software, Richmond, CA). R2 values provide an estimate of the percentage of dependent-variable variance explained by the independent variable(s). Exploratory linear regression was used to relate dose-response parameters separately in men and women. Model accuracy was affirmed by repeating the analysis on a separate set of 28 subjects studied more recently with different ACTH and cortisol assays.
Results
Table 1 summarizes the epidemiological data and primary analysis of overnight ACTH profiles. Median (range) age was 52 (18–77) yr in the full cohort with similar values in men and women (P > 0.10). BMI tended to be lower in women than men with respective median values of 24 vs. 26 kg/m2 [P = 0.083 (t test) and P = 0.045 (rank sum test)]. Arithmetic mean (8 h overnight) ACTH concentrations obtained in each of the 111 subjects had a median value of 18 (7.4–45) ng/liter. Peak and nadir ACTH values were 41 (13–161) and 6.5 (1.4–26) ng/liter for the total group without gender differences (all P values >0.10).
Table 1.
Subject characteristics and primary analysis of 111 overnight ACTH-cortisol concentration profiles
| All subjects (n = 111) | Men (n = 63) | Women (n = 48) |
P value |
||
|---|---|---|---|---|---|
| t test | Rank sum | ||||
| Epidemiology | |||||
| Age (yr) | 52 (18–77) | 51 (18–76) | 54 (20–77) | 0.30 | 0.28 |
| BMI (kg/m2) | 25 (18–42) | 26 (20–42) | 24 (18–35) | 0.083 | 0.045 |
| Algebraic values | |||||
| Mean ACTH concentration (ng/liter) | 18 (7.4–45) | 18 (9.9–45) | 16 (7.4–38) | 0.13 | 0.14 |
| Peak ACTH concentration (ng/liter) | 41 (13–161) | 43 (22–161) | 39 (13–105) | 0.38 | 0.44 |
| Nadir ACTH concentration (ng/liter) | 6.5 (1.4–26) | 6.1 (1.4–26) | 6.6 (2.2–25) | 0.86 | 0.98 |
| Mean cortisol concentration (μg/dl) | 8.4 (1.9–15) | 8.4 (3.3–15) | 8.4 (1.9–13) | 0.83 | 0.93 |
| Peak cortisol concentration (μg/dl) | 18 (5.8–38) | 17 (9.5–38) | 18 (5.8–31) | 0.38 | 1.00 |
| Nadir cortisol concentration (μg/dl) | 2.1 (0.38–8.0) | 2.0 (0.38–7.7) | 2.1 (0.55–8.0) | 0.87 | 0.82 |
| Deconvolution analysis | |||||
| ACTH bursts, n (per 8 h) | 7.0 (3.0–11) | 7.0 (4.0–11) | 7.0 (3.0–11) | 0.20 | 0.25 |
| ACTH burst mode (min) | 3.1 (3.0–23) | 3.1 (3.0–12) | 3.0 (3.0–23) | 0.21 | 0.98 |
| Basal ACTH secretion (ng/liter per 8 h) | 235 (52–798) | 235 (52–667) | 235 (70–798) | 0.53 | 0.98 |
| Pulsatile ACTH secretion (ng/liter per 8 h) | 183 (50–597) | 201 (61–597) | 164 (50–497) | 0.039 | 0.044 |
| Total ACTH secretion (ng/liter per 8 h) | 448 (178–1155) | 458 (255–1155) | 396 (178–1033) | 0.13 | 0.12 |
| Mass per burst (ng/liter) | 27 (8.8–81) | 29 (8.8–81) | 24 (10–78) | 0.13 | 0.18 |
| Cortisol bursts, n (per 8 h) | 6.0 (3.0–11) | 6.0 (3.0–11) | 6.0 (3.0–10) | 0.98 | 0.98 |
| Cortisol burst mode (min) | 13 (5.0–20) | 13 (5.0–18) | 13 (5.0–20) | 0.35 | 0.15 |
| Basal cortisol secretion (μg/dl per 8 h) | 18 (1.8–73) | 16 (1.8–68) | 21 (1.8–73) | 0.84 | 0.21 |
| Pulsatile cortisol secretion (μg/dl per 8 h) | 60 (7.2–140) | 64 (20–140) | 56 (7.2–111) | 0.064 | 0.045 |
| Total cortisol secretion (μg/dl per 8 h) | 82 (15–142) | 84 (39–142) | 80 (15–129) | 0.50 | 0.64 |
| Mass per burst (μg/dl) | 10 (1.4–26) | 11 (3.0–26) | 9.8 (1.4–18) | 0.055 | 0.045 |
Deconvolution analysis revealed 7 (3–11) ACTH bursts per 8 h, a latency to secretory-burst maximum of 3.1 (3.0–23) min after burst onset, and basal (nonpulsatile), pulsatile, and total ACTH secretion rates of 235 (52–798), 183 (50–579), and 448 (178–1155) ng/liter per 8 h. Sex determined pulsatile ACTH secretion with median values of 201 (men) and 164 (women) ng/liter per 8 h [P = 0.039 (t test), P = 0.044 (rank sum)].
Overnight mean, peak, and nadir (8 h) cortisol concentrations also did not differ by sex (Table 1). However, the size of individual cortisol secretory bursts (micrograms per deciliter) (multiply by 28 for nanomoles per liter) and the total (summed) amount of cortisol secreted in bursts (pulsatile cortisol secretion, micrograms per deciliter per 8 h) were higher in men than in women (both P = 0.045 by rank sum test). Accordingly, men secreted more ACTH and more cortisol (per liter of homonymous distribution volume) in pulses overnight than women.
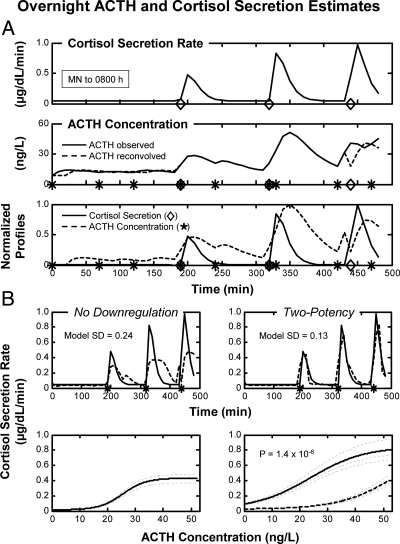
Deconvolution results were used in nonlinear analysis of ACTH's concentration-dependent drive of pulsatile cortisol secretion. Figure 1A illustrates deconvolved cortisol secretion (top panel), measured (observed), and reconvolved (calculated) ACTH concentrations (middle panel), and reconvolved ACTH concentrations with deconvolved cortisol secretion (bottom panel). Figure 1B depicts dose-response dynamics, which were estimated by relating deconvolved cortisol secretion rates to reconvolved ACTH concentrations via logistic functions. Data are for one 8-h overnight profile. To compare model fits objectively in the 111 subjects, model sd were evaluated first. There were no gender effects on model fit. Next, the potency down-regulation model (six parameters for 98 data values) was compared against the classical (no down-regulation) construct (four parameters). By generalized likelihood-ratio testing, the potency down-regulation model was strongly favored over the no-down-regulation model (P < 0.001). Corresponding median model sd were 0.16 and 0.25, respectively (P < 0.001 by signed ranks). By paired comparison, the two-potency and the no-down-regulation models yielded similar ACTH EC50 values (median 17 ng/liter for both). The down-regulation construct yielded higher estimates of ACTH efficacy (0.59 vs. 0.39) and basal cortisol secretion (0.039 vs. 0.008) than the no-down-regulation formulation (P < 0.05).
Fig. 1.
A, Profiles of deconvolved (calculated) cortisol secretion rates (top panel), measured (observed) and reconvolved (fitted) time-shifted ACTH concentrations (middle panel), and unit-normalized reconvolved ACTH concentrations and cortisol secretion rates (bottom panel) in a healthy middle-aged adult. Blood was sampled every 10 min for 8 h beginning at midnight (time zero x-axis). Asterisks (ACTH) and rhomboids (cortisol) in the bottom panel denote burst-onset times. B, Top panel, Calculated (continuous) and model-predicted (interrupted) cortisol secretion rates in the two dose-response models. Bottom panel, Dose-response analysis relating reconvolved ACTH concentrations (x-axis) to deconvolved cortisol secretion rates (y-axis) via a logistic dose-response function without (left panel) or with (right panel) allowable potency down-regulation (see Materials and Methods). Dark interrupted curve denotes down-regulated potency, whereas light-interrupted curves identify model random effects on efficacy.
Stepwise backward-elimination linear regression was used to relate dose-response parameter estimates (dependent variable) to the three independent variables: gender, age, and BMI. The no-down-regulation (no hysteresis) construct revealed decreasing ACTH efficacy with age in men (R2 = 0.11, P = 0.0086) and increasing efficacy with age in women (R2 = 0.12, P = 0.016). The gender difference in slopes was significant at P < 0.001.
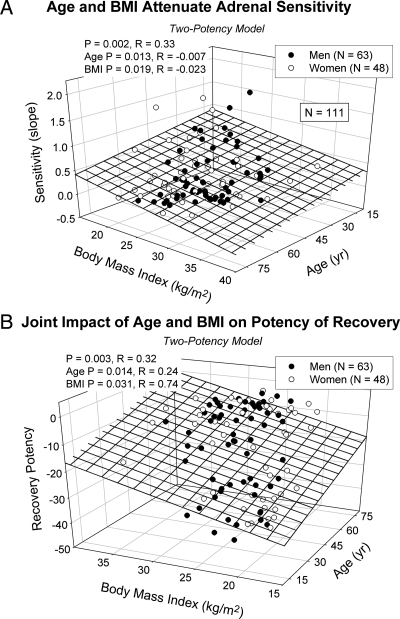
The potency down-regulation construct identified a significant increase in initial potency with age (R2 = 0.070, P = 0.005) (not plotted) and a strong joint dependence of adrenal sensitivity on age and BMI in the full cohort of 111 subjects (R2 = 0.11, P = 0.0017) (Fig. 2A). Specifically, increasing age (P = 0.013) and BMI (P = 0.019) both attenuated adrenal sensitivity (Table 2). Thus, older age was marked by reduced adrenal sensitivity to pulsatile ACTH stimulation while adjusting for BMI and vice versa in the group as a whole. The overall negative effect of age on adrenal sensitivity was explained by a sex-selective effect in men (R2 = 0.073, P = 0.034) (not plotted).
Fig. 2.
A, Age and BMI jointly attenuate adrenal sensitivity (maximal positive ACTH-cortisol dose-response slope) in a cohort of 111 healthy adults (overall P = 0.0017). Sensitivity was negatively associated with both age (P = 0.014) and BMI (P = 0.019), indicating reduced adrenal responsiveness per unit increase in ACTH concentrations. Data are from the potency-down-regulation (two potency) model. B, Age and BMI together augmented down-regulated ACTH potency in 111 healthy adults (overall P = 0.0028). Potency increased during the down-regulating phase of ACTH pulses with age (P = 0.014) and BMI (P = 0.032). Potency values are natural logarithms (exponents on base e).
Table 2.
Backward stepwise-elimination regression models vs. gender, age, and BMI in the two-potency model
| Overall significance |
Gender |
Age |
BMI |
|||||
|---|---|---|---|---|---|---|---|---|
| P value | R | P value | R | P value | R | P value | R | |
| Potency onset | 0.005 | 0.265 | 0.230 | −0.115 | 0.005 | 0.265 | 0.164 | 0.134 |
| Potency recovery | 0.0028 | 0.321 | 0.860 | −0.017 | 0.014 | 0.238 | 0.031 | 0.735 |
| Sensitivity | 0.0017 | 0.334 | 0.265 | 0.108 | 0.013 | −0.007 | 0.019 | −0.023 |
| Efficacy | NS | — | 0.656 | 0.044 | 0.827 | −0.022 | 0.675 | −0.041 |
| Basal | 0.052 | 0.185 | 0.495 | 0.066 | 0.052 | 0.185 | 0.235 | 0.114 |
| Inflection point | NS | — | 0.112 | −0.152 | 0.620 | −0.048 | 0.076 | 0.170 |
| Model sd | NS | — | 0.938 | −0.008 | 0.223 | −0.117 | 0.362 | −0.087 |
| Random effects | NS | — | 0.608 | 0.050 | 0.823 | 0.022 | 0.827 | 0.021 |
| EC50 onset | NS | — | 0.876 | −0.015 | 0.771 | −0.028 | 0.627 | 0.048 |
| EC50 recovery | NS | — | 0.938 | −0.008 | 0.321 | 0.096 | 0.765 | −0.029 |
n = 111 (63 men and 48 women). NS (not significant) denotes P > 0.10. Individual R values are partial correlations for gender, age, and BMI. A negative R value for gender denotes male greater than female. Potency is a negative exponential term. The P values in lightface type represent the penultimate probability of significance, prior to rejection of that term from backward stepwise-elimination regression, whereas boldface P values reflect the final significant model values.
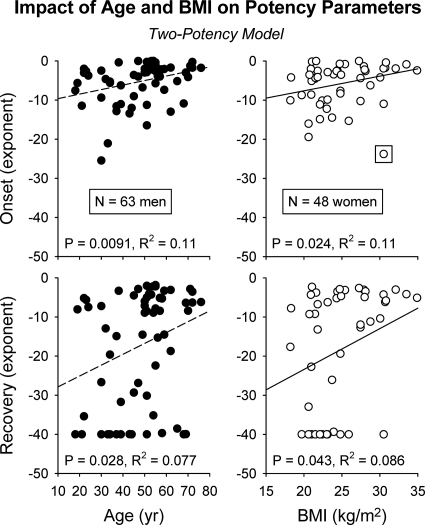
Age and BMI also jointly (i.e. when the effect of each variable was adjusted for that of the other) augmented the potency of the down-regulated phase of pulsatile ACTH's drive of cortisol secretion (R2 = 0.10, P = 0.0028, n = 111 subjects) (Fig. 2B). Exploratory (gender segmented) univariate regression analysis indicated that age positively determined ACTH potency in men (P = 0.0091 for initial and P = 0.028 for down-regulated potency) (Fig. 3, left panel). In contradistinction, BMI positively determined ACTH potency in women (P = 0.024 initial and P = 0.043 down-regulated) (Fig. 3, right panel).
Fig. 3.
Positive association of ACTH potency with age in men (left diptych) and with BMI in women (right diptych). Onset (top panel) and offset (bottom panel) denote initial and delayed (down-regulated) ACTH potencies. Data are presented as described in Fig. 3.
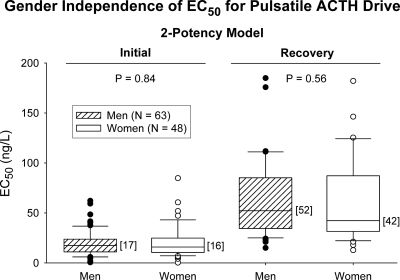
The initial EC50 onset in the two-potency model was comparable in men (17 ng/liter) and women (16 ng/liter) (Fig. 4, left panel). Analogously, the down-regulated EC50 was similar in women (42 ng/liter) and men (52 ng/liter (Fig. 4, right panel). There was a nonsignificant trend for basal cortisol secretion to rise with age (n = 111) (P = 0.052, R = 0.185) (Table 2).
Fig. 4.
Gender independence of ACTH-cortisol EC50 values during the initial and down-regulating (recovery) phases of overnight pulsatile ACTH secretion. Median values are given in brace parentheses. Data are presented as box-and-whisker plots (see Materials and Methods).
Age, gender, and BMI did not influence model-based random effects (not shown) or median time delays to down-regulation within cortisol secretory bursts [median 28 min (10–50 min)].
Discussion
A new investigative strategy was used to identify clinical factors that modulate dynamic ACTH drive of cortisol secretion in 111 normal adults, displaying a wide range of ages (18–77 yr) and BMI (18–42 kg/m2). The motivation is recent evidence that complex signaling dynamics may operate not only in vitro (8–10, 17–19) but also in healthy individuals in vivo (9, 10). Indeed, a sporadic clinical mutation of the ACTH receptor gene that attenuated the down-regulation resulted in Cushing syndrome (11). By combining overnight ACTH-cortisol sampling, automated dose-response estimation and stepwise forward-selection regression, the present analyses delineate strong correlations between age, BMI, and sex and specific dynamics of the ACTH drive. Salient outcomes include the following: 1) mathematical affirmation of a marked reduction in dose-response model variance (denoting improved fit) of paired ACTH concentration-cortisol secretion time series after allowance for reversible down-regulation of ACTH potency (P < 0.001); 2) consistent (model independent) estimates of the endogenous ACTH EC50 driving cortisol secretory bursts; 3) jointly negative effects of age and BMI on adrenal sensitivity (P = 0.0017); 4) jointly positive effects of age and BMI on down-regulated ACTH potency (P = 0.0028); 5) increased ACTH potency with age in men (P = 0.0091) and with BMI in women (P = 0.024); and 6) a median down-regulation time delay of 28 min comparable by sex within ACTH-cortisol pulses pairs. The collective findings point to a far more complex regulation of overnight ACTH-adrenal interactions than previously recognized.
Formal comparison of the precision of fit of the new potency down-regulation and the classical (no down-regulation) dose-response constructs demonstrated greatly enhanced fit [median reduction of fitted variance 60% (P < 0.001)]. This verifies an earlier analysis of 28 adults, which also reported significant model improvement (9). Although improved mathematical fit per se does not establish model validity, metyrapone administration in five men decreased the calculated ACTH efficacy by 85% viz., from 1.0 ± 0.33 to 0.15 ± 0.011 μg/dl · min (P = 0.002). During insulin-induced hypoglycemia in seven other men, the estimated potency of ACTH's drive of cortisol secretion was 7-fold greater than that for β-endorphin (P = 0.011). And in 17 adults, two-potency model estimates of efficacy, sensitivity, potency, and basal parameters did not differ when assessed using 24- and 8-h overnight data (not shown).
The median estimate of endogenous ACTH EC50 was 17 ng/liter at the onset and 47 ng/liter at the offset (response down-regulation) of coupled ACTH-cortisol pulses in the 111 subjects. Analyzed without allowable down-regulation, the median ACTH EC50 was also 17 ng/liter. Two earlier dose-response estimation methods devoid of down-regulation predicted EC50 values of 16 ± 3.3 and 24 ± 2.1 (se) ng/liter from the 24-h data (9, 20). Visual inspection of published paired ACTH-cortisol concentrations collected over several hours during insulin tolerance test, arginine vasopressin, CRH, or ACTH stimulation allowed indirect EC50 estimates of 25–59 ng/liter (21–25). However, these estimates are for peak rather than mean ACTH concentrations. If the latter are about 40% of the peak value, then an indirectly inferred range for mean ACTH EC50 would be 10–24 ng/liter. Because overnight plasma ACTH concentrations in the 111 subjects evaluated here averaged 18 ng/liter with nadir and peak values of 6.5 and 41 ng/liter, respectively, the analytically estimated EC50 of 17 ng/liter suggests highly effectual tuning of cortisol secretion by endogenous ACTH pulses (7, 23). For these values and a nominal cortisol distribution volume of 8 liter/m2 in adults (26), the overnight cortisol secretion would be about 4 mg/m2 per 8 h, which is consistent with estimates of the (24 h) cortisol production rate of 2.4–14 mg per 24 h (9, 27).
Stepwise regression analysis unveiled two prominent joint-variable effects. First, estimated adrenal sensitivity (slope of dose response) in the full cohort was negatively determined by BMI and age, together explaining 11% of the variance in this parameter. Technically this indicates that increased age and BMI predict decreased cortisol secretory responses to a unit (nanograms per liter) increment in ACTH concentrations. The clinical effect on the age-BMI interaction is substantial if one compares (calculated) adrenal sensitivity for an 18 yr old having a BMI of 18 kg/m2 with that of a 78 yr old having a BMI of 38 kg/m2. Second, recovery (down-regulated) ACTH potency was determined positively by BMI and age, together explaining 10% of the dose-response variance. This outcome favors more sustained cortisol secretion during ongoing ACTH pulses in overweight and older individuals. Exploratory univariate regression analyses within gender pointed to influences of BMI (positive effect in women only) and age (positive effect in men only) on ACTH potency. If this is verified in additional cohorts, these data would signify that each of age, BMI, and gender contributes to intersubject variability in endogenous ACTH drive.
Important confirmatory data were obtained in relation to the recently inferred gender-stratified impact of age on calculated ACTH efficacy in the non-down-regulated model. The point of testing the no-down-regulation model was to verify or to refute a previous inference under the conventional model of opposite effects of age on ACTH efficacy in men and women. In the 48 women studied here, ACTH efficacy rose with age (P = 0.016, R2 = 0.12), whereas in the 63 men studied here, ACTH efficacy declined with age (P = 0.0086, R2 = 0.11). The gender contrast was highly significant (P < 0.001). For example, predicted ACTH efficacy (maximal stimulated cortisol secretion) at age 18 yr would be 111 (men) and 47 (women), whereas at age 78 yr, it would be 88 (men) and 67 (women) mg cortisol secreted per day (nominal volume of distribution = 14 liters per 1.73 m2 in men, 13 liters per 1.6 m2 in women) (26, 28, 29). In the extant literature, age altered inferable ACTH efficacy in a gender-specified manner in some (30, 31) but not other (32, 33) acute arginine vasopressin/CRH/ACTH stimulation tests. Therefore, further clinical investigations of sex-by-age interactions are needed in this axis.
Several caveats are appropriate. First, inferences related to age, BMI, and sex ultimately need to be confirmed by randomly ordered dose-varying injections of near-physiological ACTH pulses in a prospective fashion in larger cohorts and in relation to testosterone and estradiol levels (not available here). Other factors must also determine ACTH-cortisol feed-forward. Indeed, substantial variance in dose-response parameters remains unexplained. Possible factors include intraadrenal circadian pacemakers (34); endogenous sex steroids (35), systemic and local cytokines (36), glucose intolerance or diabetes mellitus (37); and peptides like neuropeptide Y, angiotensin II, and leptin (38). These and other considerations require further investigation. Second, corticosteroid-binding globulin (CBG) availability influences cortisol kinetics (28). However, estimates of ACTH EC50 would not be affected by CBG differences (7). In addition, the age-related rise in ACTH efficacy in women would be opposite of that predicted by a menopausal fall in CBG and estrogen levels. Third, how acute or chronic stress alters endogenous ACTH-cortisol dose responsiveness has not been ascertained. And fourth, the generality of these inferences to other human pituitary hormone-target organ interactions is not yet known.
In summary, age, BMI, and gender are individually and in some cases jointly associated with endogenous ACTH's stimulation of overnight pulsatile cortisol secretion in healthy adults. Gender markedly affects how age influences ACTH efficacy (increased and decreased, respectively, in women and men), and whether age (men) or BMI (women) augments ACTH potency. Age and BMI together modulate adrenal sensitivity (both negatively) and ACTH potency (both positively). These outcomes, if confirmed longitudinally, introduce a more dynamic understanding of pituitary-adrenal glucocorticoid control.
Acknowledgments
We thank Jill Smith for support of the manuscript preparation; Ashley Bryant for data analysis and graphics; the Mayo Immunochemical Laboratory for assay assistance; and the Mayo research nursing staff for implementing the protocol.
This work was supported in part by the Center for Translational Science Activities Grant 1 UL 1 RR024150 from the National Center for Research Resources (Rockville, MD), and Grants DK73148 and DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- CBG
- corticosteroid-binding globulin.
References
- 1. Dempsher DP, Gann DS, Phair RD. 1984. A mechanistic model of ACTH-stimulated cortisol secretion. Am J Physiol 246:R587–R596 [DOI] [PubMed] [Google Scholar]
- 2. Giustina A, Veldhuis JD. 1998. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19:717–797 [DOI] [PubMed] [Google Scholar]
- 3. Jusko WJ, Slaunwhite WR, Jr, Aceto T., Jr 1975. Partial pharmacodynamic model for the circadian-episodic secretion of cortisol in man. J Clin Endocrinol Metab 40:278–289 [DOI] [PubMed] [Google Scholar]
- 4. Veldhuis JD, Keenan DM, Pincus SM. 2008. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev 29:823–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keenan DM, Licinio J, Veldhuis JD. 2001. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA 98:4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keenan DM, Alexander SL, Irvine CH, Clarke I, Scott C, Turner A, Tilbrook AJ, Canny BJ, Veldhuis JD. 2004. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements. Proc Natl Acad Sci USA 101:6740–6745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keenan DM, Veldhuis JD. 2003. Mathematical modeling of receptor-mediated interlinked systems. Encyclopedia of hormones. San Diego: Academic Press; 286–294 [Google Scholar]
- 8. Schulster D, Rafferty B, Williams B. 1984. Corticotropin-induced desensitization: steroidogenic and cyclic AMP responses in superfused adrenocortical cells. Mol Cell Endocrinol 36:43–51 [DOI] [PubMed] [Google Scholar]
- 9. Keenan DM, Roelfsema F, Veldhuis JD. 2010. Dose-response downregulation within the span of single interpulse intervals. Am J Physiol Regul Integr Comp Physiol 299:R11–R18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keenan DM, Iranmanesh A, Veldhuis JD. 2011. Analytical construct of reversible desensitization of pituitary-testicular signaling: illustrative application in aging. Am J Physiol Regul Integr Comp Physiol 300:R349–R360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swords FM, Baig A, Malchoff DM, Malchoff CD, Thorner MO, King PJ, Hunyady L, Clark AJ. 2002. Impaired desensitization of a mutant adrenocorticotropin receptor associated with apparent constitutive activity. Mol Endocrinol 16:2746–2753 [DOI] [PubMed] [Google Scholar]
- 12. Veldhuis JD, Iranmanesh A, Naftolowitz D, Tatham N, Cassidy F, Carroll BJ. 2001. Corticotropin secretory dynamics in humans under low glucocorticoid feedback. J Clin Endocrinol Metab 86:5554–5563 [DOI] [PubMed] [Google Scholar]
- 13. Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G. 1990. Twenty-four hour rhythms in plasma concentrations of adenohypophyseal hormones are generated by distinct amplitude and/or frequency modulation of underlying pituitary secretory bursts. J Clin Endocrinol Metab 71:1616–1623 [DOI] [PubMed] [Google Scholar]
- 14. Roelfsema F, van den Berg G, Frölich M, Veldhuis JD, van Eijk A, Buurman MM, Etman BH. 1993. Sex-dependent alteration in cortisol response to endogenous adrenocorticotropin. J Clin Endocrinol Metab 77:234–240 [DOI] [PubMed] [Google Scholar]
- 15. van den Berg G, Pincus SM, Veldhuis JD, Frölich M, Roelfsema F. 1997. Greater disorderliness of ACTH and cortisol release accompanies pituitary-dependent Cushing's disease. Eur J Endocrinol 136:394–400 [DOI] [PubMed] [Google Scholar]
- 16. Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD. 2009. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab 297:E538–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamberts SW, Bons EG, Zuiderwijk-van der Roest JM. 1987. Studies on the mechanism of corticotrophin-mediated desensitization of corticosterone secretion by rat adrenocortical cells. Mol Cell Endocrinol 52:243–249 [DOI] [PubMed] [Google Scholar]
- 18. Lebrethon MC, Naville D, Begeot M, Saez JM. 1994. Regulation of corticotropin receptor number and messenger RNA in cultured human adrenocortical cells by corticotropin and angiotensin II. J Clin Invest 93:1828–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rani CS, Keri G, Ramachandran J. 1983. Studies on corticotropin-induced desensitization of normal rat adrenocortical cells. Endocrinology 112:315–320 [DOI] [PubMed] [Google Scholar]
- 20. Keenan DM, Roelfsema F, Carroll BJ, Iranmanesh A, Veldhuis JD. 2009. Sex defines the age dependence of endogenous ACTH-cortisol dose responsiveness. Am J Physiol Regul Integr Comp 297:R515–R523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oelkers W, Boelke T, Bähr V. 1988. Dose-response relationships between plasma adrenocorticotropin (ACTH), cortisol, aldosterone, and 18-hydroxycorticosterone after injection of ACTH-(1–39) or human corticotropin-releasing hormone in man. J Clin Endocrinol Metab 66:181–186 [DOI] [PubMed] [Google Scholar]
- 22. Thomas MA, Rebar RW, LaBarbera AR, Pennington EJ, Liu JH. 1991. Dose-response effects of exogenous pulsatile human corticotropin-releasing hormone on adrenocorticotropin, cortisol, and gonadotropin concentrations in agonadal women. J Clin Endocrinol Metab 72:1249–1254 [DOI] [PubMed] [Google Scholar]
- 23. Tuchelt H, Dekker K, Bahr V, Oelkers W. 2000. Dose-response relationship between plasma ACTH and serum cortisol in the insulin-hypoglycaemia test in 25 healthy subjects and 109 patients with pituitary disease. Clin Endocrinol (Oxf) 53:301–307 [DOI] [PubMed] [Google Scholar]
- 24. Orth DN, Jackson RV, DeCherney GS, DeBold CR, Alexander AN, Island DP, Rivier J, Rivier C, Spiess J, Vale W. 1983. Effect of synthetic ovine corticotropin-releasing factor. Dose response of plasma adrenocorticotropin and cortisol. J Clin Invest 71:587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hensen J, Hader O, Bähr V, Oelkers W. 1988. Effects of incremental infusions of arginine vasopressin on adrenocorticotropin and cortisol secretion in man. J Clin Endocrinol Metab 66:668–671 [DOI] [PubMed] [Google Scholar]
- 26. Peterson RE. 1959. The miscible pool and turnover rate of adrenocortical steroids in man. Recent Prog Horm Res 15:231–274 [Google Scholar]
- 27. Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, Booth JD, Winterer JC, Loriaux DL. 1991. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 72:39–45 [DOI] [PubMed] [Google Scholar]
- 28. Bright GM. 1995. Corticosteroid-binding globulin influences kinetic parameters of plasma cortisol transport and clearance. J Clin Endocrinol Metab 80:770–775 [DOI] [PubMed] [Google Scholar]
- 29. Kraan GP, Dullaart RP, Pratt JJ, Wolthers BG, de Bruin R. 1997. Kinetics of intravenously dosed cortisol in four men. Consequences for calculation of the plasma cortisol production rate. J Steroid Biochem Mol Biol 63:139–146 [DOI] [PubMed] [Google Scholar]
- 30. Parker CR, Jr, Slayden SM, Azziz R, Crabbe SL, Hines GA, Boots LR, Bae S. 2000. Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J Clin Endocrinol Metab 85:48–54 [DOI] [PubMed] [Google Scholar]
- 31. Greenspan SL, Rowe JW, Maitland LA, McAloon-Dyke M, Elahi D. 1993. The pituitary-adrenal glucocorticoid response is altered by gender and disease. J Gerontol 48:M72–M77 [DOI] [PubMed] [Google Scholar]
- 32. Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. 2005. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology 30:80–91 [DOI] [PubMed] [Google Scholar]
- 33. Karaca Z, Lale A, Tanriverdi F, Kula M, Unluhizarci K, Kelestimur F. 2010. The comparison of the low and standard dose ACTH and glucagon stimulation tests in the evaluation of hypothalamo-pituitary-adrenal axis in healthy adults. Pituitary 14:134–140 [DOI] [PubMed] [Google Scholar]
- 34. Kino T, Chrousos GP. 2011. Circadian CLOCK-mediated regulation of target-tissue sensitivity to glucocorticoids: implications for cardiometabolic diseases. Endocr Dev 20:116–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polderman KH, Gooren LJ, Van der Veen EA. 1994. Testosterone administration increases adrenal response to adrenocorticotrophin. Clin Endocrinol (Oxf) 40:595–601 [DOI] [PubMed] [Google Scholar]
- 36. Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. 2009. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA 106:14716–14721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Revsin Y, van Wijk D, Saravia FE, Oitzl MS, De Nicola AF, de Kloet ER. 2008. Adrenal hypersensitivity precedes chronic hypercorticism in streptozotocin-induced diabetes mice. Endocrinology 149:3531–3539 [DOI] [PubMed] [Google Scholar]
- 38. Kempná P, Körner M, Waser B, Hofer G, Nuoffer JM, Reubi JC, Flück CE. 2010. Neuropeptide Y modulates steroid production of human adrenal H295R cells through Y1 receptors. Mol Cell Endocrinol 314:101–109 [DOI] [PubMed] [Google Scholar]