Abstract
Rationale:
Accurate diagnosis of head and neck paragangliomas is often complicated by biochemical silence and lack of catecholamine-associated symptoms, making accurate anatomical and functional imaging techniques essential to the diagnostic process.
Methods:
Ten patients (seven SDHD, three SDHB), with a total of 26 head and neck paragangliomas, were evaluated with anatomical and functional imaging. This study compares five different functional imaging techniques [18F-fluorodihydroxyphenylalanine (18F-FDOPA) positron emission tomography (PET), 18F-fluorodopamine (18F-FDA) PET/computed tomography (CT), 18F-fluoro-2-deoxy-d-glucose (18F-FDG) PET/CT, 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy, and 111In-pentetreotide scintigraphy] in the localization of head and neck paragangliomas.
Results:
Prospectively 18F-FDOPA PET localized 26 of 26 lesions in the 10 patients, CT/magnetic resonance imaging localized 21 of 26 lesions, 18F-FDG PET/CT localized 20 of 26 lesions, 111In-pentetreotide scintigraphy localized 16 of 25 lesions, 18F-FDA PET/CT localized 12 of 26 lesions, and 123I-MIBG scintigraphy localized eight of 26 lesions. Differences in imaging efficacy related to genetic phenotype, even in the present small sample size, included the negativity of 18F-FDA PET/CT and 123I-MIBG scintigraphy in patients with SDHB mutations and the accuracy of 18F-FDG PET/CT in all patients with SDHD mutations, as compared with the accuracy of 18F-FDG PET/CT in only one patient with an SDHB mutation.
Conclusion:
Overall, 18F-FDOPA PET proved to be the most efficacious functional imaging modality in the localization of SDHx-related head and neck paragangliomas and may be a potential first-line functional imaging agent for the localization of these tumors.
Head and neck paragangliomas are tumors arising from the parasympathetic ganglia in the head and neck including the carotid bifurcations, the glomus jugulare, and the glomus vagale (1). Presumably because of biochemical silence, reflected by normal catecholamine and metanephrine levels (2), patients do not often complain of catecholamine-related signs and symptoms. Genetic predisposition to head and neck paragangliomas has been identified in the succinate dehydrogenase gene subunits B, C, and D (SDHB/SDHC/SDHD). SDHC and SDHD mutations are more commonly associated with head and neck paragangliomas than are SDHB mutations; however, head and neck paragangliomas have been identified in patients with SDHB gene mutations (3, 4). Diagnostic challenges with head and neck paragangliomas, such as biochemical silence, and therefore, a lack of classical symptoms associated with paragangliomas underscore the need for accurate and tumor-specific imaging techniques.
Anatomical and functional imaging is important in the localization and subsequent treatment of head and neck paragangliomas. Anatomical imaging, such as computed tomography (CT) and magnetic resonance imaging, is used for the initial localization of tumors, but its nonspecificity limits its efficacy in diagnosing a paraganglioma over another mass. On the other hand, functional imaging modalities can be used to differentiate between specific types of tumor because they target specific cell receptors such as cell membrane norepinephrine transporters, somatostatin receptors, as well as glucose and amino acid transporters that are thought to be up-regulated on paraganglioma cells (5).
Although previous studies have looked at the efficacy of various imaging techniques in localizing pheochromocytoma/paraganglioma, few looked specifically at functional imaging in head and neck paragangliomas (6–8). The aim of this study was to evaluate the efficacy of five different functional imaging techniques currently available at the National Institutes of Health [18F-fluorodihydroxyphenylalanine (18F-FDOPA) positron emission tomography (PET), 18F-fluorodopamine (18F-FDA) PET/CT, 18F-fluoro-2-deoxy-d-glucose (18F-FDG) PET/CT, 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy, and 111In-pentetreotide scintigraphy] (9–15) in the localization of head and neck paragangliomas in a small cohort of SDHx patients and to provide recommendations to clinicians regarding the localization of these tumors.
Patients and Methods
Patients
Ten patients (nine males, one female) with head and neck paragangliomas were evaluated at the National Institutes of Health in an institutional review board-approved protocol, 00-CH-0093, between November 2007 and October 2010; informed written consent was provided and testing was performed. Of these 10 patients, five had a previous diagnosis of a head and/or neck tumor. The mean age at diagnosis was 38.4 yr (sd ± 16.8) with a range of 19–56 yr. Three patients (patients 5, 8, and 9) had metastatic disease [defined as pheochromocytomas/paragangliomas in sites outside of where chromaffin tissue is normally present, such as the lungs, bones, lymph nodes, and liver (16)] at the time of evaluation. These lesions were not analyzed in this study.
Seven patients had an underlying mutation in the SDHD gene and three patients in the SDHB gene.
Two of 26 lesions, in two patients (patients 1 and 7), were resected and pathologically confirmed as paragangliomas. Pathologic review of outside specimens from another three patients (patients 2, 3, and 10) also confirmed paragangliomas. The pathological review of the tumors of the final five patients was not performed because of the refusal of surgery (patients 4 and 6) or the contraindication for surgery due to the presence of other tumors (patients 5, 8, and 9).
Computed tomography and magnetic resonance imaging
CT of the neck was performed on all patients using high-speed multislice scanners: Philips Brilliance 64 (Best, The Netherlands), GE Lightspeed QX/I (General Electric, Milwaukee, WI), or Siemens Definition (Siemens Medical Solutions, Forchheim, Germany). CT scanning was performed after 130 ml infusion of an iv water-soluble nonionic iodine low-osmolality contrast agent (iopamidol 300 mg of iodine per milliliter [Isovue 300; Bracco Diagnostics, Princeton, NJ]) at 3–4 ml/sec, except one patient (patient 8) in whom iv contrast was not given due to impaired kidney function.
Magnetic resonance imaging (MRI) of the neck was performed on all patients using 1.5 Tesla or 3.0T Philips Achieva magnetic resonance scanners. The studies were performed after the injection of 0.2 ml/kg (0.1 mmol/kg) gadopentetate dimeglumine (Magnevist; Berlex, Wayne, NJ) or gadoversetamide (OptiMARK; Tyco Healthcare/Mallinckrodt, St. Louis, MO).
A single radiologist who was blinded to the results of other studies but was provided with a preliminary diagnosis of paraganglioma read the CT/MRI scans.
Functional imaging
18F-FDG and 18F-FDA PET/CT imaging were performed using a Discovery ST PET/CT scanner (General Electric Medical Systems) with a 15-cm axial field of view. The CT studies, used for attenuation correction and coregistration, were performed without contrast. Patients fasted for at least 6 h before injection of 555 MBq (15 mCi) 18F-FDG and for at least 6 h before the injection of 37 MBq (1 mCi) 18F-FDA. For 18F-FDG PET/CT, patients were scanned beginning 60 min after injection, whereas 18F-FDA PET/CT scanning began approximately 10 min after injection.
18F-FDOPA PET imaging was performed using an Advance scanner (General Electric Healthcare) with a 15-cm axial field of view and a rod source attenuation correction. Patients fasted for at least 6 h before injection of 444 MBq (12 mCi) of 18F-FDOPA. All patients were premedicated with 200 mg carbidopa 1 h before tracer injection. Patients were scanned starting 30 min after injection.
123I-MIBG scintigraphy was performed approximately 24 h after an injection of 370 MBq (10 mCi) 123I-MIBG. To protect the thyroid, patients were medicated with 100 mg saturated solution of potassium iodide by mouth twice a day for 4 d starting the night before the 123I-MIBG injection. Planar and single-photon emission computed tomography (SPECT) images were obtained. Some patients underwent SPECT/CT scanning.
111In-pentetreotide scintigraphy was performed approximately 4 h after an injection of 229.4 MBq (6.2 mCi) 111In-pentetreotide. Planar imaging followed by SPECT or SPECT/CT was performed at 4 h with repeat SPECT or SPECT/CT at 24 h, as needed.
Patients' medications were reviewed before imaging to ensure that they were not taking drugs that would interfere with 123I-MIBG scintigraphy or 18F-FDA PET/CT imaging studies.
All functional imaging studies were reviewed by a nuclear medicine physician. 18F-FDA PET/CT and 18F-FDOPA PET studies are considered research scans and were read in a blinded fashion. Lesions were graded on a scale of 0–5 with a grade of 4 or 5 indicating a definite abnormality and the presence of a lesion, as previously described (17).
Results
Nine patients underwent all five functional imaging scans and one patient (patient 4) underwent all scans but 111In-pentetreotide scintigraphy. All 10 patients also underwent CT and MRI. All definite head and neck foci localized with functional imaging modalities were presumed to be true positive paragangliomas. A total of 26 head and neck lesions were found and named based on their anatomical location: 14 carotid body tumors, seven glomus jugulare tumors, four glomus vagale tumors, and one in the soft tissue of the neck above the level of the right carotid body at the level of C1. Clinical data are shown in Table 1.
Table 1.
Clinical characteristics
| Pt | Sex | Age at diagnosis (yr) | Head and neck tumor location based on CT/MRI | Genotype | Biochemical phenotype |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metastatic or other primary | NE (80–498 pg/ml) | EPI (4–83 pg/ml) | DA (3–46 pg/ml) | NMN (18–112 pg/ml) | MN (12–61 pg/ml) | |||||
| 1 | M | 23 | 2.0 × 1.2 cm R carotid body tumor | SDHD | 239 | 18 | 14 | 50 | 54 | |
| 2 | M | 25 | 1.8 × 2.0 cm R glomus jugulare tumor | SDHD | Second primary, cardiac | 5324 | 24 | 203 | 1758 | 23 |
| 3 | M | 44 | 1.2 × 0.7 cm L carotid body tumor; 1.1 × 1.0 cm L carotid body tumor (two additional lesions localized on 18F-FDOPA PET: one in the neck on the right and one in the neck on the left at the level of C1) | SDHD | 639 | 14 | 425 | 291 | 21 | |
| 4 | M | 74 | 1.0 × 0.8 cm R carotid body tumor | SDHB | 228 | 62 | 14 | 51 | 51 | |
| 5 | F | 56 | 2.4 × 1.8 cm R carotid body tumor; 2.2 × 2.8 cm L carotid body tumor | SDHD | Metastatic | 1539 | <5 | 20 | 684 | 13 |
| 6 | M | 19 | 2.0 × 1.7 cm R glomus jugulare tumor; 1.0 × 1.0 cm L glomus jugulare tumor; 3.6 × 2.3 cm L glomus vagale tumor; 2.6 × 1.7 cm R carotid body tumor; 3.1 × 2.6 cm L carotid body tumor | SDHD | 296 | 10 | 437 | 97 | 31 | |
| 7 | M | 33 | 0.6 × 0.6 cm R carotid body tumor | SDHB | 175 | 8 | 8 | 22 | 27 | |
| 8 | M | 29 | 3.2 × 2.6 cm R carotid body tumor; 2.4 × 1.9 cm L carotid body tumor | SDHB | Metastatic | 12668 | <26 | 2474 | 2679 | |
| 9 | M | 38 | 3.8 × 2.8 cm R carotid body tumor; 3.1 × 2.3 cm L carotid body tumor; 4.2 × 2.5 cm R glomus vagale tumor (three additional lesions localized on 18F-FDOPA PET: two in the jugular fossa on each side and one in the soft tissue of the neck on the left) | Unknown | Metastatic | 1083 | <11 | 17 | 456 | <5 |
| 10 | M | 43 | 2.3 × 1.4 cm R carotid body tumor; 1.5 × 0.9 cm L glomus vagale tumor; 11.5 × 0.9 cm R glomus jugulare tumor (one additional lesion localized on 18F-FDOPA PET in the L jugular fossa) | SDHD | 481 | 11 | 123 | 62 | 40 | |
Clinical characteristics at the time of the imaging studies, including the presence of pheochromocytoma/paraganglioma (metastatic or second primary) outside the head and neck paraganglioma being evaluated. Bold numbers indicate a value above the upper reference limit. DA, Dopamine; EPI, epinephrine; F, female; L, left; M, male; MN, metanephrine; NE, norepinephrine; NMN, normetanephrine; Pt, patient; R, right.
Prospectively, 18F-FDOPA PET identified 26 of 26 lesions (100%); CT/MRI identified 21 of 26 lesions (81%); 18F-FDG PET/CT identified 20 of 26 lesions (77%); 111In-pentetreotide scintigraphy identified 16 of 25 lesions (64%); 18F-FDA PET/CT identified 12 of 26 lesions (46%); and 123I-MIBG scintigraphy identified eight of 26 lesions (31%). One lesion initially not localized on CT/MRI was retrospectively found after functional imaging studies. No false-positive results were seen. Results are outlined in Table 2.
Table 2.
Number of lesions identified with each imaging modality
| Pt | Sex | Age at diagnosis (yr) | Size (cm) | Lesion | CT/MRI | 18F-FDG | 18F-FDA | 18F-FDOPA | 123I-MIBG | 111In-pentetreotide | 18F-F-DOPA only |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 23 | 2.0 × 1.2 | R CBT | + | + | 0 | + | 0 | + | |
| 2 | M | 25 | 1.8 × 2.0 | R glomus jugluare | + | + | 0 | + | 0 | + | |
| 3 | M | 44 | 1.2 × 0.7 | L CBT | + | + | 0 | + | 0 | 0 | |
| 1.1 × 1.0 | L CBT (level of C1) | + | + | 0 | + | 0 | 0 | ||||
| 1.3 × 0.9 | L glomus vagale | (+) | + | 0 | + | 0 | 0 | ||||
| R above CBT level | 0 | 0 | 0 | + | 0 | 0 | x | ||||
| 4 | M | 74 | 1.0 × 0.8 | R CBT | + | 0 | 0 | + | 0 | ND | |
| 5 | F | 56 | 2.2 × 2.8 | L CBT | + | + | 0 | + | 0 | + | |
| 2.4 × 1.8 | R CBT | + | + | 0 | + | 0 | + | ||||
| 6 | M | 19 | 3.1 × 2.6 | L CBT | + | + | + | + | + | + | |
| 2.6 × 1.7 | R CBT | + | + | + | + | + | + | ||||
| 3.6 × 2.3 | L glomus vagale | + | + | + | + | + | + | ||||
| 1.0 × 1.0 | L glomus jugulare | + | + | + | + | + | + | ||||
| 2.0 × 1.7 | R glomus jugulare | + | + | + | + | 0 | 0 | ||||
| 7 | M | 33 | 0.6 × 0.6 | R CBT | + | 0 | 0 | + | 0 | 0 | |
| 8 | M | 29 | 2.4 × 1.9 | L CBT | + | + | 0 | + | 0 | + | |
| 3.2 × 2.6 | R CBT | + | + | + | + | + | + | ||||
| 9 | M | 38 | 3.1 × 2.3 | L CBT | + | + | + | + | 0 | + | |
| 3.8 × 2.8 | R CBT | + | + | 0 | + | 0 | + | ||||
| 4.2 × 2.5 | R glomus vagale | + | + | + | + | + | + | ||||
| R glomus jugulare | 0 | 0 | + | + | 0 | 0 | |||||
| L glomus jugulare | 0 | 0 | 0 | + | 0 | 0 | x | ||||
| 10 | M | 43 | 2.3 × 1.4 | R CBT | + | + | + | + | + | + | |
| 1.5 × 0.9 | L glomus vagale | + | + | + | + | 0 | + | ||||
| 1.5 × 0.9 | R glomus jugluare | + | + | + | + | + | + | ||||
| L glomus jugulare | 0 | 0 | 0 | + | 0 | 0 | x |
The plus sign indicates positive localization of the lesion. F, Female; 111In-pentetreotide, 111In-pentetreotide scintigraphy; M, male; ND, scan not performed (not done); Pt, patient.
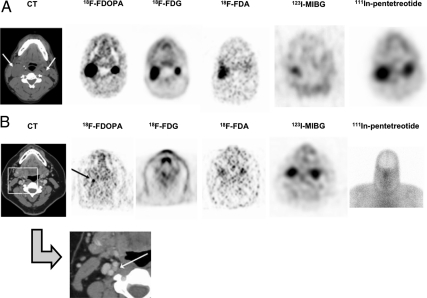
For three of the lesions, 18F-FDOPA PET was the only modality that was positive. Although no actual measurements are available for these lesions because they were localized using only a PET scanner, nuclear medicine physicians describe them to be relatively small lesions. Most lesions were detected by at least two modalities including CT/MRI. In two patients with SDHB mutations (patients 4 and 7), 18F-FDOPA PET was the only functional imaging modality to identify the carotid body tumors, although one patient (patient 4) did not undergo 111In-pentetreotide scintigraphy. In the third patient with an SDHB mutation (patient 8), bilateral carotid body tumors were identified with 18F-FDOPA PET, 18F-FDG PET/CT, and 111In-pentetreotide scintigraphy; 18F-FDA PET/CT and 123I-MIBG scintigraphy each identified only one tumor (Fig. 1). Of the seven patients with SDHD mutations, three patients (patients 6, 9, and 10) showed positive results in at least one lesion with all modalities. In the other four patients with SDHD mutations, 18F-FDA PET/CT and 123I-MIBG scintigraphy were negative in all lesions, and one patient (patient 3) had a totally negative 111In-pentetreotide scintigraphy scan.
Fig. 1.
A, Patient 8 with bilateral carotid body tumors. Transverse sections of CT scan and the five functional imaging scans are shown. The CT scan, 18F-FDOPA PET, 18F-FDG PET/CT, and 111In-pentetreotide scintigraphy all successfully show the bilateral carotid body tumors (arrows). 18F-FDA PET/CT and 123I-MIBG scintigraphy both show only the right carotid body tumor. B, Patient 7 with right 6-mm carotid body tumor. Transverse sections of the CT scan and four functional imaging modalities and an anterior planar image of the 111In-pentetreotide scan (SPECT of this level not performed) are shown. The CT and 18F-FDOPA PET successfully demonstrate the right carotid body tumor (arrows); no other functional imaging studies demonstrate this tumor.
Interestingly, the tumors of two patients with SDHB mutations and a silent biochemical phenotype showed no uptake of 18F-FDG, whereas the patient with a noradrenergic and dopaminergic secretory profile did show uptake of 18F-FDG. The biochemical phenotype did not appear to correlate with the imaging of the head and neck tumors of patients with SDHD mutations.
Discussion
We present a comparison of five functional imaging techniques in the localization of SDHx-related head and neck paragangliomas. 18F-FDOPA PET, localizing 26 of 26 tumors, was found to be the best functional imaging modality for localizing SDHx-related head and neck paragangliomas. This was followed by CT/MRI localizing 21 of 26 tumors, 18F-FDG PET/CT localizing 20 of 26 tumors, 111In-pentetreotide scintigraphy localizing 16 of 25 tumors, 18F-FDA PET/CT localizing 12 of 26 tumors, and 123I-MIBG scintigraphy localizing eight of 26 tumors. Pheochromocytomas/paragangliomas are diagnosed based on biochemical and imaging studies, both anatomical and functional, which makes choosing an accurate imaging modality very important. Functional imaging modalities target tumor cells by different mechanisms. 18F-FDG targets the glucose pathway of cells (18, 19), whereas 18F-FDA and 123I-MIBG target norepinephrine transporters (20, 21). 18F-FDOPA enters through amino acid transporters (22, 23), and 111In-pentetreotide targets somatostatin receptors (24, 25). Four of these five techniques target pheochromocytoma/paraganglioma cells specifically.
Previous studies have demonstrated the efficacy of 18F-FDOPA PET in the localization of pheochromocytomas/paragangliomas in general (6, 15, 26), but few have focused specifically on head and neck paragangliomas (8, 27, 28). A study by Hoegerle et al. (8) evaluated 10 patients with suspected head and neck paragangliomas using MRI and 18F-FDOPA PET and were able to localize such tumors in eight patients with 18F-FDOPA PET. A case report by Brink et al. (27) also demonstrated successful imaging in an SDHD patient with bilateral carotid body tumors using 18F-FDOPA PET. Taieb et al. (28) evaluated nine patients with a pheochromocytoma/paraganglioma, including three with head and neck paragangliomas, and demonstrated 18F-FDOPA uptake in all head and neck paragangliomas present. None of these studies assessed the efficacy of 18F-FDOPA PET in patients with SDHx mutations specifically. In the present report, 18F-FDOPA PET was superior to all other functional imaging techniques studied and had 100% sensitivity, regardless of biochemical phenotype, tumor location, or genetic status.
Although 18F-FDG PET/CT is not pheochromocytoma/paraganglioma specific, its efficacy in localizing these tumors is well established (29), especially in the localization of SDHB-related lesions (17). Timmers et al. (17) described localization of two SDHB-related neck tumors using 18F-FDG PET/CT, and Taieb et al. (30) described four patients with head and/or neck tumors, two of whom had an SDHB mutation and were imaged on 18F-FDG PET/CT. In our patients, 18F-FDG PET/CT localized 20 of 26 lesions (77%) in eight of 10 patients (80%). The only patients whose tumors were not imaged on 18F-FDG PET/CT were two SDHB patients with solitary, biochemically silent tumors. Interestingly, the single SDHB patient whose neck tumors were 18F-FDG avid had a noradrenergic and dopaminergic biochemical profile and metastatic disease. In comparison, one SDHD patient (patient 1) presented with a biochemically silent solitary carotid body tumor that showed an uptake with 18F-FDG. The efficacy of 18F-FDG PET/CT in localizing these tumors should be noted, and, in the absence of 18F-FDOPA PET, the functional imaging of SDHx-related head and neck paragangliomas should be performed using 18F-FDG PET/CT.
111In-pentetreotide scintigraphy is used as an imaging modality for pheochromocytomas/paragangliomas because of the presence of somatostatin receptors on these tumor cells, 73% of pheochromocytomas and 93% of paragangliomas (24). This radiopharmaceutical has demonstrated limited efficacy in primary pheochromocytomas/paragangliomas (31, 32); it has proven more useful in metastatic disease (33) and has high sensitivity in head and neck neuroendocrine tumors (34, 35). Kwekkeboom et al. (36) evaluated 25 patients with head and/or neck paraganglioma and reported a sensitivity of 94% (on a per lesion basis) for 111In-pentetreotide scintigraphy. Similarly, a study by Koopmans et al. (7) demonstrated 93% sensitivity (on a per patient basis) with 111In-pentetreotide scintigraphy in 29 patients with head and neck paragangliomas. In contrast, in the present series, 111In-pentetreotide scintigraphy detected 16 of 25 (64%) of lesions in seven of nine patients (78%).
18F-FDA PET/CT is an excellent imaging modality for the diagnosis and localization of primary and metastatic pheochromocytomas/paragangliomas derived from the sympathetic nervous system (15, 32). In contrast, its sensitivity for head and neck paragangliomas, which are derived from the parasympathetic nervous system, was only 46% in our small series and localized only 12 of 26 (46%) lesions in four of 10 patients (40%).
Historically, 123/131I-MIBG scintigraphy has been the staple functional imaging modality for the localization and diagnosis of pheochromocytomas/paragangliomas, but recent studies have demonstrated that other imaging techniques are superior (6, 15). Milardovic et al. (37) described 123I-MIBG scintigraphy in 117 pheochromocytoma/paraganglioma patients and found low efficacy in imaging head and/or neck lesions with 123I-MIBG scintigraphy localizing only 32 of 145 head and/or neck tumors. Bhatia et al. (11) also described 123I-MIBG scintigraphy in head and neck paragangliomas and reported localization of eight of 14 carotid body tumors. We also found that 123I-MIBG scintigraphy has little efficacy in these tumors, localizing only eight of 26 (31%) of the tumors in four of 10 patients (40%).
In the present study, we found that 18F-FDOPA PET imaging was most sensitive for very small tumors. Its low uptake in the brain and salivary glands was also a plus when compared with 18F-FDG PET/CT and 123/131I-MIBG scintigraphy, particularly for small tumors located adjacent to these structures. The authors are aware of the positivity of 18F-FDOPA PET in other central nervous system tumors, such as glioblastomas, but these tumors are not located in the neck or the jugular fossa, and thus, we believe that 18F-FDOPA PET positivity in the head and neck region in our patient sample represent true paragangliomas.
In this study, all definite head and neck foci localized with functional imaging modalities were presumed to be true positive paragangliomas. We were comfortable using this as our gold standard in this series for several reasons. First, one of the primary advantages of functional imaging is its specificity compared with that of anatomic imaging, which is nonspecific and yet has often been used as a gold standard. More importantly, however, most lesions were also confirmed by at least one other functional imaging study, and 22 of 26 were confirmed on CT/MRI (one was confirmed retrospectively), the commonly used but nonspecific gold standard in many imaging studies when histologic confirmation is not obtained. Only three of 26 lesions were seen with a single modality (18F-FDOPA PET). Given the typical location of these lesions for paraganglioma and the fact that no false-positive 18F-FDOPA PET results were seen in any of the other patients, we feel confident that these foci represent true positive findings.
This study suggests that there are imaging differences between head and neck paragangliomas and other pheochromocytomas/paragangliomas as well as between genotypes. These differences may be due to differing cellular receptors and transporters between the parasympathetic and sympathetic ganglia. For example, vesicular monoamine transporter expression may be dependent on cell origin, with adrenal chromaffin cells mainly expressing vesicular monoamine transporter-1, whereas cells of the nervous ganglia tend to express high levels of vesicular monoamine transporter-2 (38, 39). Such differences and those that may be due to biochemical phenotype and tumor location remain to be fully explored in larger numbers of patients. Major limitations of this study are its small size and the lack of patients with sporadic and SDHC-related head and neck paragangliomas.
Conclusion
We show that such differences can influence the choice of functional imaging studies performed and suggest 18F-FDOPA PET as the first-line imaging agent for the localization and diagnosis of head and neck paragangliomas in SDHx-mutation carriers, regardless of biochemical phenotype. Although not widely available at present, with the advent of PET imaging and the more widespread use of it for diagnostic purposes, we can only assume that more and more facilities will aim to make studies such as 18F-FDOPA PET more widely available to patients. In the absence of 18F-FDOPA PET, we recommend the use of the more readily available 18F-FDG PET/CT or 111In-pentetreotide scintigraphy in combination with CT/MRI for proper anatomical localization.
Acknowledgments
This work was supported, in part, by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CT
- Computed tomography
- 18F-FDA
- 18F-fluorodopamine
- 18F-FDG
- 18F-fluoro-2-deoxy-d-glucose
- 18F-FDOPA
- 18F-fluorodihydroxyphenylalanine
- 123I-MIBG
- 123I-metaiodobenzylguanidine
- MRI
- magnetic resonance imaging
- PET
- positron emission tomography
- SDHB
- succinate dehydrogenase gene subunit B
- SDHC
- succinate dehydrogenase gene subunit C
- SDHD
- succinate dehydrogenase gene subunit D
- SPECT
- single-photon emission CT.
References
- 1. Gujrathi CS, Donald PJ. 2005. Current trends in the diagnosis and management of head and neck paragangliomas. Curr Opin Otolaryngol Head Neck Surg 13:339–342 [DOI] [PubMed] [Google Scholar]
- 2. Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA, Young WF., Jr 2001. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab 86:5210–5216 [DOI] [PubMed] [Google Scholar]
- 3. Baysal BE, Willett-Brozick JE, Lawrence EC, Drovdlic CM, Savul SA, McLeod DR, Yee HA, Brackmann DE, Slattery WH, 3rd, Myers EN, Ferrell RE, Rubinstein WS. 2002. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet 39:178–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, Buchta M, Franke G, Klisch J, Bley TA, Hoegerle S, Boedeker CC, Opocher G, Schipper J, Januszewicz A, Eng C. 2004. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 292:943–951 [DOI] [PubMed] [Google Scholar]
- 5. Pacak K, Eisenhofer G, Goldstein DS. 2004. Functional imaging of endocrine tumors: role of positron emission tomography. Endocr Rev 25:568–580 [DOI] [PubMed] [Google Scholar]
- 6. Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, Jager PL, Elsinga PH, Dierckx RA, van der Wal JE, Sluiter WJ, de Vries EG, Links TP. 2009. 6-[F-18]Fluoro-l-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123)I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. J Clin Endocrinol Metab 94:3922–3930 [DOI] [PubMed] [Google Scholar]
- 7. Koopmans KP, Jager PL, Kema IP, Kerstens MN, Albers F, Dullaart RP. 2008. 111In-octreotide is superior to 123I-metaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. J Nucl Med 49:1232–1237 [DOI] [PubMed] [Google Scholar]
- 8. Hoegerle S, Ghanem N, Altehoefer C, Schipper J, Brink I, Moser E, Neumann HP. 2003. 18F-DOPA positron emission tomography for the detection of glomus tumours. Eur J Nucl Med Mol Imaging 30:689–694 [DOI] [PubMed] [Google Scholar]
- 9. Hoegerle S, Nitzsche E, Altehoefer C, Ghanem N, Manz T, Brink I, Reincke M, Moser E, Neumann HP. 2002. Pheochromocytomas: detection with 18F DOPA whole body PET—initial results. Radiology 222:507–512 [DOI] [PubMed] [Google Scholar]
- 10. Mann GN, Link JM, Pham P, Pickett CA, Byrd DR, Kinahan PE, Krohn KA, Mankoff DA. 2006. [(11)C]metahydroxyephedrine and [(18)f]fluorodeoxyglucose positron emission tomography improve clinical decision making in suspected pheochromocytoma. Ann Surg Oncol 13:187–197 [DOI] [PubMed] [Google Scholar]
- 11. Bhatia KS, Ismail MM, Sahdev A, Rockall AG, Hogarth K, Canizales A, Avril N, Monson JP, Grossman AB, Reznek RH. 2008. 123I-metaiodobenzylguanidine (MIBG) scintigraphy for the detection of adrenal and extra-adrenal phaeochromocytomas: CT and MRI correlation. Clin Endocrinol (Oxf) 69:181–188 [DOI] [PubMed] [Google Scholar]
- 12. Esfandiari NH, Shulkin BL, Bui C, Jaffe CA. 2006. Multimodality imaging of malignant pheochromocytoma. Clin Nucl Med 31:822–825 [DOI] [PubMed] [Google Scholar]
- 13. Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. 2007. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer 14:569–585 [DOI] [PubMed] [Google Scholar]
- 14. Meyer-Rochow GY, Schembri GP, Benn DE, Sywak MS, Delbridge LW, Robinson BG, Roach PJ, Sidhu SB. 2010. The utility of metaiodobenzylguanidine single photon emission computed tomography/computed tomography (MIBG SPECT/CT) for the diagnosis of pheochromocytoma. Ann Surg Oncol 17:392–400 [DOI] [PubMed] [Google Scholar]
- 15. Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. 2009. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 94:4757–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linnoila RI, Keiser HR, Steinberg SM, Lack EE. 1990. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol 21:1168–1180 [DOI] [PubMed] [Google Scholar]
- 17. Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. 2007. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol 25:2262–2269 [DOI] [PubMed] [Google Scholar]
- 18. Pauwels EK, Sturm EJ, Bombardieri E, Cleton FJ, Stokkel MP. 2000. Positron-emission tomography with [18F]fluorodeoxyglucose. Part I. Biochemical uptake mechanism and its implication for clinical studies. J Cancer Res Clin Oncol 126:549–559 [DOI] [PubMed] [Google Scholar]
- 19. Kayani I, Groves AM. 2006. 18F-fluorodeoxyglucose PET/CT in cancer imaging. Clin Med 6:240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sisson JC, Wieland DM. 1986. Radiolabeled meta-iodobenzylguanidine: pharmacology and clinical studies. Am J Physiol Imaging 1:96–103 [PubMed] [Google Scholar]
- 21. Glowniak JV, Kilty JE, Amara SG, Hoffman BJ, Turner FE. 1993. Evaluation of metaiodobenzylguanidine uptake by the norepinephrine, dopamine and serotonin transporters. J Nucl Med 34:1140–1146 [PubMed] [Google Scholar]
- 22. Havekes B, King K, Lai EW, Romijn JA, Corssmit EP, Pacak K. 2010. New imaging approaches to phaeochromocytomas and paragangliomas. Clin Endocrinol (Oxf) 72:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pearse AG. 1980. The APUD concept and hormone production. Clin Endocrinol Metab 9:211–222 [DOI] [PubMed] [Google Scholar]
- 24. Reubi JC, Waser B, Khosla S, Kvols L, Goellner JR, Krenning E, Lamberts S. 1992. In vitro and in vivo detection of somatostatin receptors in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab 74:1082–1089 [DOI] [PubMed] [Google Scholar]
- 25. Krenning EP, Bakker WH, Kooij PP, Breeman WA, Oei HY, de Jong M, Reubi JC, Visser TJ, Bruns C, Kwekkeboom DJ. 1992. Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J Nucl Med 33:652–658 [PubMed] [Google Scholar]
- 26. Mackenzie IS, Gurnell M, Balan KK, Simpson H, Chatterjee K, Brown MJ. 2007. The use of 18-fluoro-dihydroxyphenylalanine and 18-fluorodeoxyglucose positron emission tomography scanning in the assessment of metaiodobenzylguanidine-negative phaeochromocytoma. Eur J Endocrinol 157:533–537 [DOI] [PubMed] [Google Scholar]
- 27. Brink I, Schaefer O, Walz M, Neumann HP. 2006. Fluorine-18 DOPA PET imaging of paraganglioma syndrome. Clin Nucl Med 31:39–41 [DOI] [PubMed] [Google Scholar]
- 28. Taieb D, Tessonnier L, Sebag F, Niccoli-Sire P, Morange I, Colavolpe C, De Micco C, Barlier A, Palazzo FF, Henry JF, Mundler O. 2008. The role of 18F-FDOPA and 18F-FDG-PET in the management of malignant and multifocal phaeochromocytomas. Clin Endocrinol (Oxf) 69:580–586 [DOI] [PubMed] [Google Scholar]
- 29. Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC. 1999. Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-d-glucose PET. Nucl Med 212:35–41 [DOI] [PubMed] [Google Scholar]
- 30. Taïeb D, Sebag F, Barlier A, Tessonnier L, Palazzo FF, Morange I, Niccoli-Sire P, Fakhry N, De Micco C, Cammilleri S, Enjalbert A, Henry JF, Mundler O. 2009. 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? J Nucl Med 50:711–717 [DOI] [PubMed] [Google Scholar]
- 31. Kaltsas G, Korbonits M, Heintz E, Mukherjee JJ, Jenkins PJ, Chew SL, Reznek R, Monson JP, Besser GM, Foley R, Britton KE, Grossman AB. 2001. Comparison of somatostatin analog and meta-iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors. J Clin Endocrinol Metab 86:895–902 [DOI] [PubMed] [Google Scholar]
- 32. Ilias I, Chen CC, Carrasquillo JA, Whatley M, Ling A, Lazúrová I, Adams KT, Perera S, Pacak K. 2008. Comparison of 6–18F-fluorodopamine PET with 123I-metaiodobenzylguanidine and 111in-pentetreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. J Nucl Med 49:1613–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Harst E, de Herder WW, Bruining HA, Bonjer HJ, de Krijger RR, Lamberts SW, van de Meiracker AH, Boomsma F, Stijnen T, Krenning EP, Bosman FT, Kwekkeboom DJ. 2001. [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in benign and malignant pheochromocytomas. J Clin Endocrinol Metab 86:685–693 [DOI] [PubMed] [Google Scholar]
- 34. Muros MA, Llamas-Elvira JM, Rodríguez A, Ramírez A, Gómez M, Arráez MA, Valéncia E, Vílchez R. 1998. 111In-pentetreotide scintigraphy is superior to 123I-MIBG scintigraphy in the diagnosis and location of chemodectoma. Nucl Med Commun 19:735–742 [DOI] [PubMed] [Google Scholar]
- 35. Whiteman ML, Serafini AN, Telischi FF, Civantos FJ, Falcone S. 1997. 111In octreotide scintigraphy in the evaluation of head and neck lesions. AJNR Am J Neuroradiol 18:1073–1080 [PMC free article] [PubMed] [Google Scholar]
- 36. Kwekkeboom DJ, van Urk H, Pauw BK, Lamberts SW, Kooij PP, Hoogma RP, Krenning EP. 1993. Octreotide scintigraphy for the detection of paragangliomas. J Nucl Med 34:873–878 [PubMed] [Google Scholar]
- 37. Milardovic R, Corssmit EP, Stokkel M. 2010. Value of 123I-MIBG scintigraphy in paraganglioma. Neuroendocrinology 91:94–100 [DOI] [PubMed] [Google Scholar]
- 38. Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards RH. 1995. Differential expression of two vesicular monoamine transporters. J Neurosci 15:6179–6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fottner C, Helisch A, Anlauf M, Rossmann H, Musholt TJ, Kreft A, Schadmand-Fischer S, Bartenstein P, Lackner KJ, Klöppel G, Schreckenberger M, Weber MM. 2010. 6–18F-fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to 123I-metaiodobenzyl-guanidine scintigraphy in the detection of extraadrenal and hereditary pheochromocytomas and paragangliomas: correlation with vesicular monoamine transporter expression. J Clin Endocrinol Metab 95:2800–2810 [DOI] [PubMed] [Google Scholar]