Abstract
Context:
Pseudohypoparathyroidism (PHP) types 1a and 1b are distinguished by clinical, biochemical, and molecular features. We report extended kindred with PHP 1b in which many affected members also had growth plate defects, including brachydactyly and a Madelung-like deformity.
Design:
Analyses included clinical examination, assessment of mineral metabolism, thyroid function, skeletal radiography, and analysis of the GNAS and STX16 genes.
Setting:
Patients were studied in an academic medical center.
Results:
We studied 37 members of a family in which PHP 1b occurred in 23 individuals. Ten of 17 affected patients who were examined had brachydactyly E, including two subjects with Madelung-like defects. Five of 16 subjects had subclinical hypothyroidism; no subject showed sc ossification or short stature. None of the unaffected members had brachydactyly or an elevated serum level of PTH or TSH. Levels of immunoactive erythrocyte Gαs were normal in two affected subjects tested. Linkage analysis indicated linkage between PTH resistance and the GNAS gene locus; however, no mutations were identified in GNAS exons 1–13. Methylation analysis of genomic DNA from affected subjects showed loss of maternal epigenotype in exon 1A with normal methylation of the differentially methylated regions for XLGαs and NESP55, and PCR demonstrated heterozygosity for a 3.0-kb deletion in the STX16 gene.
Conclusion:
The segregation of brachydactyly with PHP 1b in this family indicates that an imprinting defect in GNAS can lead to growth plate defects, including brachydactyly and Madelung deformity. These features suggest that GNAS signaling plays a more extensive role in chondrocyte maturation than previously thought.
Pseudohypoparathyroidism (PHP) is a condition in which biochemical hypoparathyroidism results from decreased responsiveness of target tissues to PTH rather than PTH deficiency. In PHP type 1a, subjects have short stature; round facies; brachydactyly; mild mental retardation; and sc ossification, a clinical constellation termed Albright hereditary osteodystrophy (AHO). PHP type 1a results from heterozygous mutations on the maternal allele of the imprinted GNAS gene (20q13.2-q13.3) that reduce expression or function of the Gαs protein (1). By contrast, subjects with GNAS mutations on paternally inherited alleles have many but not all of the features of AHO (2) and lack hormonal resistance, a condition termed pseudopseudohypoparathyroidism. The tissue- and patient-specific expression of hormone resistance in patients with AHO has been attributed to tissue-specific genomic imprinting of GNAS, a highly complex genetic locus. GNAS derives considerable genetic plasticity by use of four alternative first exons, NESP55, XLαs, exon 1A, and exon 1, which splice onto exons 2–13 of GNAS. Transcripts starting with exon 1 encode Gαs and are expressed from both the maternal and paternal alleles in most tissues, and loss of one functional GNAS allele (i.e. haploinsufficency) does not cause hormone resistance in these tissues because 50% of normal Gαs activity is sufficient to ensure normal transmembrane signal transduction. By contrast, in some tissues (i.e. imprinted tissues) transcription occurs only from the maternal allele. Mutations that reduce expression or function of the maternal GNAS allele lead to a state in which little if any Gαs protein is produced, whereas mutations in a paternal allele have no effect.
Epigenetic defects that reduce expression of Gαs from the maternal GNAS allele account for the development of PTH resistance in patients with PHP type 1b (3). Subjects with PHP type 1b typically lack features of AHO and show normal expression of Gαs in tissues in which transcription of Gαs is biallelic (1). In most familial cases, the epigenetic defect results from microdeletions in the STX16 gene located approximately 220 kb centromeric of GNAS exon 1A (3) or deletions removing differentially methylated regions (DMR) of GNAS exon NESP55 or the antisense transcript (4, 5).
Recent studies have revealed that some patients with sporadic PHP type 1b may have mild features of AHO, including sc ossifications and brachydactyly (6–9). We evaluated an extended, multigenerational African-American family in which many members had PHP type 1b. Many affected subjects had brachydactyly, and two subjects had Madelung-like deformities. Our findings provide further evidence for the role of GNAS signaling in regulating growth plate physiology.
Subjects and Methods
Population
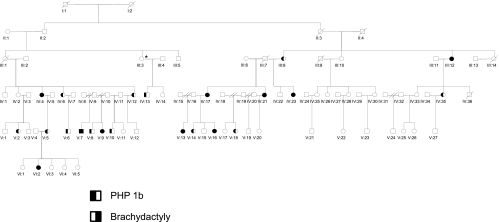
We studied an African-American kindred in which PHP type 1 occurred in four generations (Fig. 1). We reviewed the records of 37 family members, including 23 affected members (17 females and six males) and 14 unaffected subjects (10 females and four males). Height was measured using a stadiometer, and growth charts from the Centers for Disease Control and Prevention were used to determine height Z scores. A subject was considered affected if he or she had an abnormally elevated serum level of intact PTH. We evaluated all the family members, but due to sampling problems, it was not possible to perform all studies on all subjects. The protocol was approved by the appropriate institutional review boards.
Fig. 1.
Pedigree of family. Legend describes the relevant phenotypic features of male (square) and female (circle) subjects. /, Deceased individual. The numbers beneath each symbol indicate the pedigree number (see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Linkage analysis
Linkage was determined using previously described single-nucleotide polymorphisms in the PTH gene (10) and the gene encoding the PTH/PTHrP receptor (PTHR1) (10) and using microsatellites and single-nucleotide polymorphisms in GNAS (11).
Molecular analysis of the GNAS gene
The GNAS gene was analyzed by Sanger sequencing and by restriction endonuclease digestion as previously described (2, 11). We also analyzed bisulfite-modified genomic DNA after amplification by nested PCR using primers specific for DMR located in exon 1A, XLαs, and NESP55 (11). The second round of PCR employed primers that were designed with 5′ GC tags that enhanced the GC content of PCR products and facilitated successful direct-cycle sequencing (12). All primer sequences are available upon request.
Analysis of the STX16 gene
We performed PCR in a 100-μl reaction volume containing 500 ng of genomic DNA, oligonucleotide primers that annealed to sequences within exon 2 (5′-CTGATGACCGTATGGCACTG-3′) and exon 7 (5′-GCTCTGGAGTTCACCCTCAA-3′) of the STX16 gene, and TaKaRa LA Taq DNA polymerase (Takara Bio Inc., Japan), with the following cycling conditions: 94 C for 1 min followed by 30 cycles of 98 C for 5 sec, 68 C for 15 min, and 72 C for 10 min. PCR products were analyzed by PAGE.
Quantification of Gαs protein in erythrocyte cell membranes
Semiquantitative immunoblot analysis of erythrocyte Gαs protein was performed (11) using membranes from two affected family members (1000 and 1005) and two normal subjects. A pool of erythrocyte membranes from 10 normal subjects was used to generate a dose-response curve (curve not shown). Quantification of the bands was performed by phosphor imager analysis.
Results
Clinical and endocrinological evaluation
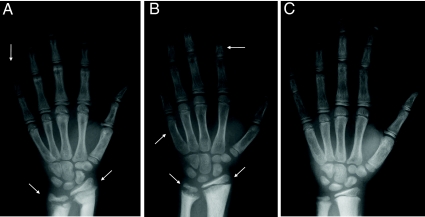
Twenty-three patients had biochemical evidence of PTH resistance, but none had short stature, round facies, obesity, or sc ossifications. Ten of 17 patients with PHP 1b who were available for analysis had brachydactyly E, often limited to the fourth metacarpals or metatarsals; no subject had brachydactyly of the first digit (i.e. brachydactyly D). Brachydactyly was not present in any unaffected subjects. Two subjects (V-7 and V1–7) had Madelung-like deformities that affected both the distal ulna and radius (Fig. 2).
Fig. 2.
Hand radiographs. A, Radiograph of subject VI-2 shows shortening of distal phalanx of digit V and marked chondrodysostosis consistent with Madelung-type deformity (arrows). B, Radiograph of subject V-7 shows marked chondrodysostosis consistent with Madelung-type deformity (lower arrows); shortening of metacarpal V and shortening of second middle phalanx of the index finger (arrow) consistent with brachydactyly E. C, Radiograph of subject V5 shows no evidence of brachydactyly.
Biochemical and molecular studies
Linkage analysis indicated discordance between PHP type 1b and specific alleles for genes encoding PTH and the PTH/PTHrP receptor (data not shown) but showed strong linkage (limit of detection = 4.17 at θ = 0.00, peak D205100) between the GNAS locus and PHP type 1. Analysis of the coding regions and flanking intronic sequences of the GNAS gene using genomic DNA from two affected subjects revealed no mutations, and levels of immunoactive Gαs protein in erythrocytes membranes were normal (93 and 112% of normal pool values, respectively). Restriction endonuclease analysis of genomic DNA from affected family members indicated an abnormal methylation pattern for the exon 1A DMR region (data not shown) that in turn indicated that the NgoMIV sites on both alleles were unmethylated. These results are consistent with a loss of methylation of the exon 1A region on the maternal allele. Comprehensive analysis of bisulfite-treated DNA from four affected subjects (V-14, V-13, III-3, and VI-2) showed complete conversion of all C's to T's within the amplified region of the exon 1A DMR, indicating that both GNAS alleles were unmethylated. By contrast, the bisulfite-treated DNA sequences of the NESP55 and XLαs DMR were similar to those of normal control DNA, indicating that the methylation defect was limited to exon 1a (data not shown). Similar methylation analyses in two unaffected subjects (III-3 and V-20) showed normal methylation patterns at all DMR.
PCR analysis of STX16
All 14 affected PHP type 1b subjects who were tested were heterozygous for the 3-kb deletion in STX16, thus confirming the basis for PHP 1b in this family. One unaffected subject, III-3, who had normal serum calcium and PTH levels and normal methylation patterns for GNAS also carried the microdeletion but was unavailable for determination of brachydactyly. This individual transmitted the STX16 mutation to her affected son, IV-13, and likely inherited the mutation from her father, II-1, an obligate carrier (Fig. 1).
Discussion
We evaluated an extensive multigenerational family in which many members had PHP type 1b (Fig. 1). Although we were unable to perform molecular and biochemical studies on all patients, we believe that affected members of this family had PHP type 1b based on the following findings: 1) normal sequence of the GNAS gene; 2) normal levels of immunoactive erythrocyte Gαs; and 3) abnormal methylation patterns in GNAS that are consistent with an epigenetic defect. The abnormal imprinting was limited to the DMR of exon 1A, and a previously described 3-kb STX16 microdeletion (13) was identified in all affected subjects who were available for analysis. Our linkage studies directly eliminated the PTH and PTHR1 genes as potential second gene defects, and the size of the kindred essentially excludes a primary role for another gene defect unless in complete linkage disequilibrium with GNAS. Thus, although we were unable to analyze genomic DNA from every member of this extended kindred, our results establish the 3-kb STX16 microdeletion as the basis for PHP type 1b in affected members of this extended kindred.
Brachydactyly was present in nearly 60% of the patients with PHP 1b who were tested and in none of the unaffected relatives. The brachydactyly was of the type E form and in many cases was limited to shortening of the fourth metacarpal and was not associated with short stature. In addition, two of the affected subjects in this study had Madelung-like defects.
The classical Madelung wrist deformity is characterized by particular prominence of the lower end of the ulna, marked shortening and bowing of the radius, and palmar and ulnar deviation of the carpal bones. The Madelung deformity is a dyschondrosteosis that results from premature fusion of the physis with cessation of longitudinal growth. The physeal lesion is always located in the ulnar zone of the distal radius but varies in the anteroposterior plane, which in part determines the precise appearance of the deformity in the patient (14, 15). The Madelung deformity, or defects that resemble the Madelung deformity, occurs in several syndromes, some of which are also associated with mesomelic short stature. Leri-Weill syndrome and Langer mesomelic dwarfism are due to heterozygous and homozygous mutations, respectively, of the short stature homeobox gene (SHOX) located on pseudoautosomal regions of the X and Y chromosomes. Other causes of Madelung deformity include Turner syndrome, multiple exostoses syndrome, multiple epiphysial dysplasia, and dysostosis multiplex of the mucopolysaccharidoses (16).
The Madelung deformity occurs when a major disruption to normal growth plate architecture occurs and leads to a reduction in the number of chondrocyte columns. The columns that remain show a breakdown of normal columnar architecture because nests of chondrocytes replace the abnormal columns. On a cellular level, within the nests, chondrocytes exist at varying stages of maturation, inappropriately sited adjacent to cells of other developmental stages. In addition, there is an apparent increase in the size of the hypertrophic zone and accelerated fusion of the physis. These histological features are reminiscent of the accelerated program of differentiation into hypertrophic chondrocytes that characterizes the severe metaphyseal dysplasia that occurs in mice and humans that lack functional alleles encoding the PTH1R or PTHrP (17–19). More recently heterozygous mutations in PTHrP have been identified as one basis for familial brachydactyly E and short stature (20, 21), providing further confirmation of the important role of the PTHrP-PTH1R signaling pathway in the growth plate. The occurrence of brachydactyly in patients with AHO, whether associated with a GNAS mutation on maternal or paternal allele, suggests that haploinsufficiency rather than imprinting accounts for a deficiency in Gαs protein that impairs PTHrP signaling in the growth plate. Nevertheless, a previous report demonstrated modest preferential expression of the maternal GNAS allele in a bone sample from one of 19 human subjects (22), thus providing limited evidence that imprinting can influence GNAS expression in bone in at least some patients. Partial imprinting of GNAS may also occur in other tissues that had previously been considered to exhibit biallelic expression of Gαs (23). Thus, the development of brachydactyly in PHP 1b may be limited to those patients in whom Gαs expression is influenced by an imprinting mechanism in growth plate cells. A similar mechanism may also explain the development of the Madelung deformity in the patients we describe here.
In summary, our findings confirm previous case reports that described brachydactyly as evidence of mild AHO in at least some patients with sporadic PHP 1b (6–9, 24). Moreover, the coexistence of a Madelung-like deformity in at least some of the PHP 1b patients represents a novel association and extends the list of conditions that can cause this unusual growth plate defect.
Supplementary Material
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Stephen Kam, Justin Cho, and Mahmood Khan. We also appreciate Michail Spiliopoulos' assistance with the pedigree drawing. We acknowledge the support of all the patients who made this research possible.
This work was supported by National Institutes of Health/National Center for Research Resources Grant M01 RR00052 (to the Johns Hopkins University School of Medicine General Clinical Research Center) and U.S. Public Health Service Grants RO1 DK34281 and DK56178 (to M.A.L.). This research was also supported by the generosity of the Bosworth and Friedman families. We acknowledge the support of all the patients who made this research possible.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHO
- Albright hereditary osteodystrophy
- DMR
- differentially methylated region
- PHP
- pseudohypoparathyroidism.
References
- 1. Levine MA, Zapalowski C, Kappy MS. 2005. Disorders of calcium, phosphate, parathyroid hormone and vitamin D. In: Kappy MS, Allen DB, Geffner ME. eds. Principles and practice of pediatric endocrinology. Springfield, IL: Charles C. Thomas; 695–814 [Google Scholar]
- 2. Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. 2007. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Gα(s) in the development of human obesity. J Clin Endocrinol Metab 92:1073–1079 [DOI] [PubMed] [Google Scholar]
- 3. Bastepe M. 2008. The GNAS locus and pseudohypoparathyroidism. Adv Exp Med Biol 626:27–40 [DOI] [PubMed] [Google Scholar]
- 4. Bastepe M, Fröhlich LF, Linglart A, Abu-Zahra HS, Tojo K, Ward LM, Jüppner H. 2005. Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type Ib. Nat Genet 37:25–27 [DOI] [PubMed] [Google Scholar]
- 5. Chillambhi S, Turan S, Hwang DY, Chen HC, Jüppner H, Bastepe M. 2010. Deletion of the noncoding GNAS antisense transcript causes pseudohypoparathyroidism type Ib and biparental defects of GNAS methylation in cis. J Clin Endocrinol Metab 95:3993–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantovani G, de Sanctis L, Barbieri AM, Elli FM, Bollati V, Vaira V, Labarile P, Bondioni S, Peverelli E, Lania AG, Beck-Peccoz P, Spada A. 2010. Pseudohypoparathyroidism and GNAS epigenetic defects: clinical evaluation of Albright hereditary osteodystrophy and molecular analysis in 40 patients. J Clin Endocrinol Metab 95:651–658 [DOI] [PubMed] [Google Scholar]
- 7. Mariot V, Maupetit-Méhouas S, Sinding C, Kottler ML, Linglart A. 2008. A maternal epimutation of GNAS leads to Albright osteodystrophy and parathyroid hormone resistance. J Clin Endocrinol Metab 93:661–665 [DOI] [PubMed] [Google Scholar]
- 8. Unluturk U, Harmanci A, Babaoglu M, Yasar U, Varli K, Bastepe M, Bayraktar M. 2008. Molecular diagnosis and clinical characterization of pseudohypoparathyroidism type-Ib in a patient with mild Albright's hereditary osteodystrophy-like features, epileptic seizures, and defective renal handling of uric acid. Am J Med Sci 336:84–90 [DOI] [PubMed] [Google Scholar]
- 9. de Nanclares GP, Fernández-Rebollo E, Santin I, García-Cuartero B, Gaztambide S, Menéndez E, Morales MJ, Pombo M, Bilbao JR, Barros F, Zazo N, Ahrens W, Jüppner H, Hiort O, Castaño L, Bastepe M. 2007. Epigenetic defects of GNAS in patients with pseudohypoparathyroidism and mild features of Albright's hereditary osteodystrophy. J Clin Endocrinol Metab 92:2370–2373 [DOI] [PubMed] [Google Scholar]
- 10. Jan de Beur SM, Ding CL, LaBuda MC, Usdin TB, Levine MA. 2000. Pseudohypoparathyroidism 1b: exclusion of parathyroid hormone and its receptors as candidate disease genes. J Clin Endocrinol Metab 85:2239–2246 [DOI] [PubMed] [Google Scholar]
- 11. Jan De Beur SM, O'Connell JR, Peila R, Cho J, Deng Z, Kam S, Levine MA. 2003. The pseudohypoparathyroidism type lb locus is linked to a region including GNAS1 at 20q13.3. J Bone Miner Res 18:424–433 [DOI] [PubMed] [Google Scholar]
- 12. Han W, Cauchi S, Herman JG, Spivack SD. 2006. DNA methylation mapping by tag-modified bisulfite genomic sequencing. Anal Biochem 355:50–61 [DOI] [PubMed] [Google Scholar]
- 13. Bastepe M, Fröhlich LF, Hendy GN, Indridason OS, Josse RG, Koshiyama H, Körkkö J, Nakamoto JM, Rosenbloom AL, Slyper AH, Sugimoto T, Tsatsoulis A, Crawford JD, Jüppner H. 2003. Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest 112:1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vickers D, Nielsen G. 1992. Madelung deformity: surgical prophylaxis (physiolysis) during the late growth period by resection of the dyschondrosteosis lesion. J Hand Surg Br 17:401–407 [DOI] [PubMed] [Google Scholar]
- 15. Munns CF, Glass IA, LaBrom R, Hayes M, Flanagan S, Berry M, Hyland VJ, Batch JA, Philips GE, Vickers D. 2001. Histopathological analysis of Leri-Weill dyschondrosteosis: disordered growth plate. Hand Surg 6:13–23 [DOI] [PubMed] [Google Scholar]
- 16. Ty JM, James MA. 2009. Failure of differentiation: part II (arthrogryposis, camptodactyly, clinodactyly, Madelung deformity, trigger finger, and trigger thumb). Hand Clin 25:195–213 [DOI] [PubMed] [Google Scholar]
- 17. Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. 1994. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev 8:277–289 [DOI] [PubMed] [Google Scholar]
- 18. Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Jüppner H, Segre GV, Kronenberg HM. 1996. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273:663–666 [DOI] [PubMed] [Google Scholar]
- 19. Jobert AS, Zhang P, Couvineau A, Bonaventure J, Roume J, Le Merrer M, Silve C. 1998. Absence of functional receptors for parathyroid hormone and parathyroid hormone-related peptide in Blomstrand chondrodysplasia. J Clin Invest 102:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maass PG, Wirth J, Aydin A, Rump A, Stricker S, Tinschert S, Otero M, Tsuchimochi K, Goldring MB, Luft FC, Bähring S. 2010. A cis-regulatory site downregulates PTHLH in translocation t(8;12)(q13;p11.2) and leads to brachydactyly type E. Hum Mol Genet 19:848–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klopocki E, Hennig BP, Dathe K, Koll R, de Ravel T, Baten E, Blom E, Gillerot Y, Weigel JF, Krüger G, Hiort O, Seemann P, Mundlos S. 2010. Deletion and point mutations of PTHLH cause brachydactyly type E. Am J Hum Genet 86:434–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mantovani G, Bondioni S, Locatelli M, Pedroni C, Lania AG, Ferrante E, Filopanti M, Beck-Peccoz P, Spada A. 2004. Biallelic expression of the Gsα gene in human bone and adipose tissue. J Clin Endocrinol Metab 89:6316–6319 [DOI] [PubMed] [Google Scholar]
- 23. Zazo C, Thiele S, Martin C, Fernandez-Rebollo E, Martinez-Indart L, Werner R, Garin I. Spanish PHP Group, Hiort O, Perez DN. 23 February 2011. Gsα activity is reduced in erythrocyte membranes of patients with pseudohypoparathyrodism due to epigenetic alterations at the GNAS locus. J Bone Miner Res 10.1002/jbmr.369 [DOI] [PubMed] [Google Scholar]
- 24. Demura M, Takeda Y, Yoneda T, Furukawa K, Tachi A, Mabuchi H. 2003. Completely skewed X-inactivation in a mentally retarded young female with pseudohypoparathyroidism type IB and juvenile renin-dependent hypertension. J Clin Endocrinol Metab 88:3043–3049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.