Abstract
Elevated concentrations of arsenic in groundwater pose a public health threat to millions of people worldwide. The authors aimed to evaluate the association between arsenic exposure and skin lesion incidence among participants in the Health Effects of Arsenic Longitudinal Study (HEALS). The analyses used data on 10,182 adults free of skin lesions at baseline through the third biennial follow-up of the cohort (2000–2009). Discrete-time hazard regression models were used to estimate hazard ratios and 95% confidence intervals for incident skin lesions. Multivariate-adjusted hazard ratios for incident skin lesions comparing 10.1–50.0, 50.1–100.0, 100.1–200.0, and ≥200.1 μg/L with ≤10.0 μg/L of well water arsenic exposure were 1.17 (95% confidence interval (CI): 0.92, 1.49), 1.69 (95% CI: 1.33, 2.14), 1.97 (95% CI: 1.58, 2.46), and 2.98 (95% CI: 2.40, 3.71), respectively (Ptrend = 0.0001). Results were similar for the other measures of arsenic exposure, and the increased risks remained unchanged with changes in exposure in recent years. Dose-dependent associations were more pronounced in females, but the incidence of skin lesions was greater in males and older individuals. Chronic arsenic exposure from drinking water was associated with increased incidence of skin lesions, even at low levels of arsenic exposure (<100 μg/L).
Keywords: arsenic, Bangladesh, cohort studies, environmental exposure, keratosis, melanosis
Globally, more than 100 million people, including approximately 28–57 million in Bangladesh, are chronically exposed to arsenic through naturally contaminated drinking water (1). The International Agency for Research on Cancer has classified arsenic as a class I human carcinogen (2). Arsenic in drinking water has been associated with increased risk of a wide range of health outcomes including cancers of the skin, lung, bladder, liver, and kidney (3–7); neurologic disease (8); cardiovascular disease (9); and other nonmalignant diseases (10, 11).
Although most arsenic-related cancers have long average latency periods, skin lesions appear within a relatively shorter period of time following exposure to arsenic (12, 13). Additionally, skin lesions are considered precursors to a majority of the arsenic-induced basal and squamous cell skin cancers (14).
Numerous epidemiologic studies have evaluated the relation between arsenic in drinking water and skin lesion prevalence in various populations, such as the recent review by Smith and Steinmaus (15). All prior studies have been cross-sectional or case-control in design, utilizing data from prevalent cases. Although these studies have clearly demonstrated increased skin lesion risk at high arsenic concentrations (>100 μg/L), the risk of skin lesions at lower arsenic exposure levels still remains in question. Additionally, to our knowledge, no prospective cohort studies have been conducted to evaluate the association between arsenic exposure in drinking water at the individual level and skin lesion incidence.
The Health Effects of Arsenic Longitudinal Study (HEALS) provides a unique opportunity to investigate the association between arsenic exposure and skin lesion incidence by using a prospective design based on individual-level assessment of arsenic exposure. In this study, we utilize data from the HEALS cohort to evaluate the incidence of skin lesions in relation to arsenic exposure, measured by individual-level well water and urinary total arsenic concentrations, as well as by daily arsenic intake.
MATERIALS AND METHODS
Study sample
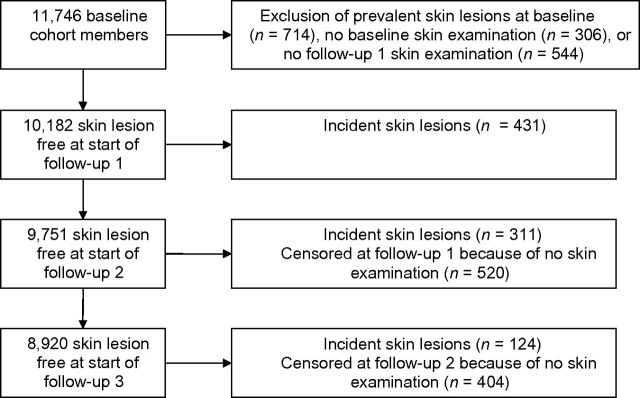
HEALS was designed to investigate the health effects of arsenic exposure through drinking water in a population-based sample of adults in Araihazar, Bangladesh. The study methods have been described previously (16). At the start of the study, we identified 12,050 eligible individuals for recruitment from the enumerated total of approximately 65,000 residents in the study area. Between October 2000 and May 2002, we sampled married individuals aged 18–75 years and residing in the study area for at least 5 years. There were 11,746 men and women enrolled into the HEALS cohort. At the baseline interview, trained study physicians blinded to the arsenic concentrations in participants’ drinking water conducted in-person interviews and clinical and skin evaluations, and they collected urine and blood samples from participants in their homes according to a structured protocol. Participants were contacted for a follow-up interview biennially thereafter, following the same protocol as that for the baseline interview. For the purposes of this analysis, we excluded individuals with prevalent skin lesions at baseline (n = 714), no baseline skin examination (n = 306), or no first follow-up skin examination (n = 544). Thus, we included 10,182 individuals in the present analysis.
Exposure assessment
At baseline, participants were asked to identify the primary well used as their main source of drinking water, from which we assigned the appropriate well water arsenic concentration exposure. Well water arsenic concentrations of all 5,966 wells in the study area were measured by graphite furnace atomic absorption spectrometry, with a detection limit of 5 μg/L. Samples below the limit of detection were subsequently reanalyzed by inductively coupled plasma-mass spectrometry, with a detection limit of 0.1 μg/L (17). Daily arsenic intake (μg/day) was calculated by multiplying the well water arsenic concentration of the primary well, μg/L, by the self-reported daily amount consumed from that well, L/day (n = 10,176). If participants drank from a secondary well, information from that well was included in the daily arsenic intake computation. To incorporate information on the duration of arsenic exposure, we calculated a cumulative arsenic index as (well water arsenic concentration of each known well, μg/L) × (daily amount consumed from each well, L/day) × (duration of well use, days), summed over all known wells. A sensitivity analysis was performed to examine the effect of using the cumulative arsenic index relative to daily arsenic dose.
Among the 9,904 individuals who provided a spot urine sample at baseline, 9,876 (99.7%) provided a spot urine sample at the first follow-up, and 9,408 (95.0%) provided a spot urine sample at the second follow-up. The urinary total arsenic concentration was measured by graphite furnace atomic absorption spectrometry, with a detection limit of 2 μg/L (18). Urinary creatinine was measured by a colorimetric method based on the Jaffe reaction described by Heinegard and Tiderstrom (19), and urinary total arsenic was subsequently divided by creatinine to obtain a creatinine-adjusted urinary total arsenic concentration, expressed as μg/g of creatinine (20).
Well water arsenic cutpoints for the first and second quintiles were adjusted to correspond with the World Health Organization's guideline for arsenic in drinking water (10 μg/L) and the national standard for arsenic in drinking water in Bangladesh (50 μg/L). The urinary total arsenic concentration and the daily arsenic intake were categorized by quintiles according to the baseline distribution of the cohort eligible for analysis.
Skin lesion status
The first follow-up wave was conducted between September 2002 and May 2004; among the 10,182 eligible participants, all completed the first follow-up interview based on the exclusion criteria of the present analysis, and 431 incident skin lesions were detected (Figure 1). The second follow-up wave was conducted between June 2004 and August 2006; among the 9,751 participants known to be free of skin lesions at the first follow-up evaluation, 9,231 (94.7%) had a completed skin examination at the second follow-up interview, of whom 311 had incident skin lesions. The third follow-up wave was conducted between January 2007 and February 2009; among the 8,920 participants known to be free of skin lesions at the second follow-up evaluation, 8,516 (95.5%) had a completed skin examination at the third follow-up interview, of whom 124 had incident skin lesions. In summary, a total of 10,182 individuals were included in these analyses, of whom 866 individuals developed incident skin lesions. Participants who did not develop skin lesions were censored at the third biennial follow-up (n = 8,392) or time of last skin examination (n = 924). For the purposes of these analyses, once an individual was censored, there was no reentry into the analysis cohort.
Figure 1.
Flowchart of study participation through follow-up 3 for skin lesion assessment, Health Effects of Arsenic Longitudinal Study, Bangladesh, 2000–2009.
A structured protocol was used to ascertain skin lesions by the study physicians who had undergone training for the detection and diagnosis of skin lesions. The study physician recorded the presence or absence of melanosis (a hyperpigmentation of the skin surface), leucomelanosis (a hypopigmentation of the skin surface), or keratosis (a thickening of the skin typically on the palms and soles) (21). For the present analysis, skin lesion incidence was constructed on the basis of the incidence of any type of skin lesion among individuals who previously had no manifestation of any type of skin lesion.
Covariates
All covariate data were derived from the baseline interview. We included sex (male, female), age (years), formal education (yes, no), attained level of education (years), smoking status (never, former, or current), and body mass index (weight (kg)/height (m)2), with height and weight measured at the baseline examination by the study physician.
Statistical analyses
Discrete-time hazard models were used to estimate discrete-time hazard ratios and their 95% confidence intervals for skin lesion incidence. These models were based on the probability (i.e., the discrete-time hazard) of skin lesion incidence at each biennial follow-up period conditional on being skin lesion free at the previous interval (22). The conditional probability was estimated by using a log-linear model with a different intercept for each study interval, but with common regression coefficients across all intervals. The regression coefficients were interpreted as log discrete-time hazard ratios, analogous to log (continuous time) hazard ratios that arise in the traditional proportional hazards model (23). Because the enrollment of participants into the cohort was clustered on household (i.e., married couples) and households were clustered on the primary well, robust standard errors computed on the basis of the primary well were used for discrete-time hazards to account for this correlation as is done in generalized estimating equations analyses (24).
Arsenic exposure quintiles were modeled by using indicator variables in regression models, initially adjusted for sex and age, and including indicators for study interval. Multivariate models included further adjustment for body mass index, smoking status, formal education, and years of attained education. These covariates were considered potential confounders on the basis of a priori causal knowledge. Tests for trend were conducted by introducing a single ordinal arsenic exposure variable in the discrete-time hazard model, and the corresponding P value of the coefficient was interpreted as the P for trend (Ptrend). We evaluated effect modification by sex and age (dichotomized at the median value) on both the additive and multiplicative scales. Additive interaction was evaluated through the relative excess risk for interaction (RERI) by using multivariate-adjusted estimates. This was calculated as
Here, β1 is the coefficient of the ordinal arsenic exposure measure, β2 is the coefficient of the ordinal effect modifier measure, and β3 is the coefficient of the cross-product of the ordinal arsenic exposure and ordinal effect modifier measures (25, 26). Bias-corrected and -accelerated 95% confidence intervals of the RERI were estimated via 1,000 bootstrap samples, where the resampling was performed at the level of well (27). Confidence intervals of the RERI were also calculated by using the delta method described by Hosmer and Lemeshow (28) with similar results (not shown). Tests for multiplicative interaction were assessed via the P value of the cross-product term of the ordinal exposure variable and the ordinal effect modifier in the discrete-time hazard model.
Using repeated urinary total arsenic concentration measures, assessed every 2 years from all participants, we also evaluated the impact of recent changes in arsenic exposure during the follow-up period on skin lesion incidence. The median urinary total arsenic concentration at baseline (273 μg/g) was used to dichotomize the baseline, first, and second follow-up measures. In the model that included baseline and follow-up 1 exposure patterns, skin lesions detected in the 2 waves subsequent to follow-up 1 were modeled. In the model that included baseline and follow-up 2 exposure patterns, skin lesions detected in the last wave subsequent to follow-up 2 were modeled. These models were also adjusted for all previously mentioned potential confounders.
Statistical analyses were performed by using the procedure GENMOD, SAS release 9.2 (SAS Institute, Inc., Cary, North Carolina), and STATA, version 11 (StataCorp LP, College Station, Texas), software.
RESULTS
Between 2000 and 2009, 866 incident skin lesion cases were identified among 10,182 individuals in the HEALS cohort—431 incident cases at the first follow-up, 311 incident cases at the second follow-up, and 124 incident cases at the third follow-up. Characteristics of the analysis cohort members according to incident skin lesion status are shown in Table 1. In unadjusted models, body mass index, years of formal education, and female sex were inversely associated with skin lesion incidence; smoking history and older age were positively associated with skin lesion incidence. Adjustment for well water arsenic concentration did not appreciably change these hazard ratios, and all sociodemographic and lifestyle characteristics remained significant risk factors for incident skin lesions (results not shown).
Table 1.
Selected Baseline Characteristics of Participants by Incident Skin Lesion Status, Health Effects of Arsenic Longitudinal Study, Bangladesh, 2000–2009
| Characteristic | Incident Skin Lesions |
HR | 95% CI | |||
| Present (n = 866) |
Absent (n = 9,316) |
|||||
| No. | % | No. | % | |||
| Well water arsenic, μg/L | ||||||
| 0.1–10 | 139 | 16.1 | 2,339 | 25.1 | 1.00 | Referent |
| 10.1–50 | 134 | 15.5 | 2,086 | 22.4 | 1.08 | 0.85, 1.38 |
| 50.1–100 | 152 | 17.5 | 1,674 | 18.0 | 1.52 | 1.19, 1.93 |
| 100.1–200 | 206 | 23.8 | 1,829 | 19.6 | 1.86 | 1.48, 2.32 |
| ≥200.1 | 235 | 27.1 | 1,385 | 14.9 | 2.69 | 2.16, 3.35 |
| Urinary total arsenic (creatinine), μg/g | ||||||
| 7–88 | 137 | 16.2 | 1,845 | 20.4 | 1.00 | Referent |
| 89–155 | 117 | 13.9 | 1,867 | 20.6 | 0.85 | 0.66, 1.09 |
| 156–240 | 169 | 20.0 | 1,803 | 19.9 | 1.25 | 0.99, 1.57 |
| 241–392 | 186 | 22.1 | 1,804 | 19.9 | 1.36 | 1.09, 1.71 |
| ≥393 | 235 | 27.8 | 1,741 | 19.2 | 1.76 | 1.42, 2.18 |
| Daily arsenic intake, μg/day | ||||||
| 0.4–19.4 | 116 | 13.3 | 1,924 | 20.7 | 1.00 | Referent |
| 19.5–100.8 | 133 | 15.4 | 1,899 | 20.4 | 1.16 | 0.90, 1.49 |
| 100.9–233.1 | 168 | 19.4 | 1,866 | 20.0 | 1.48 | 1.16, 1.88 |
| 233.2–472.0 | 185 | 21.4 | 1,849 | 19.9 | 1.63 | 1.29, 2.06 |
| ≥472.1 | 264 | 30.5 | 1,772 | 19.0 | 2.37 | 1.89, 2.97 |
| Body mass index, kg/m2 | ||||||
| <18.5 | 379 | 44.1 | 3,531 | 38.1 | 1.00 | Referent |
| 18.5–24.9 | 445 | 51.8 | 5,019 | 54.2 | 0.83 | 0.72, 0.95 |
| ≥25 | 35 | 4.1 | 709 | 7.7 | 0.47 | 0.33, 0.67 |
| Education, years | ||||||
| 0 | 424 | 49.0 | 4,060 | 43.6 | 1.00 | Referent |
| 1–5 | 248 | 28.7 | 2,778 | 29.8 | 0.85 | 0.73, 0.99 |
| ≥6 | 193 | 22.3 | 2,473 | 26.6 | 0.76 | 0.64, 0.91 |
| Sex | ||||||
| Male | 613 | 70.8 | 3,378 | 36.3 | 1.00 | Referent |
| Female | 253 | 29.2 | 5,938 | 63.7 | 0.24 | 0.21, 0.28 |
| Cigarette smoking | ||||||
| Never | 333 | 38.4 | 6,579 | 70.6 | 1.00 | Referent |
| Former | 133 | 15.4 | 467 | 5.0 | 5.10 | 4.19, 6.21 |
| Current | 400 | 46.2 | 2,267 | 24.3 | 3.39 | 2.93, 3.92 |
| Age, years | ||||||
| 18–30 | 75 | 8.7 | 3,257 | 35.0 | 1.00 | Referent |
| 31–40 | 240 | 27.7 | 3,456 | 37.1 | 2.91 | 2.23, 3.78 |
| 41–50 | 319 | 36.8 | 1,953 | 21.0 | 6.57 | 5.09, 8.49 |
| 51–75 | 232 | 26.8 | 649 | 6.9 | 13.50 | 10.29, 17.71 |
| Skin lesion severity | ||||||
| Keratosis | 197 | 22.8 | ||||
| Melanosis/leucomelanosis | 669 | 77.2 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Arsenic exposure was associated with skin lesion incidence in a dose-dependent manner for all 3 measures of exposure (Table 2). By utilization of the ordinal exposure data in the multivariate models, a 1-quintile increase in well water arsenic concentration was associated with a 31% increase in incidence of skin lesions (95% confidence interval (CI): 1.25, 1.38), with corresponding increases of 27% (95% CI: 1.21, 1.34) and 30% (95% CI: 1.24, 1.37) for urinary total arsenic concentration and daily arsenic intake.
Table 2.
Hazard Ratios for Incident Skin Lesions According to Quintiles of Arsenic Exposure, Health Effects of Arsenic Longitudinal Study, Bangladesh, 2000–2009
| Arsenic Exposure | No. of Events | Age- and Sex-adjusted Estimatea |
No. of Events | Multivariate Estimateb |
||
| HR | 95% CI | HR | 95% CI | |||
| Well water arsenic, μg/L | ||||||
| 0.1–10 | 139 | 1.00 | Referent | 137 | 1.00 | Referent |
| 10.1–50 | 134 | 1.17 | 0.93, 1.49 | 134 | 1.17 | 0.92, 1.49 |
| 50.1–100 | 152 | 1.70 | 1.34, 2.15 | 151 | 1.69 | 1.33, 2.14 |
| 100.1–200 | 206 | 2.00 | 1.60, 2.50 | 201 | 1.97 | 1.58, 2.46 |
| ≥200.1 | 235 | 3.00 | 2.41, 3.74 | 235 | 2.98 | 2.40, 3.71 |
| Ptrend | 0.0001 | 0.0001 | ||||
| Urinary total arsenic (creatinine), μg/g | ||||||
| 7–88 | 137 | 1.00 | Referent | 136 | 1.00 | Referent |
| 89–155 | 117 | 0.93 | 0.73, 1.18 | 115 | 0.90 | 0.71, 1.15 |
| 156–240 | 169 | 1.39 | 1.11, 1.74 | 167 | 1.34 | 1.07, 1.68 |
| 241–392 | 186 | 1.69 | 1.36, 2.11 | 185 | 1.62 | 1.29, 2.02 |
| ≥393 | 235 | 2.53 | 2.05, 3.14 | 233 | 2.39 | 1.92, 2.97 |
| Ptrend | 0.0001 | 0.0001 | ||||
| Daily arsenic intake, μg/day | ||||||
| 0.4–19.4 | 116 | 1.00 | Referent | 115 | 1.00 | Referent |
| 19.5–100.8 | 133 | 1.23 | 0.96, 1.58 | 132 | 1.23 | 0.96, 1.58 |
| 100.9–233.1 | 168 | 1.60 | 1.27, 2.03 | 167 | 1.57 | 1.24, 1.99 |
| 233.2–472.0 | 185 | 1.85 | 1.47, 2.32 | 183 | 1.82 | 1.45, 2.30 |
| ≥472.1 | 264 | 2.99 | 2.39, 3.74 | 261 | 2.92 | 2.34, 3.65 |
| Ptrend | 0.0001 | 0.0001 | ||||
Abbreviations: CI, confidence interval; HR, hazard ratio (discrete time).
Additionally adjusted for follow-up 2 indicator and follow-up 3 indicator.
Adjusted for sex, age, body mass index, formal education, education years, follow-up 2 indicator, follow-up 3 indicator, and smoking status (current vs. never, past vs. never).
In sensitivity analyses, inclusion of the cumulative arsenic index in skin lesion models did not show additional predictive power beyond that shown from other arsenic exposure measures (which did not include duration of well use). Because the daily arsenic dose was highly correlated with the cumulative arsenic index, results of the latter are not presented.
We evaluated whether the associations between arsenic exposure and skin lesion incidence were modified by sex and age on the additive and multiplicative scales. Estimates are presented in Tables 3 and 4 for the interpretation of multiplicative interaction; estimates interpreted for additive interaction can be derived from the information provided in the footnote. We observed significant interaction on the multiplicative scale, by sex, for the association between daily arsenic intake and skin lesion risk (χ2 = 4.60, 1 df; Pinteraction = 0.036), suggesting that the dose-response association between arsenic exposure and skin lesion incidence is stronger in females. On the additive scale, we observed that skin lesion incidence was greater in males with each 1-quintile increase in well water arsenic exposure than would be expected on the basis of the additive independent effects of sex and well water arsenic exposure alone (RERI = 0.47), as shown in Table 3. Similar departures from additivity were seen with urinary total arsenic exposure and daily arsenic intake. No significant interaction was observed on the multiplicative scale between arsenic exposure and age on skin lesion incidence (Table 4). On the additive scale, skin lesion incidence was greater in individuals aged 36 years or older with each 1-quintile increase in well water arsenic exposure than would be expected on the basis of the additive independent effects of age and well water arsenic exposure alone (RERI = 0.77), as shown in Table 4. Similar departures from additivity were also seen with urinary total arsenic exposure and daily arsenic intake.
Table 3.
Hazard Ratios for Incident Skin Lesions According to Quintiles of Arsenic Exposure by Sex, Health Effects of Arsenic Longitudinal Study, Bangladesh, 2000–2009
| Arsenic Exposure | Females |
Males |
Pinteraction | ||||
| No. of Events | HRa | 95% CI | No. of Events | HRa | 95% CI | ||
| Well water arsenic, μg/L | |||||||
| 0.1–10 | 26 | 1.00 | Referent | 111 | 1.00b | Referent | 0.10 |
| 10.1–50 | 44 | 1.79 | 1.08, 2.95 | 90 | 1.02 | 0.78, 1.34 | |
| 50.1–100 | 38 | 1.99 | 1.19, 3.32 | 113 | 1.62 | 1.25, 2.11 | |
| 100.1–200 | 57 | 2.61 | 1.62, 4.21 | 144 | 1.82 | 1.42, 2.34 | |
| ≥200.1 | 83 | 4.49 | 2.85, 7.08 | 152 | 2.58 | 2.02, 3.30 | |
| Ptrend | 0.0001 | 0.0001 | |||||
| RERI | 0.47 | 0.26, 0.74 | |||||
| Urinary total arsenic (creatinine), μg/g | |||||||
| 7–88 | 26 | 1.00 | Referent | 110 | 1.00c | Referent | 0.40 |
| 89–155 | 25 | 0.85 | 0.49, 1.50 | 90 | 0.92 | 0.70, 1.20 | |
| 156–240 | 42 | 1.42 | 0.87, 2.33 | 125 | 1.31 | 1.02, 1.69 | |
| 241–392 | 49 | 1.51 | 0.93, 2.46 | 136 | 1.67 | 1.30, 2.14 | |
| ≥393 | 93 | 2.72 | 1.74, 4.23 | 140 | 2.23 | 1.73, 2.88 | |
| Ptrend | 0.0001 | 0.0001 | |||||
| RERI | 0.47 | 0.28, 0.77 | |||||
| Daily arsenic intake, μg/day | |||||||
| 0.4–19.4 | 21 | 1.00 | Referent | 94 | 1.00d | Referent | 0.036 |
| 19.5–100.8 | 30 | 1.32 | 0.75, 2.33 | 102 | 1.22 | 0.93, 1.60 | |
| 100.9–233.1 | 45 | 1.98 | 1.16, 3.38 | 122 | 1.48 | 1.14, 1.92 | |
| 233.2–472.0 | 56 | 2.50 | 1.50, 4.17 | 127 | 1.66 | 1.27, 2.16 | |
| ≥472.1 | 96 | 4.08 | 2.50, 6.66 | 165 | 2.61 | 2.03, 3.35 | |
| Ptrend | 0.0001 | 0.0001 | |||||
| RERI | 0.48 | 0.26, 0.80 | |||||
Abbreviations: CI, confidence interval; HR, hazard ratio (discrete time); RERI, relative excess risk due to interaction.
Adjusted for age, body mass index, formal education, education years, follow-up 2 indicator, follow-up 3 indicator, and smoking status (current vs. never, past vs. never).
HR = 3.64 comparing males with females in this lowest exposure quintile.
HR = 2.87 comparing males with females in this lowest exposure quintile.
HR = 3.47 comparing males with females in this lowest exposure quintile.
Table 4.
Hazard Ratios for Incident Skin Lesions According to Quintiles of Arsenic Exposure by Age, Health Effects of Arsenic Longitudinal Study, Bangladesh, 2000–2009
| Arsenic Exposure | 18–35 Years |
36–75 Years |
Pinteraction | ||||
| No. of Events | HRa | 95% CI | No. of Events | HRa | 95% CI | ||
| Well water arsenic, μg/L | |||||||
| 0.1–10 | 17 | 1.00 | Referent | 120 | 1.00b | Referent | 0.26 |
| 10.1–50 | 25 | 1.53 | 0.81, 2.90 | 109 | 1.08 | 0.83, 1.39 | |
| 50.1–100 | 31 | 2.24 | 1.20, 4.19 | 120 | 1.54 | 1.20, 1.98 | |
| 100.1–200 | 34 | 2.37 | 1.33, 4.25 | 167 | 1.88 | 1.48, 2.39 | |
| ≥200.1 | 50 | 4.27 | 2.44, 7.48 | 185 | 2.71 | 2.15, 3.41 | |
| Ptrend | 0.0001 | 0.0001 | |||||
| RERI | 0.77 | 0.49, 1.15 | |||||
| Urinary total arsenic (creatinine), μg/g | |||||||
| 7–88 | 14 | 1.00 | Referent | 122 | 1.00c | Referent | 0.14 |
| 89–155 | 18 | 1.24 | 0.63, 2.43 | 97 | 0.85 | 0.66, 1.11 | |
| 156–240 | 28 | 1.85 | 0.98, 3.52 | 139 | 1.25 | 0.98, 1.59 | |
| 241–392 | 37 | 2.42 | 1.31, 4.47 | 148 | 1.46 | 1.15, 1.85 | |
| ≥393 | 51 | 3.47 | 1.91, 6.31 | 182 | 2.13 | 1.69, 2.68 | |
| Ptrend | 0.0001 | 0.0001 | |||||
| RERI | 0.68 | 0.41, 1.08 | |||||
| Daily arsenic intake, μg/day | |||||||
| 0.4–19.4 | 13 | 1.00 | Referent | 102 | 1.00d | Referent | 0.12 |
| 19.5–100.8 | 21 | 1.59 | 0.79, 3.21 | 111 | 1.17 | 0.90, 1.52 | |
| 100.9–233.1 | 29 | 2.24 | 1.15, 4.39 | 138 | 1.46 | 1.14, 1.88 | |
| 233.2–472.0 | 31 | 2.22 | 1.17, 4.21 | 152 | 1.79 | 1.40, 2.29 | |
| ≥472.1 | 63 | 4.54 | 2.49, 8.26 | 198 | 2.56 | 2.02, 3.24 | |
| Ptrend | 0.0001 | 0.0001 | |||||
| RERI | 0.78 | 0.48, 1.28 | |||||
Abbreviations: CI, confidence interval; HR, hazard ratio (discrete time); RERI, relative excess risk due to interaction.
Adjusted for sex, body mass index, formal education, education years, follow-up 2 indicator, follow-up 3 indicator, and smoking status (current vs. never, past vs. never).
HR = 4.50 comparing individuals aged 36–75 years with those aged 18–35 years in this lowest exposure quintile.
HR = 5.12 comparing individuals aged 36–75 years with those aged 18–35 years in this lowest exposure quintile.
HR = 4.86 comparing individuals aged 36–75 years with those aged 18–35 years in this lowest exposure quintile.
We examined the impact of 2- and 4-year changes in arsenic exposure (as measured by repeated urinary total arsenic concentrations) subsequent to baseline enrollment, shown in Table 5. The multivariate-adjusted hazard ratio for comparison of high baseline exposure with low baseline exposure was 1.70 (95% CI: 1.40, 2.08) for incident skin lesions occurring subsequent to the first follow-up. Further stratification of baseline exposure status by the first follow-up exposure levels did not appear to have a differential effect on skin lesion incidence (Table 5). The multivariate-adjusted hazard ratio for comparison of high baseline exposure with low baseline exposure was 2.09 (95% CI: 1.45, 3.01) for incident skin lesions occurring subsequent to the second follow-up. Further stratification of baseline exposure status by the second follow-up exposure levels also did not appear to have a differential effect on skin lesion incidence (Table 5).
Table 5.
Hazard Ratios for Incident Skin Lesions According to Change in Arsenic Exposure, Health Effects of Arsenic Longitudinal Study, Bangladesh, 2000–2009
| Baseline Exposurea | Follow-up Exposure | No. of Events | No. at Risk | HRb | 95% CI |
| Baseline and follow-up 1 | |||||
| Low | Low | 225 | 5,279 | 1.00c | Referent |
| Low | High | 24 | 612 | 0.99 | 0.77, 1.26 |
| High | Low | 81 | 1,370 | 1.71 | 1.37, 2.13 |
| High | High | 89 | 1,636 | 1.69 | 1.32, 2.15 |
| Baseline and follow-up 2 | |||||
| Low | Low | 61 | 4,812 | 1.00 | Referent |
| Low | High | 7 | 633 | 1.04 | 0.66, 1.64 |
| High | Low | 28 | 1,383 | 2.06 | 1.38, 3.07 |
| High | High | 26 | 1,397 | 2.15 | 1.35, 3.41 |
Abbreviations: CI, confidence interval; HR, hazard ratio (discrete time).
Low categories were based on creatinine-adjusted urinary total arsenic concentrations of <273 μg/g; high categories were based on creatinine-adjusted urinary total arsenic concentrations of ≥273 μg/g.
Adjusted for sex, age, body mass index, formal education, education years, and smoking status (current vs. never, past vs. never).
Hazard ratios were additionally adjusted for follow-up 2 indicator and follow-up 3 indicator.
DISCUSSION
We observed a dose-dependent increase in risk of incident skin lesions with increasing arsenic exposure. There was evidence of synergism on the additive scale of these associations by sex and age (with stronger effects among males and older subjects) and, to a lesser degree, on the multiplicative scale by sex (with stronger effects among females). Utilizing repeated measures of creatinine-adjusted urinary total arsenic concentration for all cohort members, we found that chronic long-term exposure to arsenic (captured from the baseline assessment of exposure) was a more important predictor of skin lesion risk than were the subsequent short-term changes in exposure (captured at the follow-up visits).
Most importantly, we observed an effect of arsenic exposure on the risk of skin lesions, even at lower levels of well water arsenic exposure in this population (50.1–100 μg/L); this finding was consistent across all major subgroups defined by sex and age. Of the 3 measures of arsenic exposure that we ascertained, the water-based measures of arsenic exposure (well water arsenic concentration and daily arsenic intake) were more strongly associated with disease risk on the basis of quintile scores. Prior studies using individual well water arsenic concentrations have shown dose-dependent associations of arsenic exposure with skin lesion prevalence by using cross-sectional designs (21, 29–31) as well as case-control studies of prevalent cases that utilized current well water arsenic concentration (32), constructed measures of lifetime arsenic exposure (13), and 20-year historical arsenic exposure (33). However, for many of these studies, the prevalence of skin lesions at the lower well water arsenic concentrations was quite low relative to the prevalence we have previously observed in our study population (21), as well as by others (33, 34). Consequently, many of these prior studies failed to show an effect of arsenic at the low dose range because of the small number of cases indentified at low exposure levels. Rahman et al.(33) showed that individuals exposed to a time-weighted mean well water arsenic concentration of 10–49 μg/L since 1970 had an increased risk of skin lesions compared with individuals exposed to <10 μg/L (male adjusted odds ratio (OR) = 3.25, 95% CI: 1.43, 7.38 and female adjusted OR = 1.66, 95% CI: 0.65, 4.24). McDonald et al.(32) in a case-control study including only female prevalent cases of skin lesions showed marginal increased risk for current well water arsenic concentrations of 11–50 μg/L (OR = 1.33, 95% CI: 0.77, 2.28) compared with ≤10 μg/L. Finally, we previously reported an increased risk of prevalent skin lesions for a time-weighted mean well water arsenic concentration of 8.1–40 μg/L (prevalence OR = 1.91, 95% CI: 1.26, 2.89) relative to ≤8.0 μg/L (21). A strength of the current analysis was that we had the unique opportunity of having a sufficient number of incident skin lesion cases among individuals exposed to arsenic concentrations less than 100 μg/L to evaluate effects at the lower end of the arsenic dose range. Variations in the incidence of skin lesions across study populations should be considered in future research and may potentially be attributable to differences in socioeconomic characteristics, smoking patterns, nutritional status, or the distribution of other modifying risk factors.
There was significant modification of the associations between arsenic exposure and skin lesion risk by sex on the additive and multiplicative scales and by age on the additive scale. Males were observed to have an increased incidence of arsenical skin lesions compared with females, which is consistent with studies of skin lesion prevalence (21, 29, 30, 33–36). It has been suggested that the increased incidence among males may be partly attributed to sun exposure (37) or differences in arsenic methylation capacity (38, 39). However, we saw that the multiplicative dose-response association between daily arsenic dose and skin lesion risk was more pronounced in females. We also observed that older individuals had an increased incidence of skin lesions, which was consistent with prior studies (33) and has also been attributed to decreased arsenic methylation capacity with increased age (38, 40–44).
Utilizing repeated measures of urinary total arsenic exposure over time, we see that once chronically exposed, decreasing exposure for up to several years did not reduce one's risk of skin lesions. Whereas short-term changes in exposure did not decrease skin lesion risk, we will continue to evaluate the modification of risk as the cohort is followed for a longer period of time. Studies from Taiwan and Chile have shown that cancer risks persist even with the cessation of arsenic exposure (45–47); therefore, it may be important to consider other chemoprevention strategies in conjunction with remediation for arsenic-exposed populations.
The major strengths of this study were the prospective design, the large size of the study cohort, the wide range of arsenic exposure, the multiple measures of baseline arsenic exposure, and the repeated prospective assessment of urinary total arsenic concentration. Whereas previous studies have demonstrated an association between arsenic exposure and skin lesions at high exposure levels, those studies relied primarily on prevalent cases and had limited power at the low exposure levels.
There are limitations of this study that we also consider. A complete lifetime historical assessment of arsenic exposure has not yet been undertaken for this study population. A major obstacle to this endeavor is that many of the wells that individuals may have used in the distant past may no longer exist or be in the same location; therefore, ascertainment of the water arsenic concentration of historical wells will not be complete for all cohort members. When we compare the mean number of years that individuals reported using their primary baseline well, individuals with incident skin lesions had reported using the baseline well for 7.96 (standard deviation, 5.98) years, and individuals without skin lesions had reported using the baseline well for 6.98 (standard deviation, 4.90) years.
The findings of this study have important public health implications for arsenic in drinking water. Prior epidemiologic research has examined the prevalence of skin lesions with arsenic concentrations. This is the first large study to examine the association of skin lesion incidence with arsenic exposure. Second, 24% of the cohort in the analysis had well water arsenic concentrations of less than 10 μg/L and 46% less than 50 μg/L, which makes the exposure levels comparable to other populations that have low level arsenic exposure.
In conclusion, we found that arsenic exposure through drinking water was associated with increased risk of skin lesion incidence, even at water concentrations less than 100 μg/L. Because prior studies have not been sufficiently powered to evaluate skin lesion risk at low levels of arsenic exposure, this work provides important evidence for arsenic toxicity at low exposure levels. Additionally, we saw persistent increased risk of skin lesions even among individuals who had reduced their arsenic exposure in recent years, which suggests that future chemoprevention interventions should be considered in conjunction with remediation of exposed populations to reduce future cancer risks.
Acknowledgments
Author affiliations: Department of Health Studies, The University of Chicago, Chicago, Illinois (Maria Argos, Tara Kalra, Brandon L. Pierce, Paul J. Rathouz, Habibul Ahsan); Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, New York (Maria Argos, Habibul Ahsan); Department of Environmental Medicine, New York University, New York, New York (Yu Chen); Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, New York (Faruque Parvez, Vesna Slavkovich, Joseph H. Graziano); Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, New York (Diane Levy); and Columbia University and The University of Chicago Research Office in Bangladesh, Mohakhali, Dhaka, Bangladesh (Tariqul Islam, Alauddin Ahmed, Rabiul Hasan, Khaled Hasan, Golam Sarwar).
This work was supported by the National Institutes of Health (grants P42 ES010349, R01 CA102484, R01 CA107431, P30 CA014599, and P30 ES009089).
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- HEALS
Health Effects of Arsenic Longitudinal Study
- OR
odds ratio
- RERI
relative excess risk for interaction
References
- 1.Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ. 2000;78(9):1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 2.IARC Monograph on Arsenic and Arsenic Compounds. Lyon, France: International Agency for Research on Cancer; 1987. (suppl 7):100. [Google Scholar]
- 3.Smith AH, Goycolea M, Haque R, et al. Marked increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147(7):660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Chen CW, Wu MM, et al. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992;66(5):888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopenhayn-Rich C, Biggs ML, Fuchs A, et al. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;7(2):117–124. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Córdoba, Argentina. Int J Epidemiol. 1998;27(4):561–569. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- 7.Tseng WP. Effects and dose-response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977;19:109–119. doi: 10.1289/ehp.7719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouwer OF, Onkenhout W, Edelbroek PM, et al. Increased neurotoxicity of arsenic in methylenetetrahydrofolate reductase deficiency. Clin Neurol Neurosurg. 1992;94(4):307–310. doi: 10.1016/0303-8467(92)90179-7. [DOI] [PubMed] [Google Scholar]
- 9.Wu MM, Kuo TL, Hwang YH, et al. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130(6):1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- 10.Rahman M, Tondel M, Ahmad SA, et al. Hypertension and arsenic exposure in Bangladesh. Hypertension. 1999;33(1):74–78. doi: 10.1161/01.hyp.33.1.74. [DOI] [PubMed] [Google Scholar]
- 11.Heck JE, Chen Y, Grann VR, et al. Arsenic exposure and anemia in Bangladesh: a population-based study. J Occup Environ Med. 2008;50(1):80–87. doi: 10.1097/JOM.0b013e31815ae9d4. [DOI] [PubMed] [Google Scholar]
- 12.Wong SS, Tan KC, Goh CL. Cutaneous manifestations of chronic arsenicism: review of seventeen cases. J Am Acad Dermatol. 1998;38(2 pt 1):179–185. doi: 10.1016/s0190-9622(98)70596-1. [DOI] [PubMed] [Google Scholar]
- 13.Haque R, Mazumder DN, Samanta S, et al. Arsenic in drinking water and skin lesions: dose-response data from West Bengal, India. Epidemiology. 2003;14(2):174–182. doi: 10.1097/01.EDE.0000040361.55051.54. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council Subcommittee to Update the 1999 Arsenic in Drinking Water Report. Arsenic in Drinking Water: 2001 Update. Washington, DC: National Academies Press; 2001. [Google Scholar]
- 15.Smith AH, Steinmaus CM. Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health. 2009;30:107–122. doi: 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahsan H, Chen Y, Parvez F, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16(2):191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Z, Zheng Y, Mortlock R, et al. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379(3):512–518. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 18.Nixon DE, Mussmann GV, Eckdahl SJ, et al. Total arsenic in urine: palladium-persulfate vs nickel as a matrix modifier for graphite furnace atomic absorption spectrophotometry. Clin Chem. 1991;37(9):1575–1579. [PubMed] [Google Scholar]
- 19.Heinegård D, Tiderström G. Determination of serum creatinine by a direct colorimetric method. Clin Chim Acta. 1973;43(3):305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- 20.Nermell B, Lindberg AL, Rahman M, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106(2):212–218. doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Ahsan H, Chen Y, Parvez F, et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study. Am J Epidemiol. 2006;163(12):1138–1148. doi: 10.1093/aje/kwj154. [DOI] [PubMed] [Google Scholar]
- 22.Singer J, Willet J. It's about time: using discrete-time survival analysis to study duration and the timing of events. J Educ Stat. 2003;18(2):155–195. [Google Scholar]
- 23.Chizmar JA. discrete-time hazard analysis of the role of gender in persistence in the economics major. J Econ Educ. 2000:107–118. [Google Scholar]
- 24.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 25.Knol MJ, van der Tweel I, Grobbee DE, et al. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36(5):1111–1118. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 26.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Efron B, Tibshirani R. An Introduction to the Bootstrap. London, United Kingdom: Chapman & Hall; 1993. [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Guha Mazumder DN, Haque R, Ghosh N, et al. Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int J Epidemiol. 1998;27(5):871–877. doi: 10.1093/ije/27.5.871. [DOI] [PubMed] [Google Scholar]
- 30.Tondel M, Rahman M, Magnuson A, et al. The relationship of arsenic levels in drinking water and the prevalence rate of skin lesions in Bangladesh. Environ Health Perspect. 1999;107(9):727–729. doi: 10.1289/ehp.99107727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahsan H, Perrin M, Rahman A, et al. Associations between drinking water and urinary arsenic levels and skin lesions in Bangladesh. J Occup Environ Med. 2000;42(12):1195–1201. doi: 10.1097/00043764-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 32.McDonald C, Hoque R, Huda N, et al. Risk of arsenic-related skin lesions in Bangladeshi villages at relatively low exposure: a report from Gonoshasthaya Kendra. Bull World Health Organ. 2007;85(9):668–673. doi: 10.2471/BLT.06.036764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman M, Vahter M, Sohel N, et al. Arsenic exposure and age- and sex-specific risk for skin lesions: a population-based case-referent study in Bangladesh. Environ Health Perspect. 2006;114(12):1847–1852. doi: 10.1289/ehp.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman M, Vahter M, Wahed MA, et al. Prevalence of arsenic exposure and skin lesions. A population based survey in Matlab, Bangladesh. J Epidemiol Community Health. 2006;60(3):242–248. doi: 10.1136/jech.2005.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindberg AL, Rahman M, Persson LA, et al. The risk of arsenic induced skin lesions in Bangladeshi men and women is affected by arsenic metabolism and the age at first exposure. Toxicol Appl Pharmacol. 2008;230(1):9–16. doi: 10.1016/j.taap.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Vahter M, Akesson A, Lidén C, et al. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Graziano JH, Parvez F, et al. Modification of risk of arsenic-induced skin lesions by sunlight exposure, smoking, and occupational exposures in Bangladesh. Epidemiology. 2006;17(4):459–467. doi: 10.1097/01.ede.0000220554.50837.7f. [DOI] [PubMed] [Google Scholar]
- 38.Lindberg AL, Ekström EC, Nermell B, et al. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res. 2008;106(1):110–120. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Hopenhayn-Rich C, Biggs ML, Smith AH, et al. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect. 1996;104(6):620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurttio P, Komulainen H, Hakala E, et al. Urinary excretion of arsenic species after exposure to arsenic present in drinking water. Arch Environ Contam Toxicol. 1998;34(3):297–305. doi: 10.1007/s002449900321. [DOI] [PubMed] [Google Scholar]
- 41.Tseng CH. Blackfoot disease and arsenic: a never-ending story. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2005;23(1):55–74. doi: 10.1081/GNC-200051860. [DOI] [PubMed] [Google Scholar]
- 42.Huang YK, Huang YL, Hsueh YM, et al. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control. 2008;19(8):829–839. doi: 10.1007/s10552-008-9146-5. [DOI] [PubMed] [Google Scholar]
- 43.Hsueh YM, Chiou HY, Huang YL, et al. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):589–596. [PubMed] [Google Scholar]
- 44.Hsueh YM, Huang YL, Huang CC, et al. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J Toxicol Environ Health A. 1998;54(6):431–444. doi: 10.1080/009841098158728. [DOI] [PubMed] [Google Scholar]
- 45.Yang CY, Chiu HF, Chang CC, et al. Bladder cancer mortality reduction after installation of a tap-water supply system in an arsenious-endemic area in southwestern Taiwan. Environ Res. 2005;98(1):127–132. doi: 10.1016/j.envres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Chang CC, Ho SC, Tsai SS, et al. Ischemic heart disease mortality reduction in an arseniasis-endemic area in southwestern Taiwan after a switch in the tap-water supply system. J Toxicol Environ Health A. 2004;67(17):1353–1361. doi: 10.1080/15287390490471451. [DOI] [PubMed] [Google Scholar]
- 47.Marshall G, Ferreccio C, Yuan Y, et al. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst. 2007;99(12):920–928. doi: 10.1093/jnci/djm004. [DOI] [PubMed] [Google Scholar]