Abstract
The tra-2 gene of the nematode Caenorhabditis elegans encodes a predicted membrane protein, TRA-2A, that promotes XX hermaphrodite development. Genetic analysis suggests that tra-2 is a negative regulator of three genes that are required for male development: fem-1, fem-2, and fem-3. We report that the carboxy-terminal region of TRA-2A interacts specifically with FEM-3 in the yeast two-hybrid system and in vitro. Consistent with the idea that FEM-3 is a target of negative regulation, we find that excess FEM-3 can overcome the feminizing effect of tra-2 and cause widespread masculinization of XX somatic tissues. In turn, we show that the masculinizing effects of excess FEM-3 can be suppressed by overproduction of the carboxy-terminal domain of TRA-2A. A FEM-3 fragment that retains TRA-2A-binding activity can masculinize fem-3(+) animals, but not fem-3 mutants, suggesting that it is possible to release and to activate endogenous FEM-3 by titrating TRA-2A. We propose that TRA-2A prevents male development by interacting directly with FEM-3 and that a balance between the opposing activities of TRA-2A and FEM-3 determines sex-specific cell fates in somatic tissues. When the balance favors FEM-3, it acts through or with the other FEM proteins to promote male cell fates.
Keywords: Sex determination, signal transduction, development, genetics, two hybrid
The nematode Caenorhabditis elegans develops as a self-fertile hermaphrodite if it has two X chromosomes per diploid cell and a male if it has only one. The hermaphrodite is female except that its germ line transiently expresses a male fate, producing a few hundred sperm before switching to oogenesis. Extensive genetic analysis suggests that the ratio of X chromosomes to sets of autosomes (the X/A ratio) determines sex by regulating a genetic cascade of masculinizing and feminizing activities (Fig. 1A; for review, see Meyer 1997; Kuwabara 1999). This paper deals with one of the interactions in the cascade, involving the tra-2 gene and the fem genes.
Figure 1.
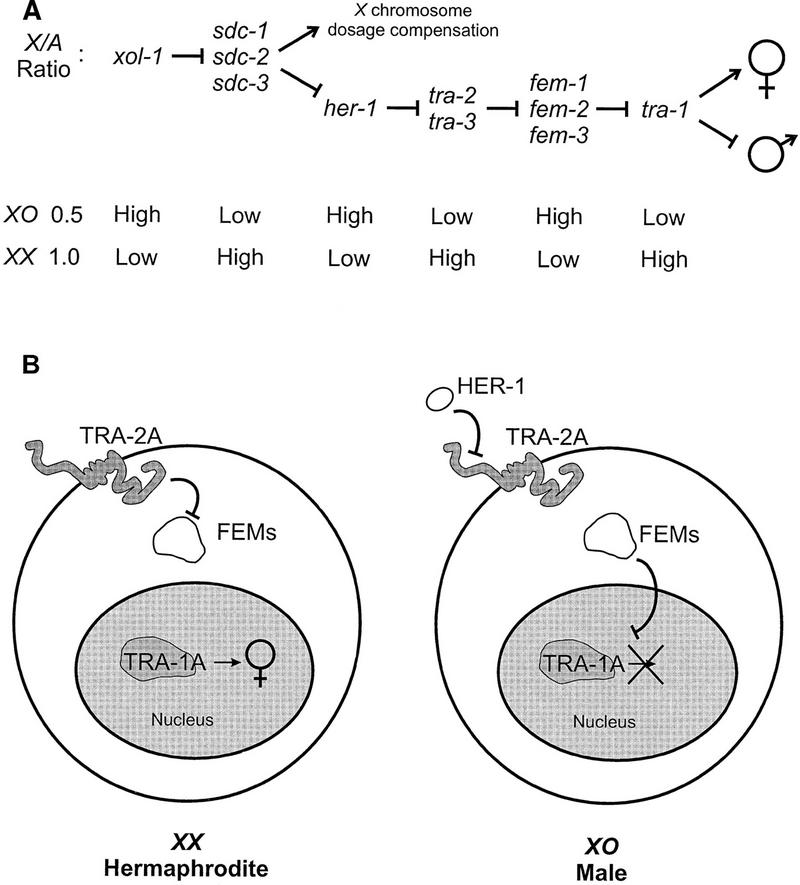
Model of somatic sex determination in C. elegans. (A) Genetic hierarchy regulating somatic sex determination. (Barred lines) Negative interactions; (arrows) positive interactions. The X/A ratio controls both X chromosome dosage compensation and sex determination via xol-1 and the sdc genes. The regulatory pathway branches at the level of the sdc genes and only the branch that controls somatic sex determination is shown. In XX animals, tra-2 negatively regulates the fem genes, allowing tra-1 to promote female development. In XO animals, elevated her-1 activity inhibits tra-2 and permits the fem genes to bring about male development by negatively regulating tra-1. The fem genes have additional targets in the germ line, because they are required for spermatogenesis irrespective of the state of tra-1 (Doniach and Hodgkin 1984; Hodgkin 1986). (B) Molecular model of somatic sex determination. (Left) A high X/A ratio prevents HER-1 synthesis, allowing TRA-2A to inhibit the FEM proteins and TRA-1A to direct female somatic development. Whether the three FEM proteins form a complex is not known. (Right) A low X/A ratio results in the synthesis of HER-1, a small, secreted protein that inactivates the membrane protein TRA-2A, thereby releasing the FEM proteins from negative regulation. The FEM proteins then inhibit the activity of the sequence-specific DNA-binding protein, TRA-1A, by an unknown mechanism to cause male development.
The activity of tra-2 is necessary for female cell fates in XX hermaphrodites (Hodgkin and Brenner 1977), whereas the fem genes are required for male cell fates both in XO males and in the germ line of XX hermaphrodites (Doniach and Hodgkin 1984; Kimble et al. 1984; Hodgkin 1986). Genetic epistasis analysis suggests that tra-2 activity promotes female development by negatively regulating the activity of at least one of the fem genes (Doniach and Hodgkin 1984; Hodgkin 1986). The major feminizing activity of tra-2 resides in a large integral membrane protein known as TRA-2A. Mutations that inactivate TRA-2A masculinize XX hermaphrodites (Kuwabara et al. 1992), and elevated expression of TRA-2A in XO animals is sufficient to transform them into fertile hermaphrodites (Kuwabara and Kimble 1995). The carboxy-terminal domain of TRA-2A, which is predicted to be intracellular, is at least partly responsible for negatively regulating fem activity, because expression of this domain alone in XO males results in partial feminization (Kuwabara and Kimble 1995).
The predicted products of the fem genes are all intracellular proteins. FEM-1 contains ANK repeats, which in many other proteins mediate specific protein–protein interactions, but its sequence is otherwise novel (Spence et al. 1990). FEM-2 is a protein serine/threonine phosphatase of Type 2C (Pilgrim et al. 1995; Chin-Sang and Spence 1996), and it interacts directly with FEM-3 (Chin-Sang and Spence 1996). The sequence of FEM-3 is unrelated to that of any other known protein (Ahringer et al. 1992).
Both genetic and molecular evidence suggest that the fem genes are post-transcriptionally regulated (Doniach and Hodgkin 1984; Hodgkin 1986; Rosenquist and Kimble 1988; Ahringer and Kimble 1991; Ahringer et al. 1992; Gaudet et al. 1996). A simple model (Fig. 1B) is that in XX animals, the carboxy-terminal domain of TRA-2A regulates fem activity by interacting with one or more of the FEM proteins (Kuwabara and Kimble 1995). Such an interaction may prevent the FEM proteins from acting on their targets and so allow female development to proceed. A candidate target of FEM activity in somatic tissues is the product of the tra-1 gene, TRA-1A. It is a sequence-specific DNA-binding protein related to the Gli proteins of vertebrates (Zarkower and Hodgkin 1992, 1993). The activity of tra-1 is sufficient to direct all somatic tissues in C. elegans to adopt female fates (Hodgkin 1987).
In XO animals, the low X/A ratio derepresses transcription of the her-1 gene (Trent et al. 1991). The product of her-1 is a secreted protein, HER-1 (Perry et al. 1993), and the model in Figure 1B proposes that it acts as an inhibitory ligand for TRA-2A (Hunter and Wood 1992; Kuwabara et al. 1992; Perry et al. 1993; Kuwabara 1996b). Freed from the influence of TRA-2A, the FEM proteins bring about male development by directly or indirectly negatively regulating TRA-1A in somatic tissues and activating spermatogenesis in the germ line. Male development requires the phosphatase activity of FEM-2 (Chin-Sang and Spence 1996; Hansen and Pilgrim 1998), but otherwise little is known about the mechanism of action of the FEM proteins. Their effect is to transduce a masculinizing signal from the cell surface to the nucleus, the likely site of action of TRA-1A. In this model, interaction between TRA-2A and one or more of the FEM proteins constitutes a key regulatory switch that determines the output of a novel signal transduction pathway.
We tested the hypothesis that the carboxy-terminal domain of TRA-2A regulates sexual fate by interacting directly with one of the FEM proteins. Here we present evidence that the carboxy-terminal domain of TRA-2A can bind to FEM-3 and that this interaction prevents male development in the somatic tissues of XX animals.
Results
FEM-3 interacts with the carboxy-terminal region of TRA-2A in yeast
We used the yeast two-hybrid system (Fields and Song 1989; Durfee et al. 1993) to test for interactions between the carboxy-terminal domain of TRA-2A and the FEM proteins. A hybrid protein consisting of FEM-3 and the Gal4 DNA-binding domain, when expressed together with a second hybrid containing a carboxy-terminal fragment of TRA-2A and the Gal4 activation domain, activated expression of Gal4-regulated HIS3 and lacZ reporter genes (Fig. 2). Expression of the lacZ reporter was ∼20-fold above background in cells expressing both hybrid proteins. Neither protein stimulated reporter expression when expressed alone or with an unrelated Gal4 hybrid protein. An independent two-hybrid screen of >2,000,000 C. elegans cDNAs substantiated the specificity of the interaction reported here (Chin-Sang and Spence 1996). The screen yielded three FEM-3-interacting clones, one of which encoded the carboxy-terminal region of TRA-2A (I. Chin-Sang and A.M. Spence, unpubl.).
Figure 2.
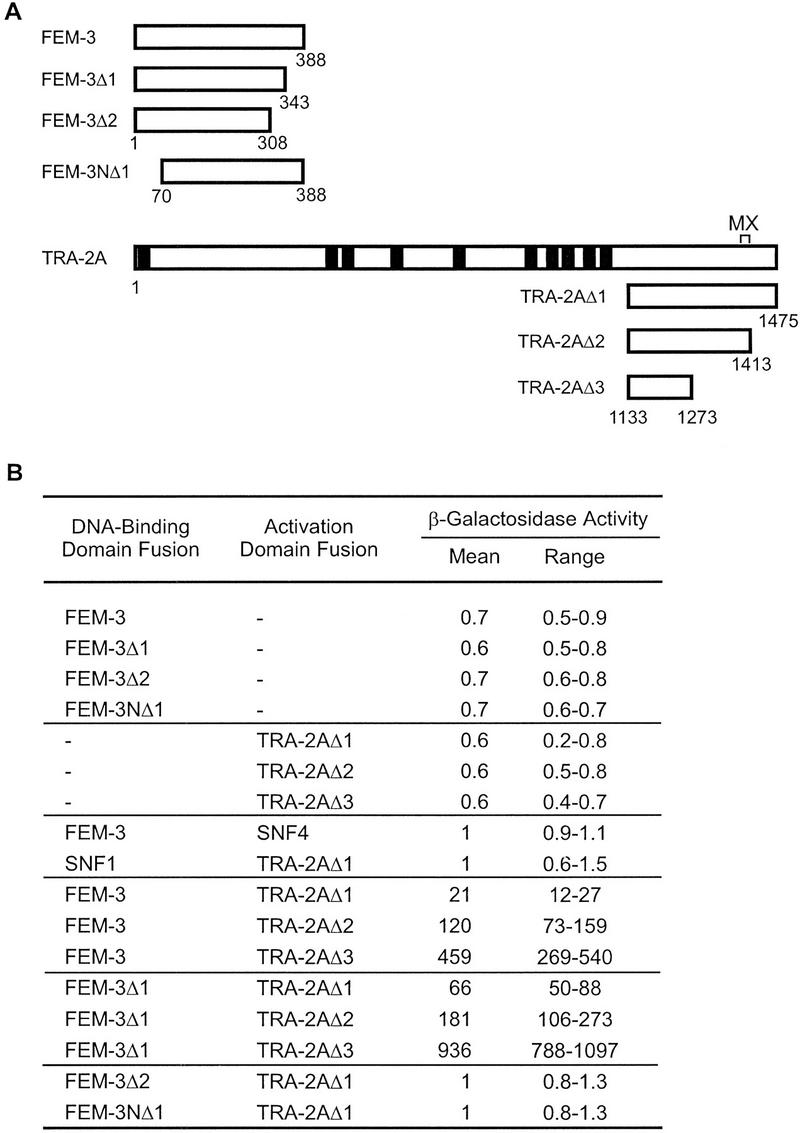
Yeast two-hybrid interaction of FEM-3 and the carboxy-terminal region of TRA-2A. (A) Diagram of fragments of FEM-3 and TRA-2A used in this study. Numbers indicate the amino acids at the termini of each fragment. The predicted signal sequence and transmembrane domains of TRA-2A are indicated as filled rectangles. The MX region is a 22-amino-acid region defined by tra-2 mutations that transform XX hermaphrodites into females but do not affect XO animals. (Kuwabara et al. 1998). (B) Interaction between FEM-3 and TRA-2A fragments in the yeast two-hybrid system. β-Galactosidase activity was measured in liquid cultures of at least four independent transformants. Activity was normalized to the level in cells coexpressing a FEM-3 DNA-binding domain fusion and a hybrid of the Gal4 activation domain and Snf4. Snf4 and Snf1 are yeast proteins that served as negative controls for interactions involving FEM-3 and TRA-2A.
The last 45 amino acids of FEM-3 were dispensable for its interaction with TRA-2A, but deletion of 80 amino acids from the carboxyl terminus, or the removal of 70 amino acids from the amino terminus of FEM-3, abolished the interaction (Fig. 2). Full-length and truncated FEM-3 fusion proteins accumulated to similar levels in yeast (data not shown). Those deletions that disrupted binding to TRA-2A may have affected the proper folding of the binding domain, or they may have eliminated sequences directly involved in the interaction.
Deletion analysis of TRA-2A identified a region of 141 amino acids that interacted with FEM-3 in the two-hybrid system (TRA-2AΔ3; see Fig. 2). The FEM-3-binding domain lies between the last predicted transmembrane domain of TRA-2A and the MX region, a 22-amino-acid region that is implicated in post-translational regulation of tra-2 activity in the germ line (Kuwabara et al. 1998). Interaction between TRA-2AΔ3 and FEM-3 stimulated β-galactosidase expression 450-fold above background (Fig. 2B). This 20-fold increase in reporter activation, compared with the level that resulted from interaction between TRA-2AΔ1 and FEM-3, may reflect an increase in the strength of the interaction, but we cannot exclude other causes (Fields and Sternglanz 1994; Estojak et al. 1995).
Association between FEM-3 and the carboxy-terminal region of TRA-2A in vitro
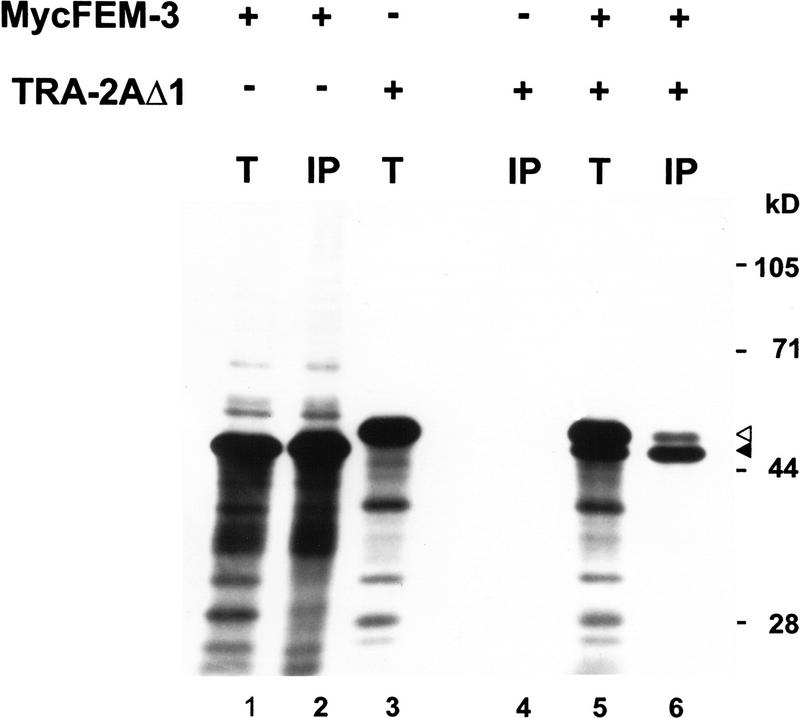
To verify that the carboxy-terminal domain of TRA-2A can associate with FEM-3, we tested for interaction between the two proteins in vitro. We produced a carboxy-terminal fragment of TRA-2A and a Myc epitope-tagged derivative of FEM-3 in reticulocyte lysates and then immunoprecipitated MycFEM-3 using the Myc monoclonal antibody, 9E10. Figure 3 shows that the carboxy-terminal domain of TRA-2A specifically coimmunoprecipitated with MycFEM-3 from reactions containing both proteins.
Figure 3.
Coimmunoprecipitation of the carboxy-terminal region of TRA-2A with MycFEM-3. [35S]Methionine-labeled MycFEM-3 (lanes 1,2), TRA-2AΔ1 (lanes 3,4) or both MycFEM-3 and TRA-2AΔ1 (lanes 5,6) were produced by in vitro translation in reticulocyte lysates. Equivalent samples were analyzed directly by SDS-PAGE and fluorography [(T) lanes 1,3,5] or subjected to immunoprecipitation with anti-Myc mAb 9E10 prior to electrophoresis [(IP) lanes 2,4,6]. The solid arrowhead marks the position of MycFEM-3; the open arrowhead marks the position of TRA-2AΔ1. Numbers at right indicate the positions and the relative molecular mass in kilodaltons of marker proteins.
A heat shock FEM-3 transgene causes male development in fem-3 XO and in XX animals
The tra-2 gene behaves as a negative regulator of the fem genes. If a direct interaction between TRA-2A and FEM-3 were important for preventing male development in XX animals, then the production of excess FEM-3 might overcome the inhibitory effect of TRA-2A and masculinize XX hermaphrodites. To test the effects of overproducing FEM-3 in transgenic nematodes, we placed a cDNA fragment encoding MycFEM-3 under the control of the nematode hsp16-2 heat shock promoter, which directs expression in most somatic tissues (Stringham et al. 1992).
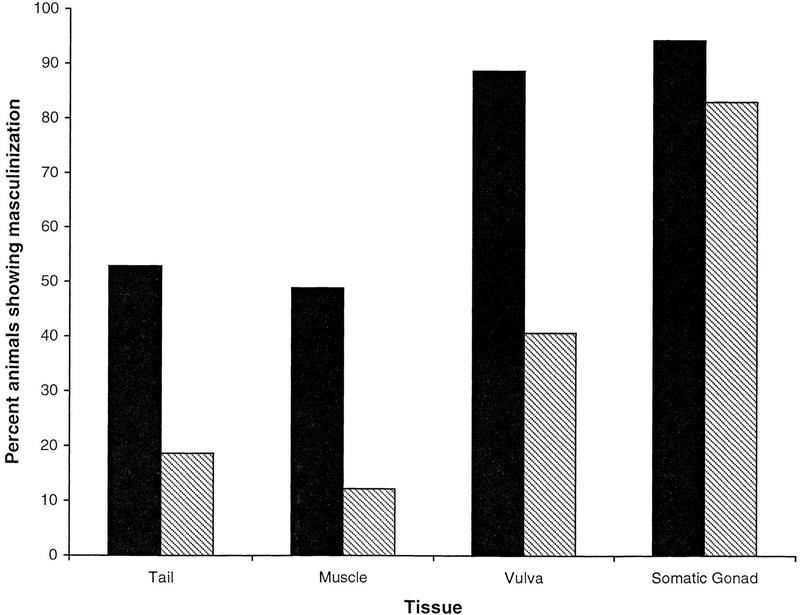
We first tested whether the HS–MycFEM-3 transgene could support male development in XO animals that lacked endogenous fem-3 activity. For these experiments, we used a HS–MycFEM-3 transgene array that had been integrated on the X chromosome (idIs4). The fem-3 allele that we used, e1996, is a putative null allele (Hodgkin 1986; Ahringer et al. 1992). Crossing fem-3; idIs4 females to heterozygous fem-3/+ males produced equal numbers of fem-3 XX and XO progeny that carried the transgene array. In the absence of heat shock, transgenic XX and XO fem-3; idIs4 mutants developed as females. Periodic induction of HS–MycFEM-3 expression throughout development caused partial masculinization in 95% of the animals, suggesting not only that it rescued male development in fem-3 XO mutants, but also that it sexually transformed fem-3 XX animals (Figs. 4, solid bars, and 5). About half of the cross progeny exhibited extensive male development of the tail, the gonad, and the ventral hypodermis. Most of the remaining animals had strongly masculinized gonads but showed few signs of masculinization of the tail. It is likely that the more strongly masculinized animals were of XO karyotype, and the less masculinized animals were XX (see below). We did not observe any effects on germ-line development, which is consistent with earlier reports that the heat shock promoter is poorly expressed in the nematode germ line (Stringham et al. 1992).
Figure 4.
Masculinization of fem-3 XO and XX mutants by HS–MycFEM-3. Animals of genotypes unc-24 fem-3; idIs4/O XO and unc-24 fem-3; idIs4/+ XX (solid bars), or unc-24 fem-3; idIs4/+ XX only (hatched bars) were subjected to repeated heat shocks during development as described in Materials and Methods. No sign of male development was observed in the absence of heat shock treatment.
To specifically examine the effects of HS–MycFEM-3 on XX fem-3 mutants, we crossed fem-3/+; idIs4/O XO males to fem-3 XX females (see Materials and Methods for complete genotypes). Because the idIs4 transgene array was X-linked, only the XX progeny of this cross inherited the array. After periodic induction of HS–MycFEM-3 expression, the transgenic fem-3 XX progeny closely resembled the less strongly masculinized class of animals observed in the previous experiment. Transformations of the tail and sex muscles in fem-3 XX mutants were infrequent, but the somatic gonad exhibited sexual transformation in 80% of transgenic animals (Figs. 4, hatched bars, and 5). This experiment proves that HS–MycFEM-3 expression is sufficient to cause sexual transformation in fem-3 XX animals.
Expression of the HS–MycFEM-3 transgene in otherwise wild-type XX nematodes resulted in strong sexual transformation of the soma: All of the somatic tissues that we examined exhibited some degree of masculinization in 70%–90% of the transgenic animals (Fig. 5; see also Table 1, line 2). Hodgkin (1986) showed that both maternal and zygotic fem-3 activity are necessary for normal male development. We suggest that maternal fem-3(+) in a wild-type genetic background may have enhanced the masculinizing effects of the HS–MycFEM-3 transgene. A similar maternal effect was probably not observed in fem-3 mutants because the HS–MycFEM-3 transgene was unlikely to have been expressed in the germ line.
Figure 5.
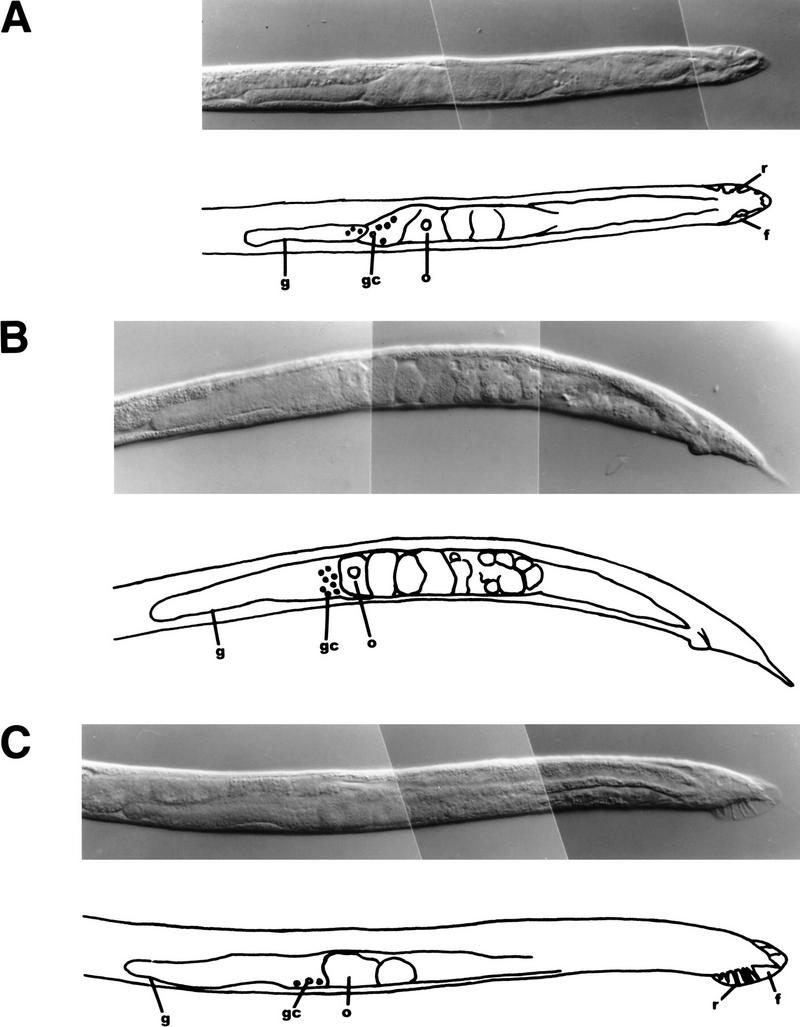
Masculinization of fem-3 and fem-3(+) XX animals by HS–MycFEM-3. (A–C) Differential interference contrast photomicrographs and tracings showing gonad and tail morphology (f) fan; (g) gonad; (gc) immature germ cells; (o) oocyte-like cell; (r) ray. (A)fem-3; idIs4/O animal, presumed XO, showing partial rescue of male development in tail (note incomplete fan with several rays), gonad, ventral hypodermis. (B) fem-3; idIs4/+ XX animal, showing male gonad morphology and absence of vulva. The tail shows little masculinization in this animal, and the germ line is unaffected. (C) fem-3(+); idIs4 XX animal, showing extensive masculinization of the tail, gonad, and ventral hypodermis.
Table 1.
Masculinization of XX animals by a TRA-2Abinding fragment of FEM-3
|
HS–MycFEM-3 varianta
|
No.
|
Percent masculinization of
|
||
|---|---|---|---|---|
|
gonad
|
ventral hypodermis
|
tail
|
||
| Nonebc | 45 | 0 | 0 | 0 |
| FEM-3 | 50 | 100 | 94 | 88 |
| FEM-3Δ1 | 167 | 42 | 23 | 7 |
| FEM-3Δ2c | 194 | 0 | 0 | 0 |
Each transgenic line carried the indicated heat inducible transgene on an extrachromosomal array. Number of independent lines analyzed: vector, 4 lines; FEM-3, 3 lines; FEM-3Δ1, 10 lines; FEM-3Δ2, 9 lines.
The extrachromosomal array carried the heat shock promoter plasmid pPD49.83 with no insert.
One animal had abnormal gonadal morphology, but it was not recognizably masculinized.
Overproduction of FEM-3 was sufficient to direct all somatic tissues in XX animals to adopt male sexual fates. These results suggest that fem-3 activity must be negatively regulated to prevent inappropriate male development in the wild-type XX soma.
Masculinization by HS–FEM-3 requires the activity of fem-1 and fem-2
Male development in C. elegans normally requires the activities of all three fem genes. We tested whether excess, unregulated FEM-3 could bypass the requirement for either FEM-1 or FEM-2 by assaying the effects of the HS–MycFEM-3 transgene in fem-1 and fem-2 mutants. Table 2 shows that HS–MycFEM-3 does not bypass the requirement for fem-1. Transgene expression caused no detectable masculinization in mutants homozygous for the putative null allele fem-1(e1991) (Doniach and Hodgkin 1984).
Table 2.
Masculinization by HS–MycFEM-3 requires fem-1 activity
| Cross: dpy-13/fem-1 unc-24; idIs4 XO × fem-1 unc-24; idIs4 XXa | |||||
|---|---|---|---|---|---|
| Non-Unc F1 | Unc F1 | ||||
| male | intersex | female | male | intersex | female |
| 214 | 171 | 12 | 0 | 3 | 350 |
unc-24 maps ∼1.5 map units from fem-1. Hence, ∼99% of the Unc F1 progeny were homozygous for fem-1.
To test whether fem-2 activity is required for masculinization by HS-MycFEM-3, we assayed the effects of the idIs4 transgene array in mutants homozygous for a putative null allele, fem-2(e2105) (Hodgkin 1986; Pilgrim et al. 1995). We examined the self progeny of maternally rescued, heat-shocked fem-2; him-5; idIs4 homozygotes. The him-5 mutation causes homozygotes to produce ∼30% XO self-progeny (Hodgkin et al. 1979). We observed no sign of masculinization among 30 animals, suggesting that fem-2 activity is also required for HS–MycFEM-3 to promote somatic male development.
Masculinization of XX hermaphrodites by a TRA-2A-binding fragment of FEM-3
A FEM-3 fragment consisting of the first 343 amino acids of the full-length protein, FEM-3Δ1, failed to rescue male development in fem-3 mutants when expressed from the heat shock promoter (data not shown). Because FEM-3Δ1 retained the ability to interact with TRA-2A in yeast (Fig. 2), we tested whether its overproduction might promote male development in wild-type XX nematodes. We reasoned that the inactive FEM-3Δ1 might saturate the available TRA-2A and release active, endogenous FEM-3.
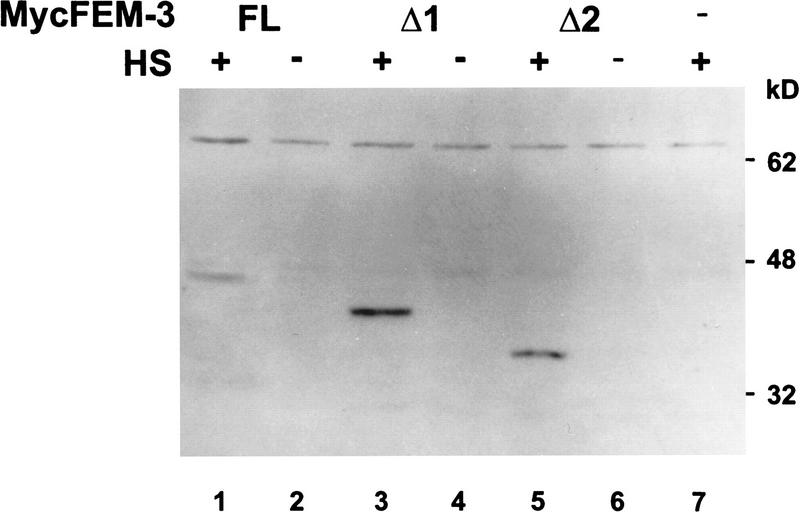
About 40% of the transgenic animals expressing HS–FEM-3Δ1 exhibited significant masculinization of the somatic gonad (Table 1, line 3). In contrast, a smaller FEM-3 fragment, FEM-3Δ2, that failed to interact with TRA-2A in yeast, failed to cause sexual transformation when expressed from the heat shock promoter in transgenic nematodes (Table 1, line 4). Figure 6 shows that both FEM-3Δ1 and FEM-3Δ2 accumulated to similar levels following heat shock.
Figure 6.
Expression of MycFEM-3 and truncated derivatives in transgenic nematodes. Lysates were prepared from animals maintained continuously at 20°C (no heat shock; HS −, lanes 2,4,6) and from animals subjected to heat shock at 33°C followed by a 2-hr recovery period at 20°C (HS +, lanes 1,3,5,7). The lysates were analyzed by SDS-PAGE and immunoblotting with antibody 9E10. Transgenic nematodes carried plasmids encoding MycFEM-3 (FL, lanes 1,2), MycFEM-3Δ1 (Δ1, lanes 3,4), or MycFEM-3Δ2 (Δ2, lanes 5,6), or empty heat shock vector (lane 7). Numbers at right indicate the relative molecular masses in kilodaltons and the positions of migration of molecular mass markers.
The carboxy-terminal domain of TRA-2A suppresses the masculinizing effects of HS–FEM-3
Kuwabara and Kimble (1995) showed that overexpression of the carboxy-terminal domain of TRA-2A partly suppresses male development in wild-type XO animals. Having found that a carboxy-terminal fragment of TRA-2A binds to FEM-3, we tested whether its overproduction might also suppress the masculinizing effects of HS–MycFEM-3 in XX animals. We compared the phenotype of XX animals coexpressing HS–MycFEM-3 and a second transgene, HS–TRA-2AΔ1, that encodes the carboxy-terminal domain of TRA-2A, to that of animals coexpressing the HS–MycFEM-3 transgene with an inactive tra-2 transgene, HS–TRA-2Afs, that carried a frameshift mutation to prevent production of TRA-2AΔ1 (see Materials and Methods).
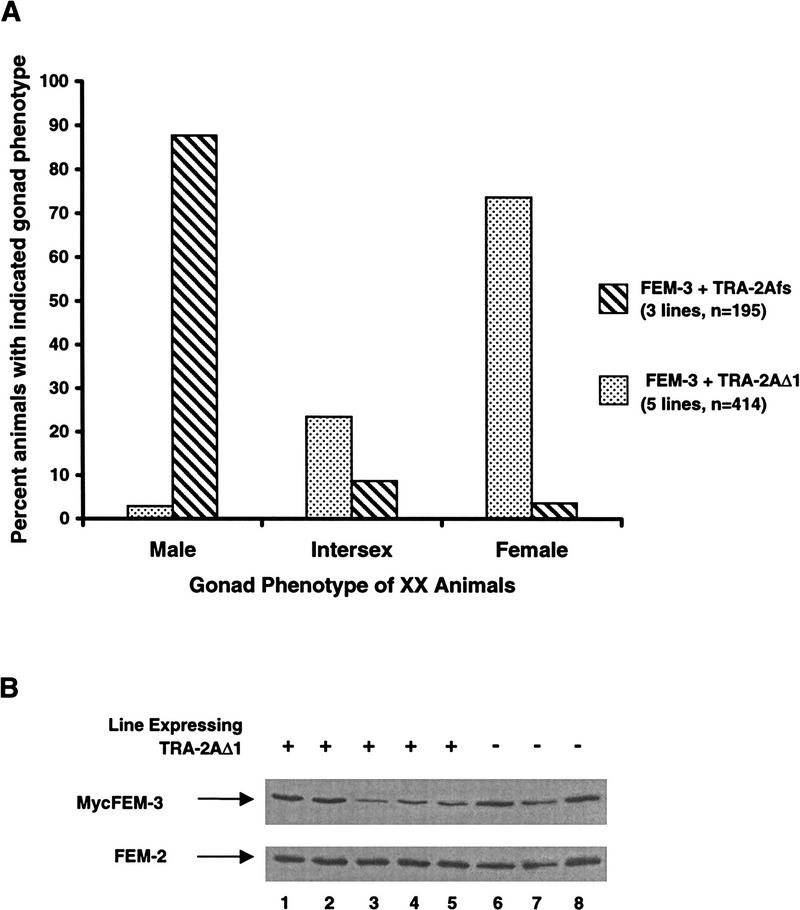
Periodic heat shock caused significant somatic masculinization of XX nematodes carrying HS–MycFEM-3 and HS–TRA-2Afs. As before, the gonad and ventral hypodermis were most strongly affected. The somatic gonad exhibited male morphology in 80%–90% of the transgenic animals in each of three independent strains (Fig. 7A). In contrast, only 3% of the animals from five independent lines (range 0%–9% for individual lines) expressing both HS–FEM-3 and HS–TRA-2AΔ1 had strongly masculinized somatic gonads. A further 23% exhibited incomplete somatic gonad masculinization, but in 74% of the animals, the gonad showed no detectable masculinization (Fig. 7A). The level of expression of MycFEM-3 varied between strains, but strains expressing only MycFEM-3 did not consistently express higher levels than those expressing both MycFEM-3 and TRA-2AΔ1 (Fig. 7B). We conclude that TRA-2AΔ1 inhibited the activity of MycFEM-3. This result suggests that the sexual fate of the somatic tissues in C. elegans depends on a balance between the opposing activities of TRA-2A and FEM-3.
Figure 7.
Suppression of the masculinizing effect of HS–MycFEM-3 by the carboxy-terminal domain of TRA-2A. (A) Masculinization of the gonad in XX animals expressing HS–MycFEM-3 with an inactive TRA-2A construct, HS–TRA-2Afs (hatched bars; three transgenic lines, n = 195) or with HS–TRA-2AΔ1 (stippled bars; five transgenic lines, n = 414). (B) Expression of MycFEM-3 in the transgenic lines used in A. Lysates from lines coexpressing MycFEM-3 and TRA-2AΔ1 (lanes 1–5) or from lines expressing MycFEM-3 and TRA-2Afs (lanes 6–8) were analyzed by SDS-PAGE and immunoblotting with the anti-Myc mAb 9E10 to test whether they contained similar levels of MycFEM-3. An equivalent blot was probed with a FEM-2 antiserum to verify that similar quantities of protein were present in each sample.
Discussion
Because male development in C. elegans requires the activity of all three fem genes (Doniach and Hodgkin 1984; Kimble et al. 1984; Hodgkin 1986), negative regulation of one is sufficient to prevent the others from acting. Previous genetic analysis has shown that tra-2 is the principal negative regulator of fem activity (Doniach and Hodgkin 1984; Hodgkin 1986; Kuwabara and Kimble 1995). Our results suggest that this regulation is achieved at the level of protein–protein interactions: TRA-2A negatively regulates FEM activity by binding directly to FEM-3.
Limiting FEM-3 activity prevents male development in the XX soma
The ability of the HS–FEM-3 transgene to cause sexual transformation of somatic tissues in XX hermaphrodites establishes that fem-3 acts as a developmental switch in the soma. In the absence of fem-3 activity, male development cannot occur, whereas elevated fem-3 activity is sufficient to cause male somatic development in an XX animal that would otherwise develop as a hermaphrodite. Overexpression of either fem-2 or fem-1, in contrast, fails to masculinize the XX soma (Chin-Sang and Spence 1996; J. Gaudet and A.M. Spence, unpubl.). These observations imply that the activity of fem-3 must ordinarily be limited to prevent XX somatic tissues from adopting male fates. Since both fem-1 and fem-2 are not only available, but essential for HS–FEM-3 to masculinize the XX soma, it appears that limiting fem-3 activity is normally the key step in preventing XX somatic tissues from adopting male fates. It is unlikely that negative regulation of FEM-3 activity is achieved via regulation of either of the other FEM proteins.
Interaction with TRA-2A limits FEM-3 activity
The observation that the carboxy-terminal region of TRA-2A interacts specifically with FEM-3 in the yeast two-hybrid system and in vitro supports the conclusion that FEM-3 is the direct regulatory target of TRA-2A. Interaction between the two proteins apparently does not require other nematode proteins. We were not able to detect interactions between the carboxy-terminal portion of TRA-2A and either of the other FEM proteins in the yeast two-hybrid system (A. Mehra, I. Chin-Sang, D. Lum, U. Vivegananthan, and A.M. Spence, unpubl.).
Two other observations favor the conclusion that TRA-2A regulates fem activity by binding to FEM-3. First, the FEM-3 fragment, FEM-3Δ1, masculinized the XX soma in a manner dependent on endogenous fem-3(+). This effect is most simply explained by proposing that FEM-3Δ1 titrated a negative regulator of endogenous FEM-3. Because FEM-3Δ1 retained TRA-2A-binding activity, and since a shorter FEM-3 fragment, FEM-3Δ2, that lacked TRA-2A-binding activity in yeast failed to masculinize transgenic nematodes, we suggest that the negative regulator in question was TRA-2A. The dependence of FEM-3Δ1 on endogenous FEM-3 for its masculinizing activity probably explains why its effects were significantly weaker than those resulting from overexpression of full-length FEM-3: The somatic tissues of XX hermaphrodites accumulate only low levels of fem-3 transcripts (Rosenquist and Kimble 1988), suggesting that the abundance of FEM-3 is likely to be low. Full-length FEM-3 also interacts with FEM-2 (Chin-Sang and Spence 1996), but FEM-3Δ1 failed to interact with FEM-2 in the yeast two-hybrid system (I. Chin-Sang and A.M. Spence, unpubl.), supporting our contention that its masculinizing activity arose entirely from its ability to titrate TRA-2A and liberate endogenous FEM-3.
Second, overexpression of the carboxy-terminal fragment of TRA-2A suppressed the masculinizing effects of excess FEM-3, suggesting that sexual fate in somatic tissues depends on a balance between the activities of FEM-3 and the carboxy-terminal region of TRA-2A. We suggest that the direct interaction of TRA-2A with FEM-3 provides a mechanism for measuring the relative levels of the two opposing activities.
Regulation of TRA-2A activity in XO males
How is TRA-2A activity regulated in XO animals to allow male development? The model of sex determination presented in Figure 1A suggests that in XO animals the secreted protein HER-1 inhibits TRA-2A and allows the FEM proteins to promote male development. One possibility is that HER-1 binding induces a conformational change in the carboxy-terminal domain of TRA-2A that disrupts the interaction between TRA-2A and FEM-3. Alternatively, HER-1 might instead destabilize TRA-2A, reducing its steady-state level and shifting the balance in favor of the masculinizing activity of FEM-3. It will be of great interest to determine whether HER-1 directly affects the affinity of the interaction between TRA-2A and FEM-3.
Significance of TRA-2/FEM-3 interaction for germ-line sex determination
Although our experiments focus on the role of the TRA-2A/FEM-3 interaction in somatic tissues, the interaction is likely also to be important in the germ line. First, the loss of tra-2 activity causes fem-dependent masculinization of both the soma and the germ line of XX animals (Hodgkin and Brenner 1977). Second, the germ line expresses both the mRNA encoding TRA-2A and a germ-line-specific tra-2 transcript encoding a protein known as TRA-2B (Okkema and Kimble 1991; Kuwabara et al. 1998). TRA-2B is identical to the cytoplasmic domain of TRA-2A (Kuwabara et al. 1998) and therefore may be expected to interact with FEM-3. Third, the sexual fate of the germ line is governed by a balance between the activities of tra-2 and fem-3 (Barton et al. 1987; Schedl and Kimble 1988). Both tra-2 and fem-3 are subject to post-transcriptional repression, mediated by their 3′ untranslated regions (3′ UTR), in the germ line (Doniach 1986; Barton et al. 1987; Ahringer and Kimble 1991; Goodwin et al. 1993). We suggest that the protein–protein interaction between TRA-2A (or TRA-2B) and FEM-3 that we have described provides a second level of control over fem-3 activity in the XX germ line. Whereas 3′ UTR-mediated repression presumably controls the levels of TRA-2A, TRA-2B, and FEM-3 in the germ line, the TRA-2(A or B)/FEM-3 interaction may serve to measure the relative levels of the proteins. Their relative levels in turn determine the sexual fate of the germ line, excess FEM-3 triggering spermatogenesis, and an excess of TRA-2 proteins resulting in oogenesis (see Gallegos et al. 1998 for further discussion).
Mechanism of inhibition of FEM-3 by TRA-2A
How does the binding of TRA-2A to FEM-3 prevent adoption of male fates in XX animals? A simple hypothesis is that the interaction sequesters FEM-3 and prevents it from gaining access to its targets or cofactors. Because TRA-2A is predicted to be a transmembrane protein (Kuwabara et al. 1992), one might expect the interaction between TRA-2A and FEM-3 to occur in vivo at the inner surface of the cell membrane. However, membrane localization of the FEM-3-binding domain of TRA-2A is probably not strictly essential for FEM-3 regulation. In our experiments, a carboxy-terminal fragment of TRA-2A that lacks apparent transmembrane sequences was nevertheless capable of suppressing the effects of HS-FEM-3. Similarly, Kuwabara and Kimble (1995) showed that overexpression of the carboxy-terminal domain of TRA-2A feminizes tra-2 XX pseudomales and tra-2(+) XO males.
FEM-3 interacts with FEM-2 (Chin-Sang and Spence 1996), and their interaction is likely to be required for male development. TRA-2A might regulate FEM-3 by preventing its interaction with FEM-2, either by direct competition or through allosteric effects. Alternatively, TRA-2A might bind to the FEM-3–FEM-2 complex and prevent its activation or its correct targeting.
Sequence requirements for the TRA-2A/FEM-3 interaction
Although the carboxy-terminal 45 amino acids of FEM-3 are dispensable for its interaction with TRA-2A, further deletion analysis failed to define a TRA-2A-binding domain in FEM-3 (Fig. 2; data not shown). Mutations in fem-3 that selectively interfere with the TRA-2A/FEM-3 interaction would be expected to cause constitutive activation of FEM-3 and inappropriate masculinization of somatic tissues in XX animals. No fem-3 gain-of-function mutations with these properties have been isolated. Such mutations might be expected to cause dominant sterility in XX animals; if so, their phenotype would have precluded their recovery in the screens performed to date. It is also possible that fem-3 mutations that disrupt TRA-2A binding inevitably compromise other aspects of FEM-3 function, such as binding to FEM-2, and result in a loss of function.
A 141-residue fragment of TRA-2A is sufficient for interaction with FEM-3 in the yeast two-hybrid system. Mutations that affect the minimal FEM-3-binding region have not yet been described. If such mutations interfered with FEM-3-binding, we would expect them to reduce tra-2 activity. Mutations have been identified that truncate TRA-2A carboxy-terminal to the FEM-3-binding region, and they reduce tra-2 activity in somatic tissues (Kuwabara et al. 1992). Their effects might be at least partly attributable to reduced stability of the mRNAs, or of the truncated proteins themselves. As well, sequences carboxy-terminal to the minimal FEM-3-binding region may affect the affinity of the interaction in vivo.
Evolutionary divergence of the FEM-3-binding region in TRA-2A
The sequence of TRA-2A from C. elegans has diverged extensively from its counterpart in the closely related nematode Caenorhabditis briggsae (Cb-TRA-2A). The predicted TRA-2A proteins from the two species exhibit only 43% amino acid identity, although the level of conservation is significantly higher in the region surrounding a potential site of HER-1 interaction known as the enhanced gain-of-function (EG) site (Kuwabara 1996a). In marked contrast to the region around the EG site, the minimal FEM-3-binding region identified in this study is particularly divergent, with only 20% identity between the two proteins and a 16-amino-acid insertion in Cb-TRA-2A. C. briggsae develops as either a male or a self-fertile hermaphrodite, as does C. elegans, and RNA interference experiments suggest that Cb-TRA-2A is required for hermaphrodite development in C. briggsae, as is Ce-TRA-2A in C. elegans (Kuwabara 1996a). The rapid sequence divergence of the carboxy-terminal region of TRA-2A during evolution may conceal a higher level of structural conservation; alternatively, it may suggest a similarly rapid divergence of the target or mechanism of action of TRA-2A. No FEM-3 homolog from C. briggsae has yet been described, but it will be interesting to investigate whether a TRA-2A/FEM-3 interaction regulates sexual fate in C. briggsae, as it does in C. elegans.
Materials and methods
C. elegans strains and culture methods
Standard methods for culture and manipulation of nematodes were used (Brenner 1974; Sulston and Hodgkin 1988). MYOB medium (Church et al. 1995) was used in place of NGM (nematode growth medium) in some experiments. The incubation temperature was 20°C unless otherwise noted. We used the standard laboratory wild-type strain of C. elegans var. Bristol, N2. All of the other strains used in this work are derivatives of N2. Following are the mutant alleles used in this study; the affected genes are described in Hodgkin (1997): LG III fem-2(e2105); LG IV dpy-13(e184), fem-1(e1991), mor-2(e1125), unc-24(e138), fem-3(e1996), dpy-20(e1282ts); and LG V him-5(e1490).
Plasmids
Standard molecular biological methods were used (Sambrook et al. 1989). Plasmids and details of their construction are available on request. J. Kimble (University of Wisconsin, Madison, WI) kindly provided a fem-3 cDNA in plasmid pJK453. Ligation of appropriate restriction fragments of pJK453 into pAS1 (Durfee et al. 1993) produced plasmids AS#1197, AS#1278, AS#1279, and AS#1283, which respectively encode FEM-3, FEM-3Δ2, FEM-3Δ1, and FEM-3NΔ1 as Gal4 DNA-binding domain fusion proteins. Full-length FEM-3 is 388 amino acids long: FEM-3Δ1 includes amino acids 1–343, FEM-3Δ2 includes amino acids 1–308, and FEM-3NΔ1 includes amino acids 70–388.
Plasmid AS#1184 is fem-3 cDNA in T7plinkTag (Bardwell and Treisman 1994), and it encodes FEM-3 with an amino-terminal Myc epitope tag (MycFEM-3).
The HS–MycFEM-3 plasmid AS#1196 was produced by subcloning the insert of plasmid AS#1184 into the C. elegans heat shock vector pPD49.83 (Mello and Fire 1995). Plasmids AS#1230 and AS#1231 are derivatives of AS#1196 that direct the expression of MycFEM-3Δ2 and MycFEM-3Δ1, respectively.
Plasmid pPK126 consists of an EcoRI fragment of tra-2 cDNA, encoding amino acids 1133–1475 of TRA-2A (referred to here as TRA-2AΔ1), in the vector T7plink (Bardwell and Treisman 1994).
Insertion of the NcoI–XhoI fragment from pPK126 into pACTII (Durfee et al. 1993, S. Elledge, pers. comm.) yielded plasmid AS#1191, encoding TRA-2AΔ1 fused to the activation domain of Gal4. Plasmids pPK165 and pPK166, respectively, encode TRA-2AΔ2 and TRA-2AΔ3 as Gal4 activation domain fusion proteins. TRA-2AΔ2 includes amino acids 1133–1413 of TRA-2A, and TRA-2AΔ3 includes amino acids 1133–1273.
The HS–TRA-2AΔ1 plasmid AS#1241 carries the NcoI–XhoI fragment from pPK126 in vector pPD49.83. Plasmid AS#1242, referred to here as HS–TRA-2Afs, differs only in that it carries a frameshift mutation in the polylinker immediately preceding the tra-2 cDNA insert, to prevent expression of TRA-2AΔ1.
Plasmid pSE1111, encoding a hybrid of the Gal4 activation domain and the yeast protein Snf4, and pSE1112, which encodes a Gal4 DNA-binding domain–Snf1 hybrid (Durfee et al. 1993), respectively served as negative controls in yeast two-hybrid tests with FEM-3 and TRA-2A derivatives.
Yeast two-hybrid tests
Yeast was cultured on standard complete and synthetic medium at 30°C (Sherman 1991). Transformation was performed by the methods of Schiestl and Gietz (1989). Two-hybrid tests were carried out in strain Y153 as described (Durfee et al. 1993). Standard methods were used to assay Β-galactosidase activity (Ausubel et al. 1989).
Immunoprecipitations
[35S]-Methionine-labeled MycFEM-3 and TRA-2AΔ1 were produced by coupled in vitro transcription and translation of AS#1184 and pPK126 in a TNT lysate (Promega). Immunoprecipitations with monoclonal antibody 9E10 (Evan et al. 1985) were carried out as described previously (Chin-Sang and Spence 1996). In some experiments, protein A–Sepharose beads (Pharmacia) were incubated with rabbit anti-mouse IgG antiserum (Jackson Laboratories) prior to use. Immunoprecipitates were analyzed by SDS-PAGE and fluorography.
Western blotting
Gal4 fusion proteins encoded by plasmids derived from pAS1 and pACTII carry the HA epitope tag recognized by mAb 12CA5 (Wilson et al. 1984). Their expression was verified by Western analysis of yeast lysates, prepared as in Ausubel et al. (1989). Western analysis was performed as described (Gaudet et al. 1996) with 12CA5 and an alkaline phosphatase-conjugated goat anti-mouse secondary antibody (GIBCO).
To assay the expression of MycFEM-3 and its truncated derivatives in nematodes after heat shock, a sample of young larvae of each transgenic line to be tested was distributed to two plates. After 3 days of incubation at 20°C, one plate in each pair was subjected to two heat shocks of 1 hr each at 33°C, separated by a rest interval of 20 min at 20°C. After the second heat shock, animals were allowed to recover at 20°C for 3 hr before harvesting. The second plate in each pair remained at 20°C until the time of harvesting. Harvesting, lysis, and Western analysis with mAb 9E10 were carried out as described (Chin-Sang and Spence 1996).
Expression of MycFEM-3 in transgenic strains coexpressing HS–MycFEM-3 and either HS–TRA-2AΔ1 or HS–TRA-2Afs was similarly assayed by Western blotting with mAb 9E10. Duplicate blots were assayed with a polyclonal anti-FEM-2 antiserum (J. Gaudet, unpubl.) to verify equal loading of the lysates.
Nematode transformation
Nematode transformation was carried out as described by Mello et al. (1991). The plasmid pRF4, carrying the rol-6(su1006) allele, served as a transformation marker: It confers a dominant Roller (Rol) phenotype. The mixture of DNA injected included pRF4 (50 μg/ml), the plasmid(s) to be tested (10 μg/ml, or in some experiments, 50 μg/ml), and pBluescript (Stratagene) as needed to adjust the total DNA concentration to 100 μg/ml. Lines carrying heritable extrachromosomal arrays were established from among the Rol F2 progeny of injected animals. At least two independent extrachromosomal arrays were tested for each fem-3 construct at a given concentration.
To produce the integrated HS–MycFEM-3 array, idIs4, animals carrying plasmid AS#1196 on an extrachromosomal array were γ-irradiated with 3600 rads from a 137Cs source. F2 Rol animals were screened for individuals that transmitted the array to all of their progeny (Mello and Fire 1995). Crosses between idIs4 Rol males and non-Rol hermaphrodites produced broods consisting entirely of non-Rol males and Rol hermaphrodites, suggesting that idIs4 was X-linked.
Heat shock experiments
Adult transgenic hermaphrodites or mated females were allowed to lay eggs for 2–16 hr and were then removed. Their progeny were subjected to periodic heat shock throughout development, beginning in late embryogenesis or early larval development. In most experiments, heat shocks were administered at 18-hr intervals, and each consisted of two 1-hr periods at 33°C separated by a 20-min period at 20°C. In the experiment shown in Figure 7, animals were heat-shocked at 33°C for 2 hr at 12-hr intervals. We examined Rol animals for signs of masculinization when they reached adulthood. In most experiments, masculinization of various tissues was assessed by differential interference contrast microscopy at a magnification of 400×. Sex muscles were scored by polarized light microscopy. The following features were taken as indicative of masculinization: an asymmetric, unilobed gonad, absence of a vulva or presence of a nonfunctional pseudovulva, presence of a tail fan, rays, or spicules, presence of sex muscles in the tail region. In some experiments, as noted, animals were scored as either female or masculinized intersexes following examination in a dissecting microscope at a magnification of 50X.
HS–MycFEM-3 in fem-3 XX animals
Roller males of genotype unc-24 fem-3/dpy-20; idIs4 were crossed to unc-24 fem-3 dpy-20 females. The Unc Rol progeny (XX animals of genotype unc-24 fem-3 dpy-20/unc-24 fem-3; idIs4/+) were scored for masculinization. In this cross and the following one, Unc recombinants heterozygous for fem-3 could be excluded because they were also Dpy.
HS–MycFEM-3 in fem-3 XX and XO animals
Males of genotype unc-24 fem-3/dpy-20 were crossed to unc-24 fem-3 dpy-20;idIs4 females. Unc Rol progeny (unc-24 fem-3 dpy-20/unc-24 fem-3; idIs4/+ XX and unc-24 fem-3 dpy-20/unc-24 fem-3; idIs4/O XO) were scored for masculinization.
HS–MycFEM-3 in fem-1 XX and XO animals
Males of genotype dpy-13/fem-1 unc-24; idIs4 were crossed to fem-1 unc-24; idIs4 females. All progeny were scored for somatic sexual phenotype with the dissecting microscope.
HS–MycFEM-3 in fem-2 XX and XO animals
Males of genotype him-5/+; idIs4 were crossed to fem-2; him-5 females. F1 Rol animals were picked to separate plates, and him-5 homozygotes were identified as those that segregated male F2 self-progeny. Several him-5 Rol hermaphrodite F2 animals were picked as L4 larvae to separate plates and were transferred to new plates daily. The first plate was incubated at 15°C, and subsequent plates were incubated at 25°C and subjected to heat shock treatment as described above. Among the F2 progeny that were homozygous for idIs4, those also homozygous for fem-2 were identified as those that produced intersexual XO F3 progeny at 15°C. Their heat shock-treated progeny were scored for masculinization.
Acknowledgments
We thank J. Kimble for generously providing a fem-3 cDNA clone, and we are grateful to Brenda Andrews and Marc Perry for critical reading of the manuscript. This research was supported by a grant to A.M.S. from the Medical Research Council of Canada.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL andrew.spence@utoronto.ca; FAX (416) 978-6885.
References
- Ahringer J, Kimble J. Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3′ untranslated region. Nature. 1991;349:346–348. doi: 10.1038/349346a0. [DOI] [PubMed] [Google Scholar]
- Ahringer J, Rosenquist TA, Lawson DN, Kimble J. The Caenorhabditis elegans sex determining gene fem-3 is regulated post-transcriptionally. EMBO J. 1992;11:2303–2310. doi: 10.1002/j.1460-2075.1992.tb05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Vol. 2. New York, NY: Wiley Interscience; 1989. [Google Scholar]
- Bardwell VJ, Treisman R. The POZ domain: A conserved protein–protein interaction motif. Genes & Dev. 1994;8:1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- Barton MK, Schedl TB, Kimble J. Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics. 1987;115:107–119. doi: 10.1093/genetics/115.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Sang ID, Spence AM. Caenorhabditis elegans sex-determining protein FEM-2 is a protein phosphatase that promotes male development and interacts directly with FEM-3. Genes & Dev. 1996;10:2314–2325. doi: 10.1101/gad.10.18.2314. [DOI] [PubMed] [Google Scholar]
- Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–2535. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- Doniach T. Activity of the sex-determining gene tra-2 is modulated to allow spermatogenesis in the C. elegans hermaphrodite. Genetics. 1986;114:53–76. doi: 10.1093/genetics/114.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniach T, Hodgkin J. A sex-determining gene, fem-1, required for both male and hermaphrodite development in Caenorhabditis elegans. Dev Biol. 1984;106:223–235. doi: 10.1016/0012-1606(84)90077-0. [DOI] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen PL, Yeh SH, Yang YZ, Kilburn AE, Lee WH, Elledge SJ. The retinoblastoma protein associates with the protein phosphatase type-1 catalytic subunit. Genes & Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Estojak J, Brent R, Golemis EA. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Fields S, Sternglanz R. The two-hybrid system: An assay for protein-protein interactions. Trends Genet. 1994;10:286–292. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- Gallegos M, Ahringer J, Crittenden S, Kimble J. Repression by the 3′ UTR of fem-3, a sex-determining gene, relies on a ubiquitous mog-dependent control in Caenorhabditis elegans. EMBO J. 1998;17:6337–6347. doi: 10.1093/emboj/17.21.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet J, VanderElst I, Spence AM. Post-transcriptional regulation of sex determination in Caenorhabditis elegans: Widespread expression of the sex-determining gene fem-1 in both sexes. Mol Biol Cell. 1996;7:1107–1121. doi: 10.1091/mbc.7.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin EB, Okkema PG, Evans TC, Kimble J. Translational regulation of tra-2 by its 3′ untranslated region controls sexual identity in C. elegans. Cell. 1993;75:329–339. doi: 10.1016/0092-8674(93)80074-o. [DOI] [PubMed] [Google Scholar]
- Hansen D, Pilgrim D. Molecular evolution of a sex determination protein: FEM-2 (PP2C) in Caenorhabditis. Genetics. 1998;149:1353–1362. doi: 10.1093/genetics/149.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. Sex determination in the nematode C. elegans: Analysis of tra-3 suppressors and characterization of fem genes. Genetics. 1986;114:15–52. doi: 10.1093/genetics/114.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— A genetic analysis of the sex-determining gene, tra-1, in the nematode Caenorhabditis elegans. Genes & Dev. 1987;1:731–745. doi: 10.1101/gad.1.7.731. [DOI] [PubMed] [Google Scholar]
- ————— . C. elegans II (ed. D.L. Riddle, T. Blumenthal, B.J. Meyer, and J.R. Priess), pp. 881–1047. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1997. Genetics. [PubMed] [Google Scholar]
- Hodgkin J, Brenner S. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics. 1977;86:275–287. [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CP, Wood WB. Evidence from mosaic analysis of the masculinizing gene her-1 for cell interactions in C. elegans sex determination. Nature. 1992;355:551–555. doi: 10.1038/355551a0. [DOI] [PubMed] [Google Scholar]
- Kimble J, Edgar L, Hirsh D. Specification of male development in Caenorhabditis elegans: The fem genes. Dev Biol. 1984;105:234–239. doi: 10.1016/0012-1606(84)90279-3. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE. Interspecies comparison reveals evolution of control regions in the nematode sex-determining gene tra-2. Genetics. 1996a;144:597–607. doi: 10.1093/genetics/144.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— A novel regulatory mutation in the C. elegans sex determination gene tra-2 defines a candidate ligand/receptor interaction site. Development. 1996b;122:2089–2098. doi: 10.1242/dev.122.7.2089. [DOI] [PubMed] [Google Scholar]
- ————— Developmental genetics of Caenorhabditis elegans sex determination. Curr Top Dev Biol. 1999;41:99–132. doi: 10.1016/s0070-2153(08)60271-9. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE, Kimble J. A predicted membrane protein, TRA-2A, directs hermaphrodite development in Caenorhabditis elegans. Development. 1995;121:2995–3004. doi: 10.1242/dev.121.9.2995. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE, Okkema PG, Kimble J. tra-2 encodes a membrane protein and may mediate cell communication in the Caenorhabditis elegans sex determination pathway. Mol Biol Cell. 1992;3:461–473. doi: 10.1091/mbc.3.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara PE, Okkema PG, Kimble J. Germ-line regulation of the Caenorhabditis elegans sex-determining gene tra-2. Dev Biol. 1998;204:251–262. doi: 10.1006/dbio.1998.9062. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA Transformation. In: Epstein HF, Shakes DC, editors. Methods in cell biology: Caenorhabditis elegans: Modern biological analysis of an organism. San Diego, CA: Academic Press; 1995. pp. 451–482. [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BJ. Sex determination and X chromosome dosage compensation. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 209–240. [PubMed] [Google Scholar]
- Okkema PG, Kimble J. Molecular analysis of tra-2, a sex determining gene in C. elegans. EMBO J. 1991;10:171–176. doi: 10.1002/j.1460-2075.1991.tb07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MD, Li WQ, Trent C, Robertson B, Fire A, Hageman JM, Wood WB. Molecular characterization of the her-1 gene suggests a direct role in cell signaling during Caenorhabditis elegans sex determination. Genes & Dev. 1993;7:216–228. doi: 10.1101/gad.7.2.216. [DOI] [PubMed] [Google Scholar]
- Pilgrim D, McGregor A, Jackle P, Johnson T, Hansen D. The C. elegans sex-determining gene fem-2 encodes a putative protein phosphatase. Mol Biol Cell. 1995;6:1159–1171. doi: 10.1091/mbc.6.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist TA, Kimble J. Molecular cloning and transcript analysis of fem-3, a sex-determination gene in Caenorhabditis elegans. Genes & Dev. 1988;2:606–616. doi: 10.1101/gad.2.5.606. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Spence AM, Coulson A, Hodgkin J. The product of fem-1, a nematode sex-determining gene, contains a motif found in cell cycle control proteins and receptors for cell-cell interactions. Cell. 1990;60:981–990. doi: 10.1016/0092-8674(90)90346-g. [DOI] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EPM. Temporal and spatial expression patterns of the small heat shock (hsp-16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Hodgkin JA. Methods. In: Wood WB, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. pp. 587–606. [Google Scholar]
- Trent C, Purnell B, Gavinski S, Hageman J, Chamblin C, Wood WB. Sex-specific transcriptional regulation of the C. elegans sex-determining gene her-1. Mech Dev. 1991;34:43–56. doi: 10.1016/0925-4773(91)90090-s. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolly ML, Lerner RA. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Zarkower D, Hodgkin J. Molecular analysis of the C. elegans sex-determining gene tra-1: A gene encoding two zinc finger proteins. Cell. 1992;70:237–249. doi: 10.1016/0092-8674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- ————— Zinc fingers in sex determination: Only one of the two C. elegans Tra-1 proteins binds DNA in vitro. Nucleic Acid Res. 1993;21:3691–3698. doi: 10.1093/nar/21.16.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]