Abstract
Background
Dermatomyositis (DM) is a multisystem autoimmune disease, in which serologic evidence of immune responses to disease-specific antigenic targets is found in approximately 50% to 70% of patients. Recently, melanoma differentiation-associated gene 5 (MDA5) has been identified as a DM-specific autoantigen that appears to be targeted in patients with DM and mild or absent muscle inflammation and with an increased risk of interstitial lung disease.
Objective
We wished to understand the role of MDA5 in DM skin inflammation by testing it to determine if a specific cutaneous phenotype is associated with MDA5 reactivity.
Methods
We retrospectively screened plasma from 77 patients with DM in the outpatient clinics at the Stanford University Department of Dermatology in California.
Results
We found that 10 (13%) patients had circulating anti-MDA5 antibodies, and had a characteristic cutaneous phenotype consisting of skin ulceration, tender palmar papules, or both. Typical areas of skin ulceration included the lateral nailfolds, Gottron papules, and elbows. Biopsy specimens of the palmar papules showed a vasculopathy characterized by vascular fibrin deposition with variable perivascular inflammation. Patients with anti-MDA5 antibodies also had an increased risk of oral pain and/or ulceration, hand swelling, arthritis/arthralgia, and diffuse hair loss. Consistent with previous reports, these patients had little or no myositis and had increased risk of interstitial lung disease.
Limitations
This study was conducted at a tertiary referral center. Multiple associations with MDA5 antibodies were tested retrospectively on a relatively small cohort of 10 anti-MDA5-positive patients.
Conclusion
We suggest that MDA5 reactivity in DM characterizes a patient population with severe vasculopathy.
Keywords: autoantibodies, clinically amyopathic dermatomyositis antibody, 140 kd (CADM-140) peptide, dermatomyositis, human, interferon-induced helicase 1 protein, interstitial, lung diseases, phenotype, ulcer
Dermatomyositis (DM) is a systemic disease characterized by chronic inflammation in the skin and muscle. Tissue destruction and injury is likely the result of an autoimmune response, as circulating, myositis-specific autoantibodies are found in 50% to 70% of patients with DM.1 In addition, many of the targets of these autoantibodies are specifically overexpressed and/or modified in muscle and lung tissue of patients with DM and thus available for immune recognition.2,3 Direct evidence for an autoimmune cause for DM skin disease, however, is lacking. Although DM skin biopsy specimens demonstrate evidence of keratinocyte injury and death along with CD4 and CD8+ lymphocyte inflammation, a direct, antigen-driven cytotoxic response has not been shown.4–6
Further evidence for the relevance of the autoimmune responses in DM has emerged with the discovery that serologic responses to specific autoantigens are associated with characteristic clinical phenotypes.7,8 For example, patients with circulating anti-tRNA synthetase antibodies are at increased risk of developing interstitial lung disease (ILD).9 It is thus of paramount importance to identify relevant autoantigens that correlate with characteristic phenotypic subsets of DM to validate the functional relevance of the autoantigen, identify the cellular target(s) of this attack, and understand the environmental conditions that initiate and perpetuate this pathologic immune response. In addition, serologic tests for autoantibodies that correlate with a specific phenotype can assist the clinician in early recognition and potentially treatment of associated complications.
Recently, melanoma differentiation-associated gene 5 (MDA5) (clinically amyopathic dermatomyositis antibody, 140 kd [CADM-140], interferon-induced helicase 1) has been described as the target of a novel, DM-specific serologic response that is seen in 19% to 35% of patients with DM.10,11 MDA5 is an RNA-specific helicase that functions in recognizing single-stranded RNA viruses.12 Recent evidence suggests that patients with anti-MDA5 serology are more likely to have absent or mild muscle disease and are at increased risk for rapidly progressive ILD.10,11,13 The cutaneous features of patients possessing this serotype have thus far not been reported to differ from other patients with DM.
This latter point is of interest, because although DM is often characterized by classic skin findings (eg, heliotrope rash, Gottron papules), cutaneous disease in DM has remarkable phenotypic heterogeneity with regard to both clinical and histologic presentation.14 It is conceivable that some of this heterogeneity can be explained by differential autoantigen targeting and/or expression, which results in injury to certain cell types and/or differentiation states that result in observable phenotypes. One specific finding is that of noninflammatory cutaneous ulcerative lesions, seen in both juvenile and adult DM.15 It is likely that these lesions represent a heterogeneous group, with potential causes being: severe interface dermatitis, vasculopathy, or inflammatory vasculitis.16–19 These lesions can be located almost any-where on the body, and are often associated with significant pain and even tissue necrosis. In addition, they can be associated with systemic ulcerative disease, mainly in the gastrointestinal tract in juvenile patients with DM.20 In addition, several small studies suggest that cutaneous necrosis is a sign of cancer-associated DM, but this has yet to be shown in large studies.21–24
We now present evidence that a specific phenotype of cutaneous ulcerations and palmar papules is associated with autoantibodies to MDA5 in adult patients with DM. The pathologic relationship between these lesions, other forms of ulceration and vasculopathy seen in DM, and ILD is discussed.
CAPSULE SUMMARY.
Melanoma differentiation-associated gene 5 (clinically amyopathic dermatomyositis antibody, 140 kd [CADM-140]) is a novel autoantigen in patients with dermatomyositis (DM) that is associated with a novel cutaneous phenotype of cutaneous ulceration, palmar papules, and oral mucosal pain.
Clinical and histopathologic evidence suggests that the immune response to melanoma differentiation-associated gene 5 in patients with DM is closely associated with a more severe cutaneous vasculopathy.
Patients with DM presenting with cutaneous ulcerations and/or palmar papules may not have characteristic muscle inflammation of DM but are at increased risk of subacute or rapidly progressive interstitial lung disease.
METHODS
Patients
All patients were seen in the outpatient clinics at the Stanford University Department of Dermatology in California between July 2004 and April 2010. The collection of plasma from patients with DM for the purposes of proteomic and antibody analysis was approved by the Stanford Institutional Review Board. The population from which plasma was collected represented approximately 80% of the total number of patients with DM seen during this time period. Patients were only included if they had a diagnosis of definite DM based on the criteria of Bohan and Peter,25 or, for patients with clinically amyopathic disease, based on the characteristic skin findings suggested by Sontheimer.14 All patients had a skin biopsy specimen with findings consistent with DM, which included at least one of the following histologic findings: vacuolar interface change, dyskeratotic keratinocytes, increased dermal mucin, dilated papillary dermal blood vessels, or superficial perivascular infiltrate in the absence of epidermal spongiosis.
Clinical data were collected as part of routine medical care. All patients had muscle enzymes performed at least once, including creatine phosphokinase, aldolase, lactate dehydrogenase, and liver transaminases. The dermatology examination consisted of a complete, 14-bullet skin examination for violaceous erythema, additionally noting for the presence of periungual telangiectasias, mechanic hands, skin ulceration, calcinosis cutis, panniculitis, and Gottron papules. Mechanic hands were defined as hyperkeratosis and scaling of the medial side of the thumb and lateral sides of the 2 to 4 digits, with decreasing severity over the more medial digits. Manual muscle testing was performed on all patients, assessing the following muscle groups on a scale of 0 to 10 (total score 150 for normal strength): neck flexors and bilateral deltoids, biceps, wrist extensors, quadriceps, ankle dorsiflexors, gluteus medius, and gluteus maximus. Beginning in June 2007, physician global assessments of skin and muscle activity were tabulated on all patients from whom tissue was collected. The physician global assessments were based on a 0-to-4 Likert scale (0 = clear; 1 = mild; 2 = moderate; 3 = severe; 4 = very severe). These detailed scores were captured in 32 of the 77 total patients. Amyopathic patients were defined as those patients with the characteristic rash of DM for at least 6 months, with neither clinical weakness attributable to inflammatory myopathy nor laboratory evidence (including muscle enzymes) indicative of active myositis.14 Clinically amyopathic patients were defined as those patients with the characteristic rash of DM for at least 6 months without clinical weakness attributable to inflammatory myopathy–patients could have positive or negative laboratory findings of myositis (including muscle enzymes).26 Patients were considered to have ILD only if they had findings consistent with fibrosis or alveolitis on normal or high-resolution computed tomography. Rapidly progressive lung disease was defined as progressive dyspnea and chest radiography changes over the course of less than 1 month.27 Age-appropriate cancer screening and/or computed tomography of the chest, abdomen, and pelvis was performed in all patients at least once either at presentation to our clinic or during follow-up. Patients were considered to have cancer-associated DM if they had a diagnosis of any malignancy (excluding nonmelanoma skin cancer) 1 year preceding or 3 years after the beginning of DM symptoms. A positive antinuclear antibody (ANA) was defined as reactivity at greater than 1:80 titer using the Crithidia luciliae kinetoplast assay.28
Assays to detect antibodies against MDA5, Mi-2, Ro60, Ro52, and Jo-1
Antibodies against MDA5, Mi-2, and Ro60 were detected by immunoprecipitation using [35S] methionine-labeled proteins generated by in vitro transcription/translation. Full-length complementary DNAs were purchased (MDA5, Origene, Rockville, MD), cloned in our laboratory (Ro60), and the Mi-2 complementary DNA has been previously described.29 [35S] methionine-labeled proteins were generated from these complementary DNAs by in vitro transcription/translation per the manufacturer’s protocol (Promega, Madison, WI), and immunoprecipitations were subsequently performed as follows. In vitro transcription/translation substrates were diluted in buffer A consisting of 1% nonidet P-40, 20 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, and 1 mmol/L EDTA pH 7.4 supplemented with a protease inhibitor cocktail. In all, 1 µL of patient serum was added and the mixture was rocked for 1 hour at 4°C, after which 35 µL of immobilized protein A agarose (Pierce, Rockford, IL) was added and rocked for an additional 20 minutes at 4°C. Samples were washed 4 times with buffer A, electrophoresed on 10% sodium dodecyl sulfate–polyacrylamide gels, and the immunoprecipitates were visualized by fluorography. The immunoprecipitations were performed on at least two separate occasions, with identical results each time. Ro-52 and Jo-1 antibodies were assayed by enzyme-linked immunosorbent assay using commercially available kits (Inova Diagnostics, San Diego, CA).
Statistics
We compared the clinical features of complementary patients with and without antibodies against MDA5 using Student t test for continuous variables and two-tailed Fisher exact test for categorical variables. P values less than .05 were considered statistically significant.
RESULTS
Patient population
We collected plasma from 77 patients with DM seen at the outpatient dermatology clinic at Stanford University School of Medicine. The characteristics of these patients are shown in Table I. At the time of plasma harvesting, patients had a median global skin and muscle disease activity of moderate and mild, respectively, on a Likert scoring system, and the median muscle strength score was 130 (maximum 150). The percentage of patients taking systemic corticosteroids (median prednisone dosage 6 mg/d), disease-modifying antirheumatic drugs, or antimalarials was 64%, 46%, and 24%, respectively, at the time of plasma harvesting. Approximately 13% of all patients had amyopathic disease, with no clinical or laboratory evidence of myositis (Table I). More than 46% of patients had a positive ANA test result at some time during their disease. Only 23 (30%) of the patients had reactivity to the myositis-specific antibodies (Mi-2, Jo-1, MDA5) that were tested (Table I).
Table I.
Characteristics of patients
| N (%) | |
|---|---|
| Gender | |
| Male | 26 (35) |
| Female | 51 (65) |
| Race | |
| Caucasian | 49 (64) |
| Latino | 9 (12) |
| Pacific Islander | 6 (7.8) |
| Asian | 5 (6.5) |
| African American | 4 (5.2) |
| Mean age at diagnosis, y | 48.2 ± 16 |
| Median disease duration, y | 1.6 (range: 53 d-36 y) |
| Autoantibody status | |
| ANA | 26 (46) |
| Mi-2 | 9 (12) |
| MDA5 | 10 (13) |
| Jo-1 | 4 (5.2) |
| Ro-52 | 15 (19) |
| Ro-60 | 5 (6.5) |
ANA, Antinuclear antibody; MDA5, melanoma differentiation-associated gene 5.
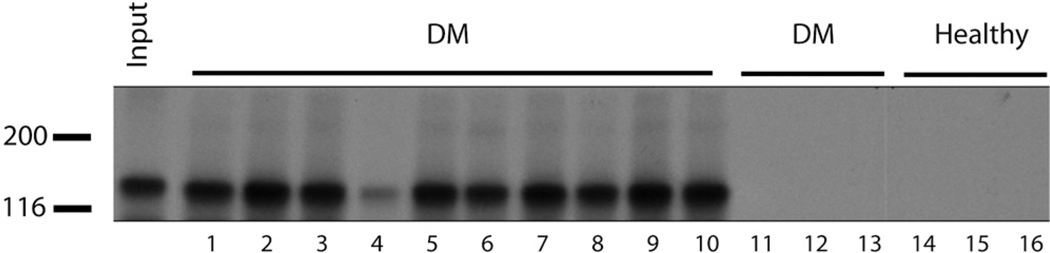
Antibodies to MDA5 were detected in 10 (13%) patients (Fig 1). Three of the anti-MDA5-positive patients were also found to have antibodies to Ro-52, whereas none had reactivity to Jo-1 or Mi-2 data (not shown). Eight of 9 of the anti-MDA5-positive patients were ANA negative (89%), a value significantly higher than the anti-MDA5-negative population (47%) (P = .029).
Fig 1.
Identification of patients with anti-melanoma differentiation-associated gene 5 (MDA5) antibodies. Plasma samples from patients with dermatomyositis (DM) and healthy control subjects were used to immunoprecipitate radiolabeled MDA5 generated by in vitro transcription/translation. All 10 of anti-MDA5 patient samples are shown (lanes 1–10), as are 3 DM samples that are anti-MDA5-negative (lanes 11–13) and 3 samples from healthy individuals (lanes 14–16). Leftmost lane (Input) refers to MDA5 protein loaded with no immunoprecipitation. Molecular weight markers (left).
The anti-MDA5 phenotype
The characteristics of the patients with and without MDA5 autoantibodies are shown in Table II. The presence of MDA5 antibodies was not significantly associated with age of disease onset, race, gender, tobacco use, or the presence of Raynaud phenomenon (Table II) (data not shown). Although 12 patients had cancer-associated DM, none of them had anti-MDA5 antibodies.
Table II.
Clinical findings of antimelanoma differentiation-associated gene 5 patients
| Total (N = 77), N (%) |
anti-MDA5-positive (N = 10), N (%) |
anti-MDA5-negative (N = 67), N (%) |
P value | |
|---|---|---|---|---|
| Age onset, y ± SD | 48.2 ± 16 | 51.5 ± 8.8 | 47.7 ± 16.8 | .50 |
| Female | 51 (65) | 7 (87.5) | 44 (63.8) | .25 |
| Cancer | 12 (15.6) | 0 (0) | 12 (17.9) | .347 |
| Tobacco use | 19 (26) | 1 (10) | 18 (29) | .438 |
| Raynaud | 13 (25) | 3 (38) | 10 (22) | .389 |
| Interstitial lung disease | 16 (25) | 6 (67) | 10 (18) | .005 |
| Rapidly progressive lung disease | 5 (6.6) | 2 (22.2) | 3 (4.5) | .104 |
| Hand swelling | 9 (13.8) | 4 (40) | 5 (9.1) | .026 |
| Arthritis/arthralgia | 20 (31.2) | 7 (70) | 13 (24) | .0076 |
| Amyopathic | 10 (13) | 3 (30) | 7 (10) | .117 |
| Clinically amyopathic | 13 (16.9) | 5 (50) | 8 (11.9) | .010 |
| Skin ulceration (any) | 20 (26.0) | 8 (80) | 12 (18) | .0002 |
| Ulceration (Gottron) | 5 (6.5) | 3 (30) | 2 (3) | .0142 |
| Ulceration (digit pulp/periungual) | 11 (14.3) | 8 (80) | 3 (4.5) | <.0001 |
| Ulceration (elbow) | 4 (5.2) | 3 (30) | 1 (1.5) | .0061 |
| Ulceration (chest/arms) | 6 (7.8) | 0 (0) | 6 (9) | 1.000 |
| Palmar papules | 7 (9.6) | 6 (60) | 1 (1.6) | <.0001 |
| Mechanic hands | 15 (22.4) | 6 (67) | 9 (15.5) | .0028 |
| Panniculitis | 2 (3.1) | 2 (20) | 0 (0) | .0216 |
| Calcinosis cutis | 3 (4.8) | 1 (11.1) | 2 (3.7) | .375 |
| Alopecia | 22 (34) | 7 (78) | 15 (27) | .0053 |
| Heliotrope rash | 42 (60) | 7 (70) | 35 (58) | .729 |
| Gottron papules | 38 (53.5) | 7 (70) | 31 (51) | .32 |
| Violaceous erythema | ||||
| Scalp | 42 (62.7) | 6 (67) | 36 (62) | 1.00 |
| Face | 57 (80.3) | 10 (100) | 47 (77) | .193 |
| V area of the chest-neck | 54 (79.4) | 8 (89) | 46 (78) | .673 |
| Elbow/knee | 48 (69.6) | 10 (100) | 46 (78) | .0259 |
| Periungual telangiectasia | 50 (73.6) | 9 (90) | 41 (71) | .27 |
| Pruritus | 44 (68.8) | 6 (67) | 38 (69) | 1.00 |
| Oral pain/ulcers | 9 (13.9) | 5 (50) | 4 (7.3) | .0029 |
Statistically significant associations (P < 0.05) are shown in bold.
MDA5, Melanoma differentiation-associated gene 5.
Extracutaneous disease
We noted a significant association with MDA5 reactivity and ILD (odds ratio 9.2, confidence interval 1.96–43.2), similar to previous observations10,13,30 (Table II). In addition, there was a significant trend that rapidly progressive lung disease occurred more commonly in the anti-MDA5-positive group. Patients with MDA5 antibodies were at higher risk for arthritis/arthralgia and hand swelling. We noted that patients with clinically amyopathic disease (no weakness but positive laboratory features of myositis) were significantly enriched in the anti-MDA5-positive cohort (50% vs 12%) (P = .010), consistent with other reports.10,11,13 There was also a difference in the percentage of patients with an elevated aldolase but normal creatine phosphokinase enzyme between the two groups; this occurred in 60% and 15% of the anti-MDA5-positive and anti-MDA5-negative patients, respectively (P = .006).
Palmar papules
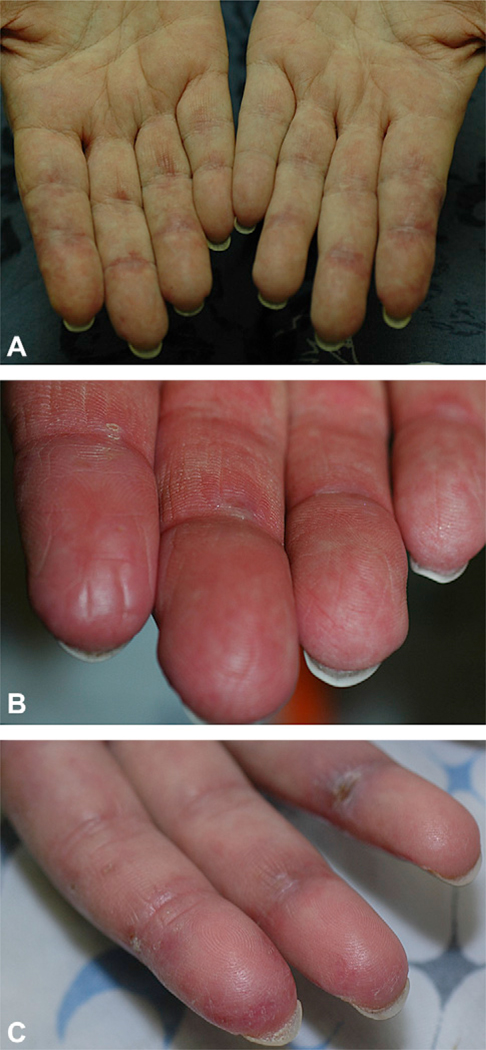
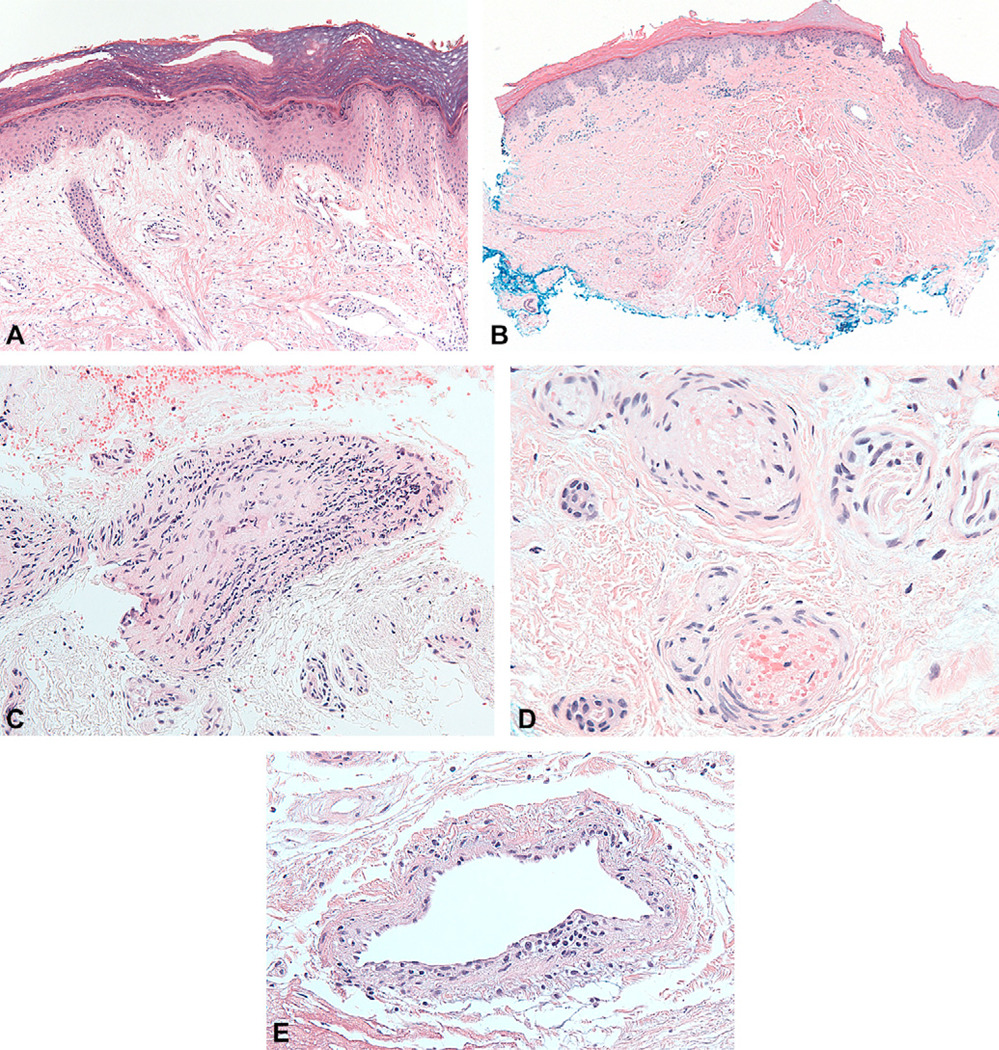
Patients with anti-MDA5 had several striking mucocutaneous features. Half (5 of 10) of the anti-MDA5-positive patients had erythematous papules, macules, or both on the palmar surfaces of the metacarpal and interphalangeal joints (Fig 2, A). Many of these lesions had a central ivory coloration, sometimes actually manifesting as two separate papules on either side of the interphalangeal joint. Some were associated with hyperkeratosis (Fig 2, B), and would sometimes ulcerate (Fig 2, C). The lesions were often painful, unlike the Gottron papules that occur on the back sides of the joints. Three of our patients underwent biopsy of these palmar papules. All of the biopsy specimens showed minimal or absent interface dermatitis, with variable increase in dermal mucin (Fig 3, A) (data not shown). Notably, both the medium and small dermal vessels show a vasculopathy that is either pauci-inflammatory (Fig 3, B) or characterized by mononuclear vessel wall infiltration (Fig 3, C). One biopsy specimen showed intraluminal thrombosis (Fig 3, D) whereas another demonstrated endothelial cell injury and fibrin deposition in the vessel wall (Fig 3, E).
Fig 2.
Palmar papules of melanoma differentiation-associated gene 5–positive patients. A, Typical erythematous palmar macules or papules on either side of interphalangeal (IP) joints. B, Characteristic hyperkeratosis that is associated with palmar papules. C, Ulceration over palmar/lateral IP joint surface in second and fourth digits.
Fig 3.
Histology of palmar papules demonstrates vasculopathy; hematoxylin-eosin stain. A, Mild interface activity with increased dermal mucin. B, Pauci-inflammatory pattern in perivascular region. C, Medium vessel wall infiltration with mononuclear cells. D, Intravascular fibrin and thrombus involving small vessels. E, Endothelial cell swelling and ballooning with fibrin deposition in vessel walls.
Ulceration
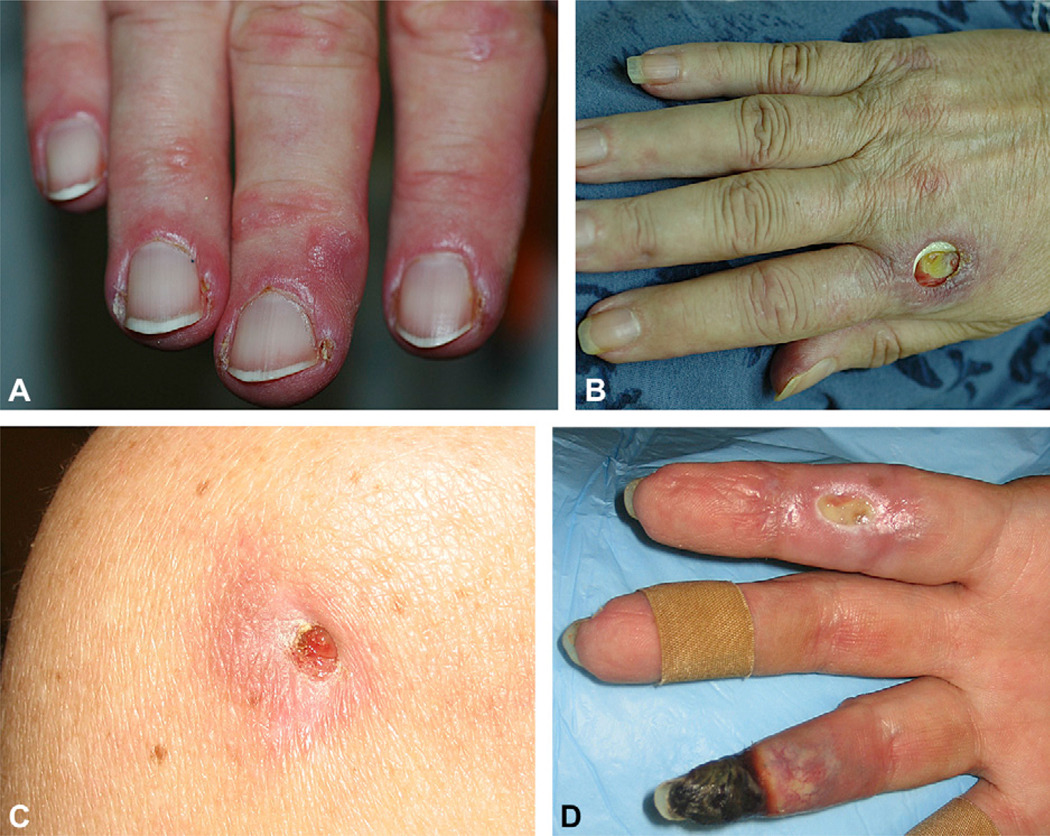
We noted that the presence of MDA5 antibodies was significantly associated with cutaneous ulceration with an odds ratio of 18.3 (confidence interval 3.5–98) (Table II). MDA5 antibodies were associated with several specific forms of ulceration: hyperkeratotic digital pulp lesions, and ulcerations located on the lateral nailfolds (Fig 4, A), within Gottron papules (Fig 4, B), and over the elbows and knees. Rarely, patients were observed to have them in other areas (eg, the ear helix, back of the feet and toes). Interestingly, the shallow erosions that can be seen in sun-exposed areas (eg, chest and upper aspect of arms) in patients with DM did not appear to be associated with antibodies to MDA5 (Table II). In one particularly severe case the patient had diffuse, non-inflammatory, “punched-out” ulcerations diffusely and ischemic digital necrosis (Fig 4, C and D). This patient was found to have coexisting partial protein-S deficiency (48% of normal), whereas protein C, factor V Leiden, cryoglobulins/cryofibrinogens, homocysteine, antiphospholipid antibody, lupus anticoagulant, prothrombin, and partial thromboplastin times were all negative or normal. This patient did not respond to warfarin therapy, aspirin, pentoxifylline, azathioprine, methotrexate, or intravenous immunoglobulin. She did experience some improvement with cyclophosphamide but was only able to tolerate a low dose (approximately 0.5 mg/kg daily) because of leukopenia.
Fig 4.
Different patterns of cutaneous ulceration in melanoma differentiation-associated gene 5–positive dermatomyositis patients. A, Lateral nailfold erythema and crusting. B, Deep ulceration overlying metacarpophalangeal joint. C, Back of left shoulder demonstrating noninflammatory, deep, “punched out” ulceration. D, Extensive ulceration and ischemic necrosis of digits.
Other mucocutaneous findings
We noted that 4 of the 10 anti-MDA5-positive patients reported tender gums and/or oral erosions, significantly more than the anti-MDA5-negative group. In addition, diffuse alopecia, mechanic hands, and elbow/knee erythema (Gottron sign) were significantly more common in the anti-MDA5-positive population (Table II). The prevalence of other classic skin signs and symptoms of DM (Gottron papules, heliotrope rash, pruritus) did not appear to be associated with MDA5 antibodies (Table II).
DISCUSSION
Antibodies to MDA5 have been recently described to be specifically associated with DM.10,11,13 Originally termed “CADM-140,” MDA5 reactivity initially was described as marking a population of patients with DM that was “clinically amyopathic.”10,11,13 However, the definition of “clinically amyopathic” is not universally agreed upon. This designation was intended to identify patients with strictly no evidence of myositis based only on what the clinician can see in the examination room (eg, history and physical examination).14 However, patients fitting this description but demonstrating elevation of muscle enzymes are variably included in this group.14,26 We have elected to include this latter group of patients in “clinically amyopathic,” as these patients tend to have very low level elevation of muscle enzymes and this has begun to be adopted more commonly in the literature.31,32 Using this definition, our results are consistent with previous studies, and it is clear that patients with anti-MDA5 antibodies have absent or very mild muscle disease compared with patients with typical DM. This is not an absolutely sensitive marker for amyopathic disease, as we had many other amyopathic patients that did not have this reactivity (data not shown). Why patients seem to have attenuated muscle disease is unclear, but may relate to differential expression and/or antigenicity of MDA5 in muscle fibers.
To our knowledge, we describe for the first time a link between a constellation of mucocutaneous findings (palmar papules, cutaneous ulcers, and gum pain) and reactivity to MDA5. The complex of cutaneous ulceration and gum pain may be explained by a vasculopathy that is associated with this serotype. Skin biopsy specimens from these lesions all showed some evidence of vascular injury or plugging with variable levels of inflammation. An autoimmune response to MDA5 is likely not the only mechanism for vasculopathy in DM, as we noted that 18% of anti-MDA5-negative patients had evidence of skin ulcers (Table II). In fact, it has been suggested that patients with DM, in general, have a high prevalence of cutaneous vasculopathy in skin biopsy specimens.16 It is likely that other mechanisms are involved in the vasculopathy of DM. However, it is interesting that half of those anti-MDA5-negative patients who had skin ulcerations had more superficial, painless erosions on the chest and arms. This is a very different phenotype from the digital and elbow ulcers in the anti-MDA5-positive group, and may represent an alternative mechanism such as severe interface activity resulting in dermoepidermal separation. Still, there were several MDA5-negative patients with digital pits or ulcerated Gottron papules. Interestingly, two of our patients with cancer-associated DM and ulcerations did not have antibodies to MDA5; thus, the potential connection of cutaneous necrosis with malignancy might be related to a different mechanism.21–24
In general, we found the cutaneous necrosis in our anti-MDA5-positive patients very challenging to treat. The few patients in whom we have used either antiplatelet agents or anticoagulants generally have not responded well. One report in the literature describes a patient who is likely anti-MDA5-positive (ulcers with rapidly progressive ILD) who was treated with cyclosporine with a good outcome for both the ILD and the ulcerations.33
The origin of vasculopathy in the anti-MDA5-positive population is unclear. A complete hypercoagulable workup was performed in only one patient with the most severe ulcerations, and she was found to be partially protein-S deficient. Most other patients only underwent testing for prothrombin/partial thromboplastin times, as the vasculopathy was subtle and not a dominant theme of their clinical presentation. It is tempting to speculate that this connection with ulcers may relate to overexpression, expression, or both of a modified (eg, immunogenic) form of MDA5 by cell(s) that make up the vascular parenchyma, thus resulting in a targeted immune response that compromises vascular function. It is also intriguing that MDA5 is induced by type I interferons, which are known to have vasculopathic effects of their own.34 It is possible that blood vessel exposure to local interferon might induce mild endothelial cell injury that leads to overexpression and/or specific modification of MDA5, resulting in loss of tolerance and an anti-MDA5 response in a permissive genetic (eg, HLA) background.
To our knowledge, palmar papules have been described twice before in the context of DM. One publication described mucinous, flesh-colored papules that were scattered on the arms and across the palmar joints of a patient with DM.35 It seems unlikely that the papules we describe correspond to these lesions, given the discrepancy clinically and histopathologically. However, another description concerns a patient with rapidly progressive lung disease with tender, palmar hyperkeratotic papules in the same location as we note.36 Biopsy specimen showed follicular keratotic plugging without interface dermatitis, but no mention is made of the vasculature. Interestingly, this patient also was devoid of muscle disease and had rapidly progressive lung disease. It is likely that the papules described in this latter report correspond to the same lesions that we describe in our cohort.
In agreement with previous reports, we also found that anti-MDA5 antibodies identify a population of patients with DM at increased risk for ILD.10,11,13 Nonetheless, all patients with DM should be considered at significant risk for ILD, and early screening should be performed in all patients with DM–pulmonary function testing, high-resolution chest computed tomography scans, or both are useful although their relative predictive value has yet to be determined in patients with DM.37 It is possible that this association is indirectly the result of the vasculopathy that we believe is closely associated with anti-MDA5 antibodies. The concomitant finding of cutaneous necrosis and pulmonary fibrosis in DM was reported more than 30 years ago.38 The association of antiendothelial cell antibodies and ILD has been reported,39 and it is possible that anti-MDA5 antibodies target endothelial cells in the appropriate context. It is hypothesized that endothelial cell damage leads to the production of various mediators of fibrosis–one report of patients with DM and polymyositis demonstrated that levels of transforming growth factor-β (a profibrotic cytokine) correlate closely with other markers of endothelial cell damage and provides a mechanistic link between endothelial cell damage and fibrosis.40
It is possible that MDA5 reactivity identifies a patient population at relatively low risk for malignancy-associated DM. Including our cohort, a total of 52 patients has been reported with reactivity to MDA5 that have data on associated malignancy and only one malignancy has been reported.10,13,41 It will be important to test this hypothesis prospectively among a larger cohort of patients with DM.
Commercial testing for anti-MDA5 antibodies is now available (http://www.rdlinc.com/contact.html) and we suggest should be considered for all patients with DM. If not feasible, using these clinical clues to identify patients with DM likely to be anti-MDA5-positive has important clinical consequences–these patients have a good prognosis in terms of myositis, may have a low risk of cancer, but are at high risk for ILD, including rapidly progressive ILD that can lead to patient mortality. In addition to clues provided on the cutaneous examination, we also noted most anti-MDA5-positive patients are ANA negative. Finally, an isolated elevation of the aldolase (with normal creatine phosphokinase levels) is more commonly seen in anti-MDA5-positive patients. It is likely that these clinical clues will help the clinician stratify prognostic risk in a patient given the diagnosis of with DM.
Acknowledgments
Supported by the Scleroderma Research Foundation (Dr Chung), National Institutes of Health (NIH) RO1 R37-DE-12354 (Dr Rosen), and NIH RO1 AR-44684 (Dr Casciola-Rosen). We thank the Johns Hopkins University Rheumatic Diseases Research Core Center (P30-AR-053503) for assays.
Abbreviations used
- ANA
antinuclear antibody
- DM
dermatomyositis
- ILD
interstitial lung disease
- MDA5
melanoma differentiation-associated gene 5
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Gunawardena H, Betteridge ZE, McHugh NJ. Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology (Oxford) 2009;48:607–612. doi: 10.1093/rheumatology/kep078. [DOI] [PubMed] [Google Scholar]
- 2.Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005;201:591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine SM, Raben N, Xie D, Askin FB, Tuder R, Mullins M, et al. Novel conformation of histidyl-transfer RNA synthetase in the lung: the target tissue in Jo-1 autoantibody-associated myositis. Arthritis Rheum. 2007;56:2729–2739. doi: 10.1002/art.22790. [DOI] [PubMed] [Google Scholar]
- 4.Caproni M, Torchia D, Cardinali C, Volpi W, Del Bianco E, D’Agata A, et al. Infiltrating cells, related cytokines and chemokine receptors in lesional skin of patients with dermatomyositis. Br J Dermatol. 2004;151:784–791. doi: 10.1111/j.1365-2133.2004.06144.x. [DOI] [PubMed] [Google Scholar]
- 5.Magro CM, Segal JP, Crowson AN, Chadwick P. The phenotypic profile of dermatomyositis and lupus erythematosus: a comparative analysis. J Cutan Pathol. 2010;37:659–671. doi: 10.1111/j.1600-0560.2009.01443.x. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel J, Schmidt R, Proelss J, Zahn S, Bieber T, Tuting T. Type I interferon-associated skin recruitment of CXCR3+ lymphocytes in dermatomyositis. Clin Exp Dermatol. 2006;31:576–582. doi: 10.1111/j.1365-2230.2006.02150.x. [DOI] [PubMed] [Google Scholar]
- 7.Love LA, Leff RL, Fraser DD, Targoff IN, Dalakas M, Plotz PH, et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine (Baltimore) 1991;70:360–374. doi: 10.1097/00005792-199111000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Targoff IN. Idiopathic inflammatory myopathy: autoantibody update. Curr Rheumatol Rep. 2002;4:434–441. doi: 10.1007/s11926-002-0089-7. [DOI] [PubMed] [Google Scholar]
- 9.Yoshifuji H, Fujii T, Kobayashi S, Imura Y, Fujita Y, Kawabata D, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity. 2006;39:233–241. doi: 10.1080/08916930600622884. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 2010;49:433–440. doi: 10.1093/rheumatology/kep375. [DOI] [PubMed] [Google Scholar]
- 11.Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Kuwana M. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60:2193–2200. doi: 10.1002/art.24621. [DOI] [PubMed] [Google Scholar]
- 12.Barral PM, Sarkar D, Su ZZ, Barber GN, DeSalle R, Racaniello VR, et al. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol Ther. 2009;124:219–234. doi: 10.1016/j.pharmthera.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571–1576. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 14.Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. 2002;20:387–408. doi: 10.1016/s0733-8635(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 15.Kawachi Y, Maruyama H, Furuta J, Fujisawa Y, Nakamura Y, Takahashi T, et al. Cutaneous deep necrosis with dermatomyositis: correlation with interstitial pneumonia. Eur J Dermatol. 2007;17:345–346. doi: 10.1684/ejd.2007.0220. [DOI] [PubMed] [Google Scholar]
- 16.Crowson AN, Magro CM. The role of microvascular injury in the pathogenesis of cutaneous lesions of dermatomyositis. Hum Pathol. 1996;27:15–19. doi: 10.1016/s0046-8177(96)90132-x. [DOI] [PubMed] [Google Scholar]
- 17.Kissel JT, Halterman RK, Rammohan KW, Mendell JR. The relationship of complement-mediated microvasculopathy to the histologic features and clinical duration of disease in dermatomyositis. Arch Neurol. 1991;48:26–30. doi: 10.1001/archneur.1991.00530130034016. [DOI] [PubMed] [Google Scholar]
- 18.Miller M, Carton FX. Vasculitis in children’s dermatomyositis (author’s transl) [in French] Ann Dermatol Venereol. 1980;107:841–845. [PubMed] [Google Scholar]
- 19.Yamamoto T, Ohkubo H, Katayama I, Nishioka K. Dermatomyositis with multiple skin ulcers showing vasculitis and membrano-cystic lesion. J Dermatol. 1994;21:687–689. doi: 10.1111/j.1346-8138.1994.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 20.Gennaro AR, Bacon HE. Dermatomyositis and associated lesions of the gastrointestinal tract. Dis Colon Rectum. 1969;12:256–260. doi: 10.1007/BF02617280. [DOI] [PubMed] [Google Scholar]
- 21.Burnouf M, Mahe E, Verpillat P, Descamps V, Lebrun-Vignes B, Picard-Dahan C, et al. Cutaneous necrosis is predictive of cancer in adult dermatomyositis [in French] Ann Dermatol Venereol. 2003;130:313–316. [PubMed] [Google Scholar]
- 22.Mahe E, Descamps V, Burnouf M, Crickx B. A helpful clinical sign predictive of cancer in adult dermatomyositis: cutaneous necrosis. Arch Dermatol. 2003;139:539. doi: 10.1001/archderm.139.4.539-a. [DOI] [PubMed] [Google Scholar]
- 23.Martorell-Calatayud A, Serra Guillen C, Ciudad-Blanco C, Sanmartin O. Skin necrosis as a predictive factor for neoplasia in dermatomyositis [in Spanish] Actas Dermo-Sif. 2010;101:459–460. [PubMed] [Google Scholar]
- 24.Mautner GH, Grossman ME, Silvers DN, Rabinowitz A, Mowad CM, Johnson BL., Jr Epidermal necrosis as a predictive sign of malignancy in adult dermatomyositis. Cutis. 1998;61:190–194. [PubMed] [Google Scholar]
- 25.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 26.Gerami P, Schope JM, McDonald L, Walling HW, Sontheimer RD. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis sine myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54:597–613. doi: 10.1016/j.jaad.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Ideura G, Hanaoka M, Koizumi T, Fujimoto K, Shimojima Y, Ishii W, et al. Interstitial lung disease associated with amyopathic dermatomyositis: review of 18 cases. Respir Med. 2007;101:1406–1411. doi: 10.1016/j.rmed.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Slater NG, Cameron JS, Lessof MH. The Crithidia luciliae kinetoplast immunofluorescence test in systemic lupus erythematosus. Clin Exp Immunol. 1976;25:480–486. [PMC free article] [PubMed] [Google Scholar]
- 29.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–826. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gono T, Kawaguchi Y, Sugiura T, Furuya T, Kawamoto M, Hanaoka M, et al. Interferon-induced helicase (IFIH1) polymorphism with systemic lupus erythematosus and dermatomyositis/polymyositis. Mod Rheumatol. 2010;20:466–470. doi: 10.1007/s10165-010-0311-9. [DOI] [PubMed] [Google Scholar]
- 31.Klein RQ, Teal V, Taylor L, Troxel AB, Werth VP. Number, characteristics, and classification of patients with dermatomyositis seen by dermatology and rheumatology departments at a large tertiary medical center. J Am Acad Dermatol. 2007;57:937–943. doi: 10.1016/j.jaad.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morganroth PA, Kreider ME, Okawa J, Taylor L, Werth VP. Interstitial lung disease in classic and skin-predominant dermatomyositis: a retrospective study with screening recommendations. Arch Dermatol. 2010;146:729–738. doi: 10.1001/archdermatol.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimojima Y, Ishii W, Kato T, Hoshi K, Matsuda M, Hashimoto T, et al. Intractable skin necrosis and interstitial pneumonia in amyopathic dermatomyositis, successfully treated with cyclosporin A. Intern Med. 2003;42:1253–1258. doi: 10.2169/internalmedicine.42.1253. [DOI] [PubMed] [Google Scholar]
- 34.Feldman D, Goldstein AL, Cox DC, Grimley PM. Cultured human endothelial cells treated with recombinant leukocyte A interferon: tubuloreticular inclusion formation, antiproliferative effect, and 2’,5’ oligoadenylate synthetase induction. Lab Invest. 1988;58:584–589. [PubMed] [Google Scholar]
- 35.del Pozo J, Almagro M, Martinez W, Yebra-Pimentel MT, Garcia-Silva J, Pena-Penabad C, et al. Dermatomyositis and mucinosis. Int J Dermatol. 2001;40:120–124. doi: 10.1046/j.1365-4362.2001.01060.x. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki Y, Okuyama R, Tsunoda T, Tagami H, Aiba S. Keratotic palmar papules in a dermatomyositis patient preceding the development of interstitial pneumonia. Dermatology. 2007;215:169–170. doi: 10.1159/000104272. [DOI] [PubMed] [Google Scholar]
- 37.Fathi M, Dastmalchi M, Rasmussen E, Lundberg IE, Tornling G. Interstitial lung disease, a common manifestation of newly diagnosed polymyositis and dermatomyositis. Ann Rheum Dis. 2004;63:297–301. doi: 10.1136/ard.2003.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herreman G, Godeau P, Marteau R, Herson S, Sqalli S, Serdaru M. Dermatomyositis: pulmonary fibrosis and cutaneous necrosis–a new entity; apropos of 2 cases [in French] Ann Med Interne (Paris) 1977;128:773–779. [PubMed] [Google Scholar]
- 39.Cervera R, Ramirez G, Fernandez-Sola J, D’Cruz D, Casademont J, Grau JM, et al. Antibodies to endothelial cells in dermatomyositis: association with interstitial lung disease. BMJ. 1991;302:880–881. doi: 10.1136/bmj.302.6781.880-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funauchi M, Shimadsu H, Tamaki C, Yamagata T, Nozaki Y, Sugiyama M, et al. Role of endothelial damage in the pathogenesis of interstitial pneumonitis in patients with polymyositis and dermatomyositis. J Rheumatol. 2006;33:903–906. [PubMed] [Google Scholar]
- 41.Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T. Anti-MDA5 and anti-TIF1-{gamma} antibodies have clinical significance for patients with dermatomyositis. Rheumatology (Oxford) 2010;49:1726–1733. doi: 10.1093/rheumatology/keq153. [DOI] [PubMed] [Google Scholar]