In a 1991 review article titled “The pancreatic islet as Rubik’s cube. Is phospholipid hydrolysis a piece of the puzzle?” (26), Stewart Metz concluded that “an obligate role for phospholipase activation in glucose-induced insulin secretion is not yet rigorously established, despite tantalizing, inferential evidence.” This statement remained accurate for more than 15 years. Recent studies by Bao and colleagues (2, 5) and Jacobson et al. (13), including a recent article appearing in AJP-Endocrinology and Metabolism (2), provide key supportive evidence for a role of Group VIA phospholipase A2 (iPLA2β) in insulin secretion.
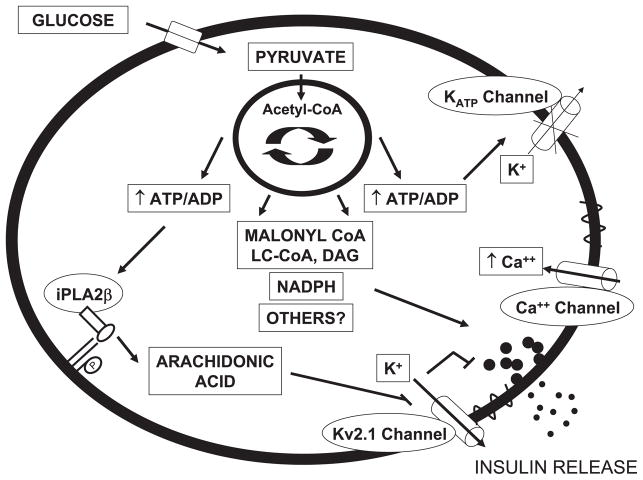
Defective insulin secretion from the pancreatic β-cell causes type 2 diabetes, a disease estimated to affect 6% of the world’s population (11a). The molecular nature of the β-cell defect is still elusive, in part due to our incomplete understanding of the complex physiological mechanisms regulating insulin secretion. Glucose-stimulated insulin secretion (GSIS) involves a triggering pathway capable of inducing insulin release and an amplifying pathway that operates only when the triggering pathway is activated (11). The triggering pathway, also called ATP-sensitive potassium (KATP) channel-dependent pathway, involves an increase in ATP/ADP ratio following mitochondrial glucose metabolism, closure of the KATP channel, membrane depolarization, opening of the voltage-gated Ca2+ channel, influx of intracellular Ca2+, and insulin exocytosis. The nature of the amplifying pathway remains debated, probably because it involves several coupling factors (21–23, 27). In fact, signals derived from the malonyl-CoA/long-chain acyl-CoA pathway (29), NADPH (7, 20), citrate derived from cataplerotic reactions (8), glutamate (24), and GTP (15) have all been proposed as coupling factors for GSIS (Fig. 1). A role for lipid signaling molecules derived from phospholipase A2-mediated hydrolysis of membrane phospholipids was first suspected some 25 years ago (16) but has remained uncertain until now.
Fig. 1.
Schematic representation of stimulus-secretion coupling mechanisms in the pancreatic β-cell. Mitochondrial metabolism of glucose generates coupling factors that all contribute to stimulating insulin release. The triggering pathway of insulin secretion involves an increase in ATP/ADP ratio, closure of the ATP-sensitive potassium (KATP) channel, membrane depolarization, opening of the voltage-gated Ca2+ channels, Ca2+ influx, and insulin exocytosis. The voltage-gated delayed rectifier Kv2.1 channel contributes to repolarizing the cell membrane and provides an “off ” signal for insulin release. Mitochondrial signals, such as those derived from metabolism of long-chain acyl-CoA (LC-CoA), NADPH, and others, amplify the triggering pathway and potentiate insulin secretion. Bao and colleagues (2, 5) and Jacobson et al. (13) now provide evidence for an additional amplifying pathway involving Group VIA phospholipase A2 (iPLA2β)-mediated hydrolysis of membrane phospholipids, arachidonic acid release, and inhibition of the Kv2.1 channel. DAG, diacylglycerol.
Several lines of evidence support a role for phospholipid hydrolysis in insulin secretion. First, arachidonic acid (AA) represents ~30% of the fatty acid mass in islet glycerophospholipids (30). Second, glucose stimulates the release of AA in islets (34); blockade of AA release suppresses insulin secretion (31, 33), and both AA and lysophospholipids (released upon phospholipase A-mediated hydrolysis of phospholipids) have well-documented signaling properties. Gross et al. (10) identified a cytosolic, Ca2+-independent, and ATP-stimulated phospholipase A2 activity that prefers AA in the sn-2 position of the glycerol backbone, and Ma and colleagues (17, 19) cloned the 84-kDa isoforms of iPLAβ in islets. Inhibition of the enzyme by its suicide substrate bromoenol lactone also inhibits insulin secretion (32). In addition, overexpression (18) or siRNA knockdown (1) of iPLAβ in insulin-secreting cells respectively amplifies and reduces GSIS. Although clearly insightful, these studies did not provide conclusive evidence for a role of iPLAβ in insulin secretion in physiologically relevant models, and interpretation of experiments using bromoenol lactone is confounded by the ability of this compound to inhibit other lipases (14).
Recent studies by Bao and colleagues have used genetic mouse models to further examine the functional roles of iPLAβ in vivo. First, they generated iPLAβ-knockout (KO) mice and reported reduced male fertility (4) and altered macrophage function (3). Second, they examined islet membrane phospholipid composition by electrospray ionization mass spectrometry and showed a virtually identical proportion of arachidonate-containing phosphatidylcholine species in iPLAβ-KO and wild-type (WT) islets, arguing against a housekeeping role for iPLAβ in regulating arachidonate incorporation into phosphatidylcholine (5). Third, they observed that isolated islets from iPLAβ-KO mice have reduced insulin secretion in response to glucose and the cAMP-elevating agent forskolin (5). Fourth, although female iPLAβ-KO mice had normal fasting and fed blood glucose levels, they developed more severe hyperglycemia upon administration of the β-cell toxin streptozotocin and became more glucose intolerant in response to high-fat feeding than WT mice (5). In a recent article appearing in AJP-Endocrinology and Metabolism, Bao et al. (2) complemented their loss-of-function strategy with a gain-of-function approach and generated iPLAβ-transgenic (Tg) mice overexpressing iPLAβ under the rat insulin 1 promoter. They demonstrated that iPLAβ activity in iPLAβ-Tg islets was elevated severalfold over that of the endogenous enzyme but that the morphology of the islets was not altered. Male iPLAβ-Tg mice had higher circulating insulin and lower blood glucose levels than WT animals both in the fasting state and after glucose administration, yet insulin tolerance was similar in iPLAβ-Tg and WT animals. Unexpectedly, insulin secretion in response to glucose was not increased in islets from iPLAβ-Tg mice, although potentiation of GSIS by forskolin was greater in iPLAβ-Tg islets. Conversely, as previously shown (5), forskolin potentiation of GSIS was reduced in islets from iPLAβ-KO mice. GSIS in the absence of forskolin also tended to be reduced, although, in contrast to that study, the difference was not significant. Also in contrast to previous results using female mice (5), male iPLAβ-KO animals had impaired fasting glucose and abnormal glucose tolerance. Neither the rate of incorporation of labeled arachidonic acid nor membrane phospholipid composition was significantly different between islets from iPLAβ-Tg and -WT mice. The mechanism by which altered iPLAβ activity affects GSIS appears to involve modulation of the delayed rectifier potassium channel Kv2.1. Kv2.1 is a voltage-gated channel that repolarizes the cell membrane after depolarization, thereby providing an “off” signal for insulin release (28). Previously, the same group of investigators (13) demonstrated that glucose inactivation of the Kv2.1 channel was mediated by AA. They now show that, in islets from iPLAβ-Tg mice, inactivation of the Kv2.1 channel in response to glucose is more pronounced, Kv currents are reduced, and the increase in cytosolic Ca2+ is more sustained. These changes are similar to those observed in islets from Kv2.1-KO mice (12). On the basis of these results, those authors proposed that glucose activates iPLAβ-mediated AA release from membrane phospholipids, which restrains the activity of the Kv2.1 channel and results in amplification of intracellular Ca2+ influx and potentiation of insulin secretion (Fig. 1).
These results provide important information and also raise a number of questions. First, the role of iPLAβ in the maintenance of glucose homeostasis under normal conditions is not entirely clear due to differences between males and females and between in vivo and in vitro situations (2, 5). In this regard, examining insulin secretion in vitro in dynamic perifusion studies may reveal differences in the phasic release of insulin that may not be apparent in static incubations. Since the mechanisms of action of iPLAβ appear to involve the Kv2.1 channel, one might predict that first-phase insulin secretion will be prolonged or shortened in islets from iPLAβ-Tg and -KO, respectively. Second, the relationship between iPLAβ and the cAMP/protein kinase A pathway remains unclear. Alterations of forskolin potentiation of GSIS upon modulation of iPLAβ activity might be interpreted either as a direct interaction between iPLA2β and the cAMP/potein kinase A pathway, which remains to be characterized, or as an indirect consequence of altered GSIS. Examining whether the response to other amplifying agents and nonglucose secretagogues is also changed in iPLAβ-KO and -Tg islets might help clarify this point. Third, it might be informative to assess the insulin response to glucose in iPLAβ-KO and -Tg islets under conditions in which intracellular Ca2+ is elevated and the KATP channel is held open [i.e., in the presence of KCl and diazoxide (9)] to better ascertain the specific contribution of iPLAβ to the KATP-independent pathway. Fourth, elucidating the exact mechanism(s) by which iPLA2β/AA modulates the Kv2.1 channel may also be of interest, e.g., direct physical interaction to alter channel structure, intermediary factors, etc. Finally, although the assumption is that the changes in insulin secretion observed upon gain- or loss-of-function of iPLAβ are mediated by changes in glucose-induced AA release and the resulting modulation of the Kv2.1 channel, intracellular AA levels were not directly measured in these studies. In this regard, the second product of the iPLAβ-mediated phospholipid hydrolysis, lysophosphatidic acid, is known to also influence insulin secretion (25), and its potential contribution to the changes in insulin secretion in islets from iPLAβ-KO and -Tg mice remains to be determined.
Nevertheless, these studies provide a significant advancement to our understanding of the role of iPLAβ in insulin secretion. First, they demonstrate that iPLAβ has a signaling function rather than a housekeeping role in β-cells. Second, although iPLAβ appears dispensable for the maintenance of blood glucose levels under normal conditions, it is one of the (probably many) mechanisms by which the β-cell mounts a compensatory response to the increased secretory demand imposed, for example, by high-fat feeding. Third, the mechanism of action of iPLAβ involves modulation of the Kv2.1 channel activity and Ca2+ currents. These findings provide a positive answer to Stewart Metz’s question (26) by demonstrating that, indeed, phosholipid hydrolysis is a piece of the Rubik’s cube. Although an obligate role for phospholipase activation in GSIS is still uncertain, the results of Bao and colleagues (2, 5) clearly demonstrate that iPLAβ belongs to the growing list of intracellular pathways contributing to the regulation of insulin secretion.
Acknowledgments
Many thanks to Dr. Marc Prentki (Montreal Diabetes Research Center, University of Montreal, Montreal, QC, Canada) for critical reading of the manuscript.
References
- 1.Bao S, Bohrer A, Ramanadham S, Jin W, Zhang S, Turk J. Effects of stable suppression of Group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells. J Biol Chem. 2006;281:187–198. doi: 10.1074/jbc.M509105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao S, Jacobson DA, Wohltmann M, Bohrer A, Jin W, Philipson LH, Turk J. Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2β in pancreatic β-cells and in iPLA2β-null mice. Am J Physiol Endocrinol Metab. 2007 Oct;Sep; doi: 10.1152/ajpendo.0638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao S, Li Y, Lei X, Wohltmann M, Jin W, Bohrer A, Semenkovich CF, Ramanadham S, Tabas I, Turk J. Attenuated free cholesterol loading-induced apoptosis but preserved phospholipid composition of peritoneal macrophages from mice that do not express group VIA phospholipase A2. J Biol Chem. 2007;282:27100–27114. doi: 10.1074/jbc.M701316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao S, Song H, Wohltmann M, Ramanadham S, Jin W, Bohrer A, Turk J. Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express Group VIA phospholipase A2 and effects of metabolic stress on glucose homeostasis. J Biol Chem. 2006;281:20958–20973. doi: 10.1074/jbc.M600075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem. 2004;279:44370–44375. doi: 10.1074/jbc.M311842200. [DOI] [PubMed] [Google Scholar]
- 8.Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000;49:718–726. doi: 10.2337/diabetes.49.5.718. [DOI] [PubMed] [Google Scholar]
- 9.Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992;89:1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross RW, Ramanadham S, Kruszka KK, Han X, Turk J. Rat and human pancreatic islet cells contain a calcium ion independent phospholipase A2 activity selective for hydrolysis of arachidonate which is stimulated by adenosine triphosphate and is specifically localized to islet beta-cells. Biochemistry. 1993;32:327–336. doi: 10.1021/bi00052a041. [DOI] [PubMed] [Google Scholar]
- 11.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 11a.International Diabetes Federation. Diabetes Atlas. 3. Brussels, Belgium: International Diabetes Federation; 2006. [Google Scholar]
- 12.Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ammälä CE, Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 2007;6:229–235. doi: 10.1016/j.cmet.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson DA, Weber CR, Bao S, Turk J, Philipson LH. Modulation of the pancreatic islet beta-cell-delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J Biol Chem. 2007;282:7442–7449. doi: 10.1074/jbc.M607858200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 15.Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5:253–264. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laychock SG. Phospholipase A2 activity in pancreatic islets is calcium-dependent and stimulated by glucose. Cell Calcium. 1982;3:43–54. doi: 10.1016/0143-4160(82)90036-7. [DOI] [PubMed] [Google Scholar]
- 17.Ma Z, Ramanadham S, Kempe K, Chi XS, Ladenson J, Turk J. Pancreatic islets express a Ca2+-independent phospholipase A2 enzyme that contains a repeated structural motif homologous to the integral membrane protein binding domain of ankyrin. J Biol Chem. 1997;272:11118–11127. [PubMed] [Google Scholar]
- 18.Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu FF, Turk J. Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress group VIA phospholipase A2 (iPLA2beta) indicate a signaling rather than a housekeeping role for iPLA2beta. J Biol Chem. 2001;276:13198–13208. doi: 10.1074/jbc.M010423200. [DOI] [PubMed] [Google Scholar]
- 19.Ma Z, Wang X, Nowatzke W, Ramanadham S, Turk J. Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J Biol Chem. 1999;274:9607–9616. doi: 10.1074/jbc.274.14.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald M. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets: further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995;270:20051–20058. [PubMed] [Google Scholar]
- 21.MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005;288:E1–E15. doi: 10.1152/ajpendo.00218.2004. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci. 2005;360:2211–2225. doi: 10.1098/rstb.2005.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maechler P, Carobbio S, Rubi B. In beta-cells, mitochondria integrate and generate metabolic signals controlling insulin secretion. Int J Biochem Cell Biol. 2006;38:696–709. doi: 10.1016/j.biocel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis (see comments) Nature. 1999;402:685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 25.McDonald P, Veluthakal R, Kaur H, Kowluru A. Biologically active lipids promote trafficking and membrane association of Rac1 in insulin-secreting INS 832/13 cells. Am J Physiol Cell Physiol. 2007;292:C1216–C1220. doi: 10.1152/ajpcell.00467.2006. [DOI] [PubMed] [Google Scholar]
- 26.Metz SA. The pancreatic islet as Rubik’s Cube. Is phospholipid hydrolysis a piece of the puzzle? Diabetes. 1991;40:1565–1573. doi: 10.2337/diab.40.12.1565. [DOI] [PubMed] [Google Scholar]
- 27.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55(Suppl 2):S16–S23. doi: 10.2337/db06-s003. [DOI] [PubMed] [Google Scholar]
- 28.Philipson LH, Rosenberg MP, Kuznetsov A, Lancaster ME, Worley JF, 3rd, Roe MW, Dukes ID. Delayed rectifier K+ channel overexpression in transgenic islets and β-cells associated with impaired glucose responsiveness. J Biol Chem. 1994;269:27787–27790. [PubMed] [Google Scholar]
- 29.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem. 1992;267:5802–5810. [PubMed] [Google Scholar]
- 30.Ramanadham S, Bohrer A, Mueller M, Jett P, Gross RW, Turk J. Mass spectrometric identification and quantitation of arachidonate-containing phospholipids in pancreatic islets: prominence of plasmenylethanolamine molecular species. Biochemistry. 1993;32:5339–5351. doi: 10.1021/bi00071a009. [DOI] [PubMed] [Google Scholar]
- 31.Ramanadham S, Gross RW, Han X, Turk J. Inhibition of arachidonate release by secretagogue-stimulated pancreatic islets suppresses both insulin secretion and the rise in beta-cell cytosolic calcium ion concentration. Biochemistry. 1993;32:337–346. doi: 10.1021/bi00052a042. [DOI] [PubMed] [Google Scholar]
- 32.Ramanadham S, Song H, Bao S, Hsu FF, Zhang S, Ma Z, Jin C, Turk J. Islet complex lipids: involvement in the actions of group VIA calcium-independent phospholipase A(2) in beta-cells. Diabetes. 2004;53(Suppl 1):S179–S185. doi: 10.2337/diabetes.53.2007.s179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turk J, Colca JR, Kotagal N, McDaniel ML. Arachidonic acid metabolism in isolated pancreatic islets. II. The effects of glucose and of inhibitors of arachidonate metabolism on insulin secretion and metabolite synthesis. Biochim Biophys Acta. 1984;794:125–136. doi: 10.1016/0005-2760(84)90305-9. [DOI] [PubMed] [Google Scholar]
- 34.Wolf BA, Pasquale SM, Turk J. Free fatty acid accumulation in secretagogue-stimulated pancreatic islets and effects of arachidonate on depolarization-induced insulin secretion. Biochemistry. 1991;30:6372–6379. doi: 10.1021/bi00240a004. [DOI] [PubMed] [Google Scholar]