Abstract
Chronic Obstructive Pulmonary Disease (COPD) is a cigarette smoke (CS)-driven inflammatory airway disease with an increasing global prevalence. Currently there is no effective medication to stop the relentless progression of this disease. It has recently been shown that an activator of the P2X7/inflammasome pathway, ATP, and the resultant products (IL-1β/IL-18) are increased in COPD patients. The aim of this study was to determine whether activation of the P2X7/caspase 1 pathway has a functional role in CS-induced airway inflammation. Mice were exposed to CS twice a day to induce COPD-like inflammation and the role of the P2X7 receptor was investigated. We have demonstrated that CS-induced neutrophilia in a pre-clinical model is temporally associated with markers of inflammasome activation, (increased caspase 1 activity and release of IL-1β/IL-18) in the lungs. A selective P2X7 receptor antagonist and mice genetically modified so that the P2X7 receptors were non-functional attenuated caspase 1 activation, IL-1β release and airway neutrophilia. Furthermore, we demonstrated that the role of this pathway was not restricted to early stages of disease development by showing increased caspase 1 activation in lungs from a more chronic exposure to CS and from patients with COPD. This translational data suggests the P2X7/Inflammasome pathway plays an ongoing role in disease pathogenesis. These results advocate the critical role of the P2X7/caspase 1 axis in CS-induced inflammation, highlighting this as a possible therapeutic target in combating COPD.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is an inflammatory disease of the airways, associated primarily with cigarette smoke (CS) exposure, and characterised by a progressive and irreversible decline in lung function caused by airflow obstruction, destruction of parenchyma and emphysema [1], [2]. It is one of the few leading global causes of death that is still increasing in prevalence and is predicted to be the third leading cause of mortality by the year 2020 [3]. Studies examining the lungs of COPD patients have demonstrated the infiltration of immune cells including CD8+ T-cells, neutrophils and macrophages and as such inflammatory cells have been implicated in the pathogenesis of COPD [4]. This hypothesis, however, is yet to be tested because there are currently no medications that will reduce the airway inflammation. Indeed, even high systemic doses of glucocortoid treatment have limited effects [5]. Therefore there is an urgent need to develop effective anti-inflammatory drugs for patients suffering from COPD. To do this we would argue that it is essential to determine the mechanisms by which exposure to CS drives the airway inflammation.
Recently there has been growing evidence to implicate the NLRP3 inflammasome and its products in the inflammation observed in COPD patients. The NLRP3 inflammasome is a multi-meric protein complex important in stimulating caspase-1 activation and subsequently the release of the mature form of the inflammatory cytokines IL-1β and IL-18. Elevated IL-1β levels are found in induced sputum and BAL fluid from COPD patients [6], [7]. Adult mice over-expressing IL-1β in lung epithelium display a COPD-like phenotype consisting of lung inflammation, emphysema and airway fibrosis [8]. Elevated IL-18 levels have also been found in COPD patients and mouse models [9], [10]. Furthermore, IL-18 knockout mice show significantly decreased inflammation and emphysema compared to wild-type mice following CS exposure [11]; whereas mice over-expressing IL-18 in the lung display a COPD-like phenotype [12]. Therefore, a mechanism attenuating both cytokines may provide a substantial clinical benefit.
The NLRP3 inflammasome can be activated a number of ways; one of which is through ATP acting on the P2X7 receptor [13], [14], [15], [16]. Extracellular concentrations of ATP are maintained at low physiological concentrations by ectonucleotidases, but these concentrations increase under conditions such as infection or inflammation. This increase can be due to either greater release of ATP from cells such as epithelial or leukocytes, and/or down-regulation of ectonucleotidases [17], [18]. Recently, increases in ATP levels have been reported in in vitro/in vivo models of COPD [19], [20] and in clinical samples [21], [22]. This increase in ATP levels has been suggested to play a role in the chemotaxis and activation of inflammatory cells, such as neutrophils, through P2Y receptors [22], [23]. We suggest, however, that as the expression of the P2X7 receptor is increased in disease tissues/cells [22], [24] an alternative hypothesis could be that the ATP is acting on the P2X7 receptor leading to NLRP3 inflammasome and caspase 1 activation, which in turn cleaves the pro-form of IL-1β and IL-18 allowing them to be released. Indeed, Churg et al have recently shown that caspase 1 inhibition to attenuate a smoke driven airway inflammation [25]. These cytokines then play a central role in the inflammation observed in COPD. Our hypothesis is that modulation of this P2X7/inflammasome axis would attenuate CS-induced inflammation.
Using in vitro and in vivo pre-clinical modelling systems we show a temporal correlation between markers of the P2X7/inflammasome pathway activation and airway inflammation. We demonstrate that modulation of this pathway, using either a selective P2X7 inhibitor or P2X7 knockout mice, attenuates the airway inflammation in this in vivo model. This pathway, however, was not involved in the IL-1β release observed after the activation of innate host defence mechanisms triggered by an endotoxin insult. Finally, using samples from patients with COPD, we show our pre-clinical results are translational in that caspase 1 activity levels are higher in lung tissue from COPD patients and smokers compared to non-smoking donors.
Methods
Male C57BL/6 mice were obtained from Harlan UK Limited. P2X7 receptor knockout (KO) mice (backcrossed at least 7 times) were provided by Professor Jean Kanellopoulos (Université Paris-Sud, France), originally these mice were produced in Professor Gabel's lab. For full details of how they were developed see Solle et al, 2001 [26]. Parallel wild type controls were bred in-house. The experiments were performed in accordance with the UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) act 1986 under a project licence (PPL 70/6681).
Development and characterisation of CS driven model of airway inflammation
A whole body cigarette smoke exposure system consisting of a time-set pinch valve (C Lee Machining, Horsham, UK), exposure chambers (Teague Enterprises, CA, USA), extraction unit (Grainger Industrial Supply, USA) and TSP Sampling Unit (Teague Enterprises, CA, USA). Animals were exposed to either room air or cigarette smoke using 3R4F cigarettes (Cigarette filter removed, Tobacco Health Research Institute, University of Kentucky, Lexington, KY). CS is generated using a negative pressure system (flow-rate set at 1500 ml/min through the system) and timer pinch-valve to pump in smoke for pre-determined times based on the concentration of smoke required within the chambers. Room air is continuously pumped into the chamber for the remaining period between puffs. The duration of exposure periods was 50 minutes followed by a 10 minute venting period at the end where the flow is increased to maximum. Exposures for each group will take place in one of the Teague chambers (136L) chambers. A fan is placed at the bottom of the chamber on the left side where the smoke enters the chamber to ensure that the smoke is well dispersed throughout the chamber. Total suspended particulate (TSP) levels are assessed for each chamber at 15 minute intervals (15, 30, 45 minutes - 1 min sampling period) in order to validate the consistency of the smoke concentration within the chambers.
Dose response
Mice were exposed to either room air (1500 ml/minute) or CS (250, 500 or 750 ml/min in a total of 1500 ml/minute) using 3R4F cigarettes (Tobacco Health Research Institute) for 50 minutes, twice daily, for three consecutive days. BAL fluid white cell counts (neutrophils, monocytes/macrophages, eosinophils and lymphocytes) were determined as previously described [27].
Time course
Temporal changes in airway inflammation following CS exposure (500 ml/min) were assessed (samples taken 2, 6, 24, 48, 72, 96 and 168 hours after last challenge).
Lung tissue (cytosolic fraction) caspase 1 activity levels were assessed using an assay from Enzo Life Sciences UK Limited. mRNA expression levels were measured using either Super array Custom RT2 Profiler PCR arrays from Tebu-Bio or real-time PCR using standard techniques and validated primers/probes [27]. Cytokines were assessed using either ultrasensitive plates from Mesoscale Discovery or standard ELISA from R&D systems.
Development and characterisation of lipopolysaccharide (LPS) driven model of airway inflammation
Parallel experiments were performed using a stimulus of the normal innate defence system, LPS [27]. Briefly, LPS challenging was performed using a Perspex chamber (600×240×350 mm) and a System 22 nebuliser (Medic-Aid Ltd., Pagham, Sussex) driven by a high-flow-rate compressor (Medic-Aid Ltd., Pagham, Sussex). Animals were exposed to either aerosolised LPS (Escherichia coli, serotype 0111:B4, Sigma-Aldrich Ltd. Poole, UK) or endotoxin free saline (Fresenius Kabi, Warrington, UK) blown into the Perspex chamber for a 30 minute challenge period.
Role of P2X7 receptor in CS/LPS driven airway inflammation
Wild-type and P2X7−/− mice were exposed to the previously established CS or LPS exposure protocol. Twenty-four or six hours after the final exposure airway inflammation was assessed.
Effect of P2X7 receptor antagonist on CS-induced airway inflammation
Mice were treated with vehicle (0.5% methyl cellulose and 0.2% tween80 in distilled H2O, 10 ml/kg, p.o.) or A-438079, 1 hour prior to the first exposure and 1 hour after the second exposure each day. Animals were then exposed to either room air or CS as above. Airway inflammation was assessed 24 hours after the final exposure.
Effect of P2X7 receptor antagonist on LPS induced airway inflammation
Mice were treated with vehicle or A 438079 orally, 1 hour prior to challenge. Airway inflammation was assessed 6 hours after the LPS challenge.
Characterisation of a more chronic CS model
Male mice were exposed to room air or cigarette-smoke (500 ml/min), twice daily. Airway inflammation was determined 24 hours after 3, 7, 10, 14, 17, 21, 24 or 28 days of challenging.
Role of P2X7 receptor in the more chronic CS driven airway inflammation
Wild-type and P2X7−/− mice were exposed to room air or CS twice a day for 28 days. Twenty-four after the final exposure airway inflammation was assessed.
Human tissue
Human lung tissue samples were obtained from a transplant programme. Informed written consent was obtained for the use of human tissues for research. Ethical approval for the study was obtained from the Royal Brompton and Harefield ethics committee (09/H0708/72).
Compounds and materials
AZ 11645373 was kindly provided by Astra Zeneca Pharmaceuticals. A 438079 was synthesized by Peakdale Molecular. All other agents were purchased from Sigma-Aldrich unless otherwise described.
Data analysis
Data is expressed as mean ± s.e.m of n observations. Statistical significance was determined using either Student's t-test or One-way ANOVA followed by an appropriate post-hoc test. A P value <0.05 was taken as significant and all treatments were compared with the appropriate control group.
Results
Development and characterisation of CS driven model of airway inflammation
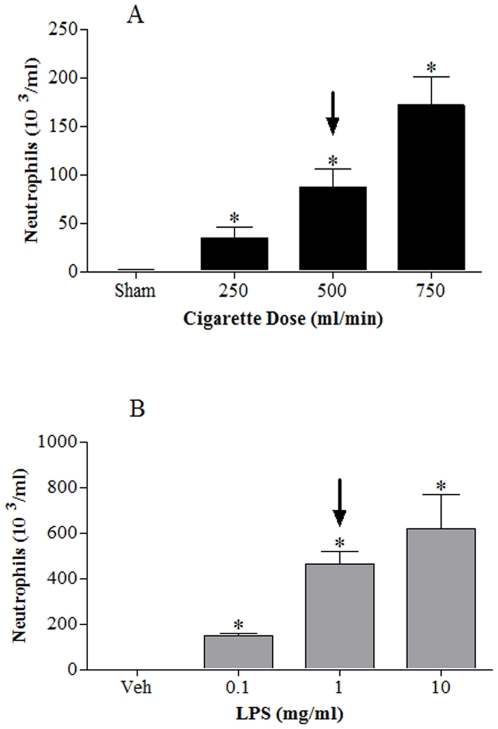
Exposure to CS resulted in a dose-related increase in white blood cells in the airway lumen; predominantly neutrophils (Figure 1A). The 500 ml/min dose of CS was selected as a sub-maximal stimulant dose and used throughout this project.
Figure 1. Characterisation of CS and LPS challenge models.
Mice were challenged with air or CS (1 hour, twice a day, for 3 days) and lungs lavaged 24 hours after final exposure; neutrophil numbers are presented in panel A. Mice were challenged with aerosolised saline or LPS (30 minutes) and lungs lavaged 6 hours after exposure; neutrophil numbers are presented in panel B. The arrows indicate the challenge dose we chose to continue our studies. Data represent mean ± s.e.mean, n = 6. * indicates statistically significant difference from air/saline control group (One-way ANOVA with a Dunn's post test).
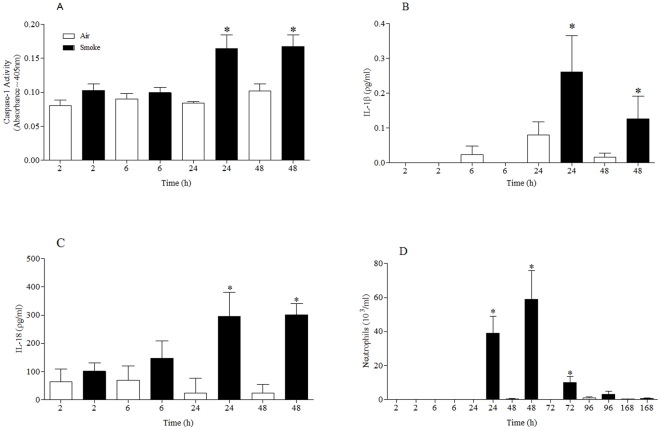
Temporal characterisation of the effects of CS exposure revealed that there appeared to be a correlation between the increase in caspase 1 activity, IL-1β/IL-18 release and airway neutrophilia (Figure 2). Caspase 1 activity in the cell free BALF was not altered (data not shown). Further analysis showed that CS challenge did not greatly alter lung tissue levels of IL-1β, IL-18, pro-caspase 1 and P2X7 mRNA (Text S1; Figure S1).
Figure 2. Temporal characterisation of P2X7 – NLRP3 inflammasome associated end points in the CS driven murine model.
Mice were challenged with air or CS (1 hour, twice a day, for 3 days) and samples collected at various times after the last challenge. (A) Caspase 1 activity in the lung tissue (B) IL-1β levels in the BALF (C) IL-18 levels in the BALF (D) numbers of neutrophils in the BALF. Data represent mean ± s.e.mean, n = 6. * indicates statistically significant difference from time matched control group (Mann-Whitney test).
Development and characterisation of LPS driven model of airway inflammation
Exposure to endotoxin, a trigger of the innate host defence mechanism, resulted in a dose-related increase in white blood cells in the airway lumen; again the profile of cell type increased was predominantly neutrophilia (Figure 1). The 1 mg/ml dose of LPS was selected as a sub-maximal stimulant dose and used throughout this project.
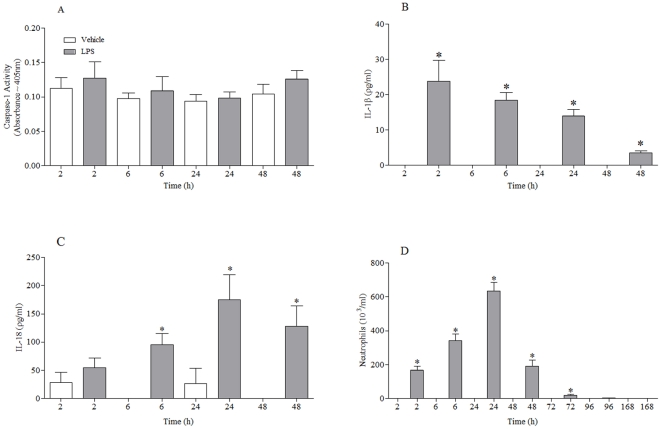
Temporal characterisation of the effects of LPS exposure revealed that there appeared to be a correlation between the increase in IL-1β/IL-18 release and airway neutrophilia (Figure 3), however unlike the CS model there was no increase in caspase 1 activity. Caspase 1 activity in the cell free BALF was not altered (data not shown). Further analysis showed that mRNA levels of pro-caspase 1 and IL-1β increased dramatically in the lung tissue after LPS challenge (Text S1; Figure S2). Whereas IL-18 and P2X7 mRNA levels were either not altered or decreased (Figure S2).
Figure 3. Temporal characterisation of P2X7 – NLRP3 inflammasome associated end points in the LPS driven murine model.
Mice were challenged with vehicle (endotoxin free sterile saline for 30 minutes) or LPS (1 mg/ml) and samples collected at various times after the last challenge (A) Caspase 1 activity in the lung tissue (B) IL-1β levels in the BALF (C) IL-18 levels in the BALF (D) numbers of neutrophils in the BALF. Data represent mean ± s.e.mean, n = 6. * indicates statistically significant difference from time matched control group (Mann-Whitney test).
Role of P2X7 receptor in CS driven airway inflammation
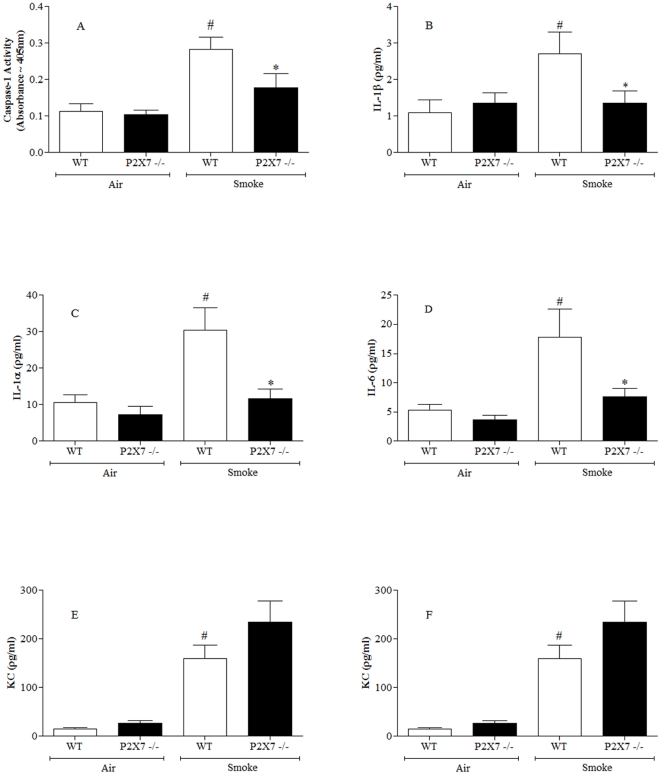
Having established there was temporal correlative data to suggest a role for the P2X7-inflammasome pathway in CS driven inflammation, we further investigated its role by comparing wild-type and P2X7−/− mice. As can be seen in Figure 4, CS exposure failed to increase caspase 1 activity, IL-1β and airway neutrophilia in P2X7−/− mice. This is strong evidence for a role of the P2X7-inflammasome axis in CS-induced airway neutrophilia. Interestingly, levels of IL-1α and IL-6 were also attenuated in the knockout mice, whereas KC levels were not altered (Figure 4).
Figure 4. Role of the P2X7 – NLRP3 inflammasome axis in the CS driven murine model.
Wild type or P2X7 knockout mice were challenged with air or CS and samples collected 24 hours after the last challenge. (A) Caspase 1 activity in the lung tissue (B) IL-1β levels in the BALF (C) IL-1α levels in the BALF (D) IL-6 levels in the BALF (E) KC levels in the BALF (F) numbers of neutrophils in the BALF. Data represent mean ± s.e.mean, n = 8. # indicates statistically significant difference from air challenge control group. * indicates statistically significant difference from CS challenged control group (Mann-Whitney test).
Role of P2X7 receptor in LPS driven airway inflammation
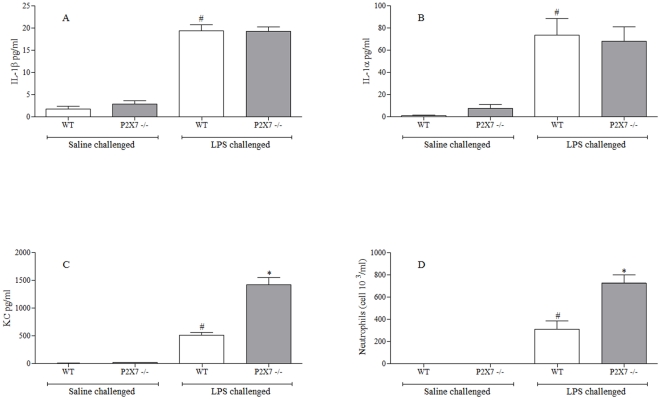
Figure 3 indicated that in the endotoxin driven inflammation there was not a role for the P2X7-inflammasome axis. To investigate this further wild-type and P2X7−/− mice were challenged with LPS. As expected P2X7−/− mice did not have altered levels of the inflammasome linked IL-1β, or indeed IL-1α (Figure 5). Interestingly, we did observe a statistical significant increase in BAL KC levels and neutrophilia (Figure 5).
Figure 5. Role of the P2X7 – NLRP3 inflammasome axis in the LPS driven murine model.
Wild type or P2X7 knockout mice were challenged with saline or LPS and samples collected 6 hours after the last challenge. (A) IL-1β levels in the BALF (B) IL-1α levels in the BALF (C) KC levels in the BALF (D) numbers of neutrophils in the BALF. Data represent mean ± s.e.mean, n = 8. # indicates statistically significant difference from air challenge control group. * indicates statistically significant difference from CS challenged control group (Mann-Whitney test).
Establishing optimum P2X7 inhibitor
To confirm the data with the P2X7−/− mice we wanted to parallel the study using a selective small molecular weight receptor inhibitor. Prior to doing this we needed to establish an appropriate tool to use in our murine model. To do this we first selected appropriate human and mouse cells to develop a P2X7-inflammasome driven in vitro assay. We detected P2X7 receptor and pro-caspase 1 expression at the mRNA and protein level in human (THP-1) and mouse (J774) monocytes/macrophages (Text S1; Figure S3). Using these cell types we established sub-maximal concentrations of ATPγS (P2X7 agonist) or LPS that induced IL-1β release (data not shown). Combination of these two stimuli resulted in enhanced release of inflammasome linked cytokines, IL-1β and IL-18, but not other cytokines such as TNFα and IL-6 (data not shown). Using these systems we determine the impact of 2 structurally distinct P2X7 inhibitors. The combination of ATPγS and LPS leads to enhanced IL-1β release (Text S1; Figure S4). Treatment with AZ 11645373 attenuated the enhanced release of IL-1β in human cells, suggesting successful blockade of the human P2X7 receptors, but not murine cells (Text S1; Figure S4). Conversely, A 438079 failed to have an impact in the human cell assay but did block the enhanced release of IL-1β from murine cells (Text S1; Figure S4). A 438079 did not alter levels of TNFα or IL-6 (data not shown). We did not detect any IL-1α release in either of the cell systems (data not shown).
Effect of P2X7 receptor antagonist on CS/LPS-induced airway inflammation
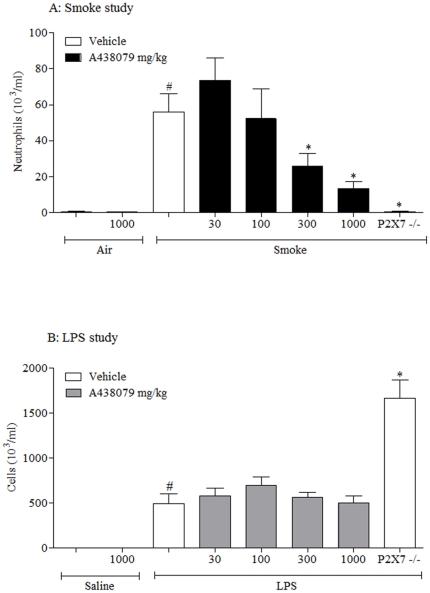
Having selected an appropriate tool to block murine P2X7 receptors we profiled it in the CS driven model. Consistent with the P2X7−/− mice, the compound caused a dose related inhibition in airway neutrophilia (Figure 6A). Lymphomononuclear cell numbers: Air/Vehicle−78.0±5.9; CS/Vehicle−119.4±11.7; CS/compound (1000 mg/kg)−138.9±9.3; CS/P2X7 −/− −120.9±10.9 cell 103/ml.
Figure 6. Role of the P2X7 – NLRP3 inflammasome axis in the CS or LPS driven murine models.
(A) Wild type or P2X7 −/− mice were challenged with air or CS and received vehicle (0.5% methyl cellulose and 0.2% tween80 in distilled water, 10 ml/kg, orally) or A 438079 twice a day and one hour prior to the cull. Numbers of neutrophils in the airway lumen was measured twenty-four hours after the final challenge. (B) Wild type or P2X7 −/− mice were challenged with saline or LPS and received vehicle or A 438079 1 hour prior to challenge. Numbers of neutrophils in the airway lumen was measured six hours after the challenge. Data represent mean ± s.e.mean, n = 8. # indicates statistically significant difference from air/saline challenge control group. * indicates statistically significant difference from CS/LPS challenged control group (One-way ANOVA with a Dunn's post test).
In the LPS driven model, unlike in the P2X7−/− mice, the compound had no impact on neutrophil levels (Figure 6B). Lymphomononuclear cell numbers: Saline/Vehicle−69.3±5.2; LPS/Vehicle−38.9±6.4; LPS/compound (1000 mg/kg)−34.1±3.6; LPS/P2X7 −/− −34.1±7.3 cell 103/ml.
Characterisation of a more chronic CS model
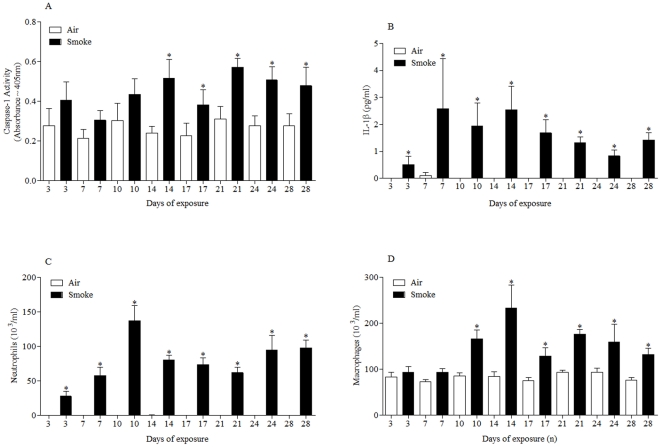
The data so far strongly suggested that the P2X7-inflammasome axis is central to the inflammation induced after CS exposure. To determine if this axis is important in ongoing inflammation, we measured markers of the pathway in a more chronic model. Temporally correlated with the increase in neutrophils and macrophages observed (Figure 7), we measured an increase in caspase 1 activity and IL-1β levels. This suggests that the P2X7-inflammasome axis is not only central to the induction of CS-induced inflammation but also in the ongoing inflammation.
Figure 7. Temporal characterisation of P2X7 – NLRP3 inflammasome associated end points in the sub-chronic CS driven murine model.
Mice were challenged with air or CS (1 hour, twice a day, for 3–28 days) and lungs lavaged 24 hours after final exposure. A) Caspase 1 activity in the lung tissue (B) IL-1β levels in the BALF (C) numbers of neutrophils in the BALF (D) numbers of macrophages in the BALF. Data represent mean ± s.e.mean, n = 6. * indicates statistically significant difference from time matched control group (Mann-Whitney test).
Role of P2X7 receptor in a more chronic CS driven airway inflammation
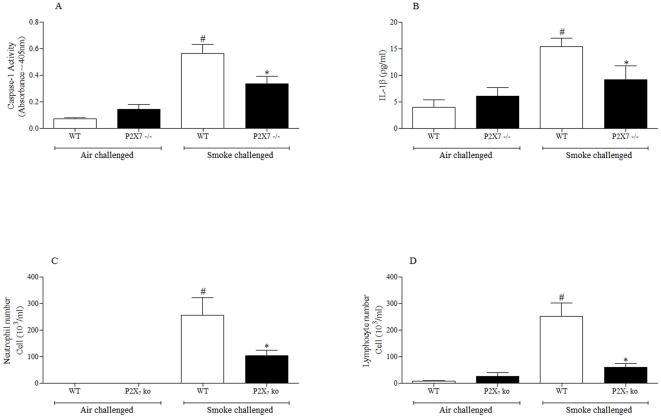
To further investigated the P2X7-inflammasome axis in the more chronic model we compared the inflammation in wild-type and P2X7−/− mice after 28 days of CS exposure. As can be seen in Figure 8 P2X7−/− mice had significantly reduced caspase 1 activity, BAL IL-1β, neutrophils and lymphocytes. Macrophages numbers were also reduced (data not shown).
Figure 8. Role of the P2X7 – NLRP3 inflammasome axis in the sub-chronic CS driven murine model.
Wild type or P2X7 knockout mice were challenged with air or CS twice a day for 28 days and samples collected 24 hours after the last challenge. (A) Caspase 1 activity in the lung tissue (B) IL-1β levels in the BALF (C) numbers of neutrophils in the BALF (D) numbers of lymphocytes in the BALF. Data represent mean ± s.e.mean, n = 5–8. # indicates statistically significant difference from air challenge control group. * indicates statistically significant difference from CS challenged control group (Mann-Whitney test).
Expression of P2X7-inflammasome axis in human tissue
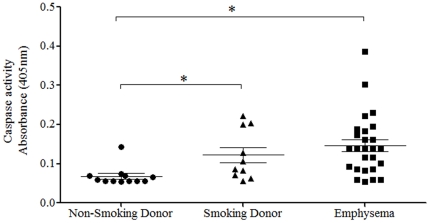
To determine if our observations in the pre-clinical models translated into the human disease, we measured caspase 1 activity in lung tissue from non-smoking donors, smoking donors and emphysema patients (details of which are listed in Table 1). Although the sample numbers and patient information are limited, it can be clearly seen that, like in our pre-clinical models, caspase 1 activity is increased in diseased tissue (Figure 9).
Table 1. Details on the donor/recipient.
| Disease Group | Age (years) | Sex (M/F) |
| Non-Smoking Donor | 27–72 | 3/9 |
| Smoking Donor | 22–62 | 7/3 |
| Emphysema | 49–69 | 17/8 |
Figure 9. Caspase 1 activity levels in human lung samples.
Lung samples were collected from transplant surgery either donor tissue (non-smokers or smokers) or diseased tissue. Caspase 1 activity in the cytoplasmic fraction of the extracted lung tissue was assessed using a specific assay. Data represent as individual data with bar indicating mean ± s.e.mean. * indicates statistically significant difference from non-smoking donor lung tissue levels (One-way ANOVA with a Dunn's post test).
Discussion
COPD is a progressive condition associated with exposure to CS and is currently reported to be the fourth leading cause of death worldwide and is predicted to rise to the third [3]. Furthermore, COPD-related costs outstrip those of other respiratory diseases such as asthma and as yet there are currently no pharmacological therapies to combat the pathogenesis and relentless progression of this disease [5]. Understanding the mechanisms driving the chronic inflammation seen in patients with the disease would undoubtedly aid the development of effective medication. In the inflammatory milieu present in the lungs of smoke-exposed mice, and human patients with COPD, are increased levels of cytokines linked to the activation of the NLRP3 inflammasome i.e. IL-1β and IL-18 [6], [7], [8], [9], [10], [28]. There is some evidence to suggest that these cytokines are central to the inflammation seen in models of COPD [11], [29]. Recently it has been shown that levels of an activator of the NLRP3 inflammasome, ATP, are increased in pre-clinical smoke-exposure models [23], [24]. Additionally, increased ATP levels in the lungs of patients with COPD have been shown to be associated with a decline in lung function and an increase in inflammatory cellular burden [21]. We suggest that exposure to CS leads to the release of endogenous danger signals (e.g. ATP) which activates the NLRP3 inflammasome via the P2X7 purinergic receptor [13] and thus causes the maturation and subsequent release of IL-1β and IL-18. We hypothesise that blockade of the P2X7 – NLRP3 inflammasome pathway will attenuate the inflammation present in CS-induced airway inflammation. Through the use of in vitro and in vivo pre-clinical modelling systems we showed a temporal correlation between markers of the P2X7/inflammasome pathway activation and airway inflammation. Furthermore, we demonstrate that modulation of this pathway, using either a selective P2X7 inhibitor or P2X7 knockout mice, attenuates the airway inflammation in this disease model. This data is consistent with data recently published by Lucattelli et al [24]. This pathway, however, was not involved in a similar inflammatory response (i.e. IL-1β release) observed after the normal innate defence mechanism is triggered by an endotoxin insult suggesting that this pathway is only activated in disease settings. Finally, using lung samples collected from patients with COPD, we demonstrate that markers of inflammasome activation are evident in tissue from diseased patients with COPD.
Exposure to CS, whether it be acute (induction of the inflammation) or more chronic (up to 28 days), resulted in an increase in caspase 1 activity and release of NALP-3 inflammasome linked cytokine IL-1β. A similar increase in caspase 1 activity was observed in lung tissue from patients with COPD so severe/chronic that they required a lung transplant which suggests that the pre-clinical model reflects the clinical disease and that the role of caspase 1 is not restricted to the induction of the inflammation but is chronically elevated. We postulate that the increase in activity was not due to an increased amount of caspase 1 because we did not observe an increase in mRNA expression. On caveat is that the increase in caspase activity observed in the human lung samples may not be through activation of the P2X7 receptor as other things (like uric acid or reactive oxygen species) than can activate NLRP3. Further assessment of the CS models revealed no change in IL-1β mRNA levels which is intriguing because we did measure an increase in this cytokine at the protein level. As the mechanism of action of glucocorticoids is generally thought to be blockade of transcription/translation of inflammatory cytokines, this could be one reason why the inflammation observed in COPD patients is resistant to glucocortoid treatment. If the inflammation is driven via a mechanism which does not rely on transcription/translation it could explain the limited impact of a steroid treatment.
Further evidence for a central role of the P2X7 – NLRP3 inflammasome pathway in CS-induced neutrophilia came from the studies we performed with either mice missing functional P2X7 receptors or a small molecular weight inhibitor. The fact that we obtained very similar data using both mechanisms of blocking the P2X7 receptor makes the results more compelling. It was interesting to note that cytokines not thought to be linked to the inflammasome/caspase 1 were also reduced after blockade of the P2X7 receptor. We suggest that the reduction in IL-1α and IL-6 could be a function of blocking the neutrophilia, rather than a direct effect on the production/release of the cytokines themselves. Indeed in the cell based systems cytokines such as IL-6 and TNFα were not affected by the P2X7 inhibitor. We could not detect any IL-1α release in our human and mouse cell based systems which, although possibly different to that reported by others [30], would indicate further that this cytokine is not controlled by the mechanisms which govern IL-1β/IL-18 release. IL-1α release has been suggested to be controlled by IL-1β, thus it could be that the inhibition of IL-1β release accounts for the reduction we observed in IL-1α [31]. The observation that the levels of KC were not affected by blockade of the P2X7–inflammasome axis is worthy of note. It would suggest that the CS-induced production/release of KC is via another mechanism, its source being cells present in the lung, rather than the neutrophils being recruited.
To establish an appropriate pharmacological P2X7 tool to use in the murine models we used human and murine cell based assays. We selected the cell type based on the expression of targets of interest i.e. the P2X7 receptor and caspase 1 and from the literature [26], [32]. We found that human THP-1 cells and murine equivalent J774 cells expressed both targets at the mRNA and protein level, whereas the epithelial cell lines (A549 and LA4) did not which is consistent to that reported by other groups [31]. This would suggest that the inflammasome-linked cytokines in the airways are more likely to be produced by macrophages/monocytes rather than epithelial cells. When we stimulated the THP-1/J774 cells with both LPS and ATP the level of IL-1β release was greater than the sum of the stimuli separately. Using these modelling systems we demonstrated that the P2X7 receptor was central to this enhanced release of IL-1β; this has been demonstrated by many other groups [16], [26], [32], [33], [34]. Of interest to us was that AZ 11645373 failed to impact on the murine cells, whereas A 438079 only impacted on the murine system, even though it is reported to act on the human receptor [35]. Despite the apparent lack of activity in the human cell system, we were able to use A 438079 as a tool in our murine models.
Interestingly although IL-1β (and IL-1α) levels after LPS challenge was not altered in the P2X7 −/− mice, we did observe an increase in airway KC and neutrophilia. The fact that we did not parallel this increase with the P2X7 inhibitor may indicate that the phenomenon is related to either the genetic manipulation or long term/complete blockade of this receptor.
In conclusion these results advocate the critical role of the P2X7/inflammasome axis in cigarette smoke induced inflammation. What is more the data suggests that the pathway has an ongoing role in the pathogenesis of COPD and highlights it as possible therapeutic targets in combating this deadly disease.
Supporting Information
Temporal characterisation of P2X7 – NLRP3 inflammasome associated end points in the CS driven murine model. Mice were challenged with air or CS (1 hour, twice a day, for 3 days) and samples collected at various times after the last challenge. (A) IL-1β mRNA levels in the lung tissue (B) IL-18 mRNA levels in the lung tissue (C) caspase 1 mRNA levels in the lung tissue (D) P2X7 mRNA levels in the lung tissue. Data represent mean ± s.e.mean, n = 6. * indicates statistically significant difference from time matched control group (Mann-Whitney test).
(TIF)
Temporal characterisation of P2X7 – NLRP3 inflammasome associated end points in the LPS driven murine model. Mice were challenged with saline or LPS (30 minutes) and samples collected at various times after the last challenge. (A) IL-1β mRNA levels in the lung tissue (B) IL-18 mRNA levels in the lung tissue (C) caspase 1 mRNA levels in the lung tissue (D) P2X7 mRNA levels in the lung tissue. Data represent mean ± s.e.mean, n = 6. * indicates statistically significant difference from time matched control group (Mann-Whitney test).
(TIF)
Caspase 1 and P2X7 mRNA levels in a range of human and mouse cell type. Cells were collected after standard culture conditions, mRNA extracted and RT-PCR performed to establish mRNA expression levels of caspase 1 and P2X7. Caspase 1 mRNA levels in human and murine cells are shown in A and C, respectively. P2X7 mRNA levels in human and murine cells are shown in B and D, respectively. Data represent mean ± s.e.mean, n = 3–6.
(TIF)
Determining the effectiveness of P2X7 receptor antagonist using human and mouse monocytes. Cultured cells were exposed to either sub-maximal concentration of ATPγs (1 mM) or LPS (0.1 µg/ml) or a combination of the two. Cells were pre-treated with vehicle or one of the inhibitors; IL-1β levels were measured by ELISA. (A) Effect of AZ 11645373 in THP-1 cells (B) Effect of AZ 11645373 in J774 cells (C) Effect of A 438079 in THP-1 cells (D) Effect of A 438079 in J774 cells. Data represent mean ± s.e.mean, 3 experimental runs each with n = 2. * indicates statistically significant difference from the respective control group (Mann-Whitney test and one-way ANOVA followed by a Dunn's post test).
(TIF)
(DOCX)
Footnotes
Competing Interests: MC is employed by Union chimique belge Pharma and CS by Roche. The other authors have declared that no competing interests exist. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: M. Birrell was funded by a project grant from the Medical Research Council (MRC), (United Kingdom) (MAB, G0800196). SE and CS were funded by a Capacity Building Award in Integrative Mammalian Biology funded by the Biotechnology and Biological Sciences Research Council, BPS, Higher Education Funding Council for England, KTN & MRC. JR and ND were funded by MRC studentships. The human tissue experiments in this manuscript were undertaken with the support of the National Institute for Health Research (NIHR) Biomedical Research Unit in Advanced Lung Disease at the Royal Brompton and Harefield National Health Service Foundation, Trust and Imperial College, London, and partly funded by the NIHR Biomedical Research Unit funding scheme. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.MacNee W. Pathogenesis of Chronic Obstructive Pulmonary Disease. The Proceedings of the American Thoracic Society. 2005;2:258–266. doi: 10.1513/pats.200504-045SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez AD, Murray CJL. The global burden of disease, 1990–2020. Nature Medicine. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 4.Grumelli S, Corry DB, Song LZ, Song L, Green L, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Medicine. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PJ, Stockley RA. COPD: current therapeutic interventions and future approaches. Eur Respir J. 2005;25:1084–1106. doi: 10.1183/09031936.05.00139104. [DOI] [PubMed] [Google Scholar]
- 6.Ekberg-Jansson A, Andersson B, Bake B, Boijsen M, Enanden I, et al. Neutrophil-associated activation markers in healthy smokers relates to a fall in DL(CO) and to emphysematous changes on high resolution CT. Respiratory Medicine. 2001;95:363–373. doi: 10.1053/rmed.2001.1050. [DOI] [PubMed] [Google Scholar]
- 7.Zeidel A, Beilin B, Yardeni I, Mayburd E, Smirnov G, et al. Immune response in asymptomatic smokers. Acta Anaesthesiol Scand. 2002;46:959–964. doi: 10.1034/j.1399-6576.2002.460806.x. [DOI] [PubMed] [Google Scholar]
- 8.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. American Journal of Respiratory Cell and Molecular Biology. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 9.Petersen AM, Penkowa M, Iversen M, Frydelund-Larsen L, Andersen JL, et al. Elevated levels of IL-18 in plasma and skeletal muscle in chronic obstructive pulmonary disease. Lung. 2007;185:161–171. doi: 10.1007/s00408-007-9000-7. [DOI] [PubMed] [Google Scholar]
- 10.Imaoka H, Hoshino T, Takei S, Kinoshita T, Okamoto M, et al. Interleukin-18 production and pulmonary function in COPD. European Respiratory Journal. 2008;31:287–297. doi: 10.1183/09031936.00019207. [DOI] [PubMed] [Google Scholar]
- 11.Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. Journal of Immunology. 2007;178:1948–1959. doi: 10.4049/jimmunol.178.3.1948. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino T, Kato S, Oka N, Imaoka H, Kinoshita T, et al. Pulmonary inflammation and emphysema: role of the cytokines IL-18 and IL-13. Am J Respir Crit Care Med. 2007;176:49–62. doi: 10.1164/rccm.200603-316OC. [DOI] [PubMed] [Google Scholar]
- 13.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 14.Sutterwala FS, Ogura Y, Zamboni DS, Roy CR, Flavell RA. NALP3: a key player in caspase-1 activation. J Endotoxin Res. 2006;12:251–256. doi: 10.1177/09680519060120040701. [DOI] [PubMed] [Google Scholar]
- 15.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 16.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 17.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 18.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohsenin A, Blackburn MR. Adenosine signaling in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2006;12:54–9. doi: 10.1097/01.mcp.0000199002.46038.cb. [DOI] [PubMed] [Google Scholar]
- 20.Polosa R, Blackburn MR. Adenosine receptors as targets for therapeutic intervention in asthma and chronic obstructive pulmonary disease. Trends Pharmacol Sci. 2009;30:528–535. doi: 10.1016/j.tips.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 22.Cicko S, Lucattelli M, Müller T, Lommatzsch M, De Cunto G, et al. Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol. 2010;185:688–697. doi: 10.4049/jimmunol.0904042. [DOI] [PubMed] [Google Scholar]
- 23.Mortaz E, Braber S, Nazary M, Givi ME, Nijkamp FP, et al. ATP in the pathogenesis of lung emphysema. Eur J Pharmacol. 2009;619:92–96. doi: 10.1016/j.ejphar.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Lucattelli M, Cicko S, Müller T, Lommatzsch M, de Cunto G, et al. P2X7 Receptor Signalling in the Pathogenesis of Smoke-induced Lung Inflammation and Emphysema. Am J Respir Cell Mol Biol. 2010;44(3):423–9. doi: 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- 25.Churg A, Zhou S, Wang X, Wang R, Wright JL. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol. 2009;40:482–490. doi: 10.1165/rcmb.2008-0038OC. [DOI] [PubMed] [Google Scholar]
- 26.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 27.Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, et al. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J. 2005;19:840–841. doi: 10.1096/fj.04-2691fje. [DOI] [PubMed] [Google Scholar]
- 28.Hoshino Y, Nakamura T, Sato A, Mishima M, Yodoi J, et al. Neurotropin demonstrates cytoprotective effects in lung cells through the induction of thioredoxin-1. Am J Respir Cell Mol Biol. 2007;37:438–446. doi: 10.1165/rcmb.2006-0402OC. [DOI] [PubMed] [Google Scholar]
- 29.Lucey EC, Keane J, Kuang P, Snider GL, Goldstein RH. Severity of Elastase-Induced Emphysema Is Decreased in Tumor Necrosis Factor-α and Interleukin-1β Receptor-Deficient Mice. Laboratory Investigation. 2002;82:79–85. doi: 10.1038/labinvest.3780397. [DOI] [PubMed] [Google Scholar]
- 30.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 32.Grahames CB, Michel AD, Chessell IP, Humphrey PP. Pharmacological characterization of ATP- and LPS-induced IL-1beta release in human monocytes. Br J Pharmacol. 1999;127:1915–1921. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151:571–579. doi: 10.1038/sj.bjp.0707265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Temporal characterisation of P2X7 – NLRP3 inflammasome associated end points in the CS driven murine model. Mice were challenged with air or CS (1 hour, twice a day, for 3 days) and samples collected at various times after the last challenge. (A) IL-1β mRNA levels in the lung tissue (B) IL-18 mRNA levels in the lung tissue (C) caspase 1 mRNA levels in the lung tissue (D) P2X7 mRNA levels in the lung tissue. Data represent mean ± s.e.mean, n = 6. * indicates statistically significant difference from time matched control group (Mann-Whitney test).
(TIF)
Temporal characterisation of P2X7 – NLRP3 inflammasome associated end points in the LPS driven murine model. Mice were challenged with saline or LPS (30 minutes) and samples collected at various times after the last challenge. (A) IL-1β mRNA levels in the lung tissue (B) IL-18 mRNA levels in the lung tissue (C) caspase 1 mRNA levels in the lung tissue (D) P2X7 mRNA levels in the lung tissue. Data represent mean ± s.e.mean, n = 6. * indicates statistically significant difference from time matched control group (Mann-Whitney test).
(TIF)
Caspase 1 and P2X7 mRNA levels in a range of human and mouse cell type. Cells were collected after standard culture conditions, mRNA extracted and RT-PCR performed to establish mRNA expression levels of caspase 1 and P2X7. Caspase 1 mRNA levels in human and murine cells are shown in A and C, respectively. P2X7 mRNA levels in human and murine cells are shown in B and D, respectively. Data represent mean ± s.e.mean, n = 3–6.
(TIF)
Determining the effectiveness of P2X7 receptor antagonist using human and mouse monocytes. Cultured cells were exposed to either sub-maximal concentration of ATPγs (1 mM) or LPS (0.1 µg/ml) or a combination of the two. Cells were pre-treated with vehicle or one of the inhibitors; IL-1β levels were measured by ELISA. (A) Effect of AZ 11645373 in THP-1 cells (B) Effect of AZ 11645373 in J774 cells (C) Effect of A 438079 in THP-1 cells (D) Effect of A 438079 in J774 cells. Data represent mean ± s.e.mean, 3 experimental runs each with n = 2. * indicates statistically significant difference from the respective control group (Mann-Whitney test and one-way ANOVA followed by a Dunn's post test).
(TIF)
(DOCX)