Abstract
Long inverted repeats (palindromes) are ubiquitous among prokaryotic and eukaryotic genomes. Earlier work has implicated both DNA breaks and short inverted repeats (IRs) in the formation of long palindromes in yeast and Tetrahymena by a proposed mechanism of intramolecular recombination. Here we report that long-palindromic linear plasmids are formed in Streptomyces following double strand DNA breakage by a nonrecombinational intra-strand annealing process that also involves IRs. By modification of palindrome-generating linear plasmids and development of a novel procedure that enables the sequencing of palindrome junctions, we show that long-palindrome formation occurs by unimolecular intra-strand annealing of IRs followed by 3′ extension of the resulting DNA fold-back. The consequent hairpin structures serve as templates for synthesis of duplex linear plasmids containing long palindromes. We suggest that this model for long-palindrome formation in Streptomyces may represent a generally applicable mechanism for generating DNA palindromes.
Keywords: Palindrome, inverted repeat, linear plasmid, telomere, DNA damage
Long inverted repeat sequences (DNA palindromes) are a ubiquitous feature of chromosomal and extrachromosomal DNAs of plants, animals, and bacteria where they have been found to have a prominent role in genetic instability and gene amplification (for reviews, see Fried et al. 1991; Rayko 1997). Long palindromes constitute the limbs of bacterial transposable elements (Kopecko and Cohen 1975; for reviews, see Berg and Howe 1989), the telomeres of some linear replicons (Sakaguchi 1990), and the arms of amplified DNA segments associated with drug resistance (Beverley et al. 1984; Walton et al. 1986) or neoplasia (Bishop 1991; Brison 1993; Stark 1993). They are the substrates for multiple pathways of DNA recombination in Escherichia coli (Cromie et al. 2000) and the products of processes as diverse as the repair of chromosome breaks (Windle et al. 1991; Coquelle et al. 1997) and programmed cell differentiation (Coyne et al. 1996) in mammalian cells.
Long DNA palindromes have been studied extensively in both prokaryotes and eukaryotes and earlier investigations have led to multiplicity of suggestions about the mechanism of their formation (Kikuchi et al. 1985; Ellis and Day 1986; Ford and Fried 1986; Passananti et al. 1987; Hyrien et al. 1988; Kunes et al. 1990; Fried et al. 1991; Ma et al. 1993; Butler et al. 1995, 1996; Lin et al. 1997; Lyu et al. 1999). Short inverted repeats (IRs) have been implicated in two particularly appealing models considered by Butler et al. (1995, 1996); these involve (1) intramolecular homologous recombination at cruciform structures formed by short IRs located adjacent to double-strand DNA breaks (Butler et al. 1995, 1996), or (2) the formation of palindromic DNA by single strand annealing (SSA) of IRs followed by removal of nonhomologous DNA and gap-filling DNA replication (Butler et al. 1995). Investigations in the ciliate Tetrahymena have led to the conclusion that formation of DNA palindromes by the latter mechanism is unlikely (Butler et al. 1995), and subsequent work in yeast has provided experimental support for a model of palindrome formation by intramolecular homologous recombination (Butler et al. 1996).
Although most plasmids isolated from prokaryotic and eukaryotic cells exist as DNA circles, some replicate as linear DNA (Kinashi et al. 1987; Sakaguchi 1990; Hinnebusch and Tilly 1993). Streptomyces is a Gram-positive filamentous spore-forming eubacterial genus that contains linear chromosomes (Lin et al. 1993) as well as a variety of stably-inherited linear plasmids ranging in length from 12 kb to >1000 kb (Kinashi et al. 1987; Keen et al. 1988; Sakaguchi 1990; Pandza et al. 1998). pSLA2 is a 17-kb high-copy-number linear Streptomyces rochei plasmid (Hirochika and Sakaguchi 1982; Hirochika et al. 1984) whose replication is initiated internally and proceeds bidirectionally from the origin towards the telomeres, where a leading strand 3′ overhang is filled in to produce blunt-ended duplex molecules (Chang and Cohen 1994; Chang et al. 1996; Qin and Cohen 1998). As the termini of Streptomyces linear chromosomes closely resemble those of pSLA2 and other linear Streptomyces plasmids, chromosomal DNA replication is presumed to occur by a similar mechanism (Fischer et al. 1998; Huang et al. 1998; Qin and Cohen 1998).
Whereas lengthy deletions within one of the two pSLA2 telomeres normally prevent pSLA2 replication as a linear plasmid (Qin and Cohen 1998), we observed that such damaged plasmids can survive by becoming large palindromic linear DNA replicons containing a normal telomere at each end. The ability to create collections of independently formed palindromic derivatives of the same molecular species in Streptomyces provided an opportunity to investigate in detail the mechanism of palindrome formation. Our findings indicate that palindromic linear replicons can be produced in Streptomyces by unimolecular nonrecombinational events involving (1) intra-strand annealing of short IRs located on a single strand DNA segment, and (2) replication/extension from the 3′ terminus using a strand of the folded back DNA molecule as a template.
Results
Formation of palindromic linear replicons following telomere damage in linear plasmids
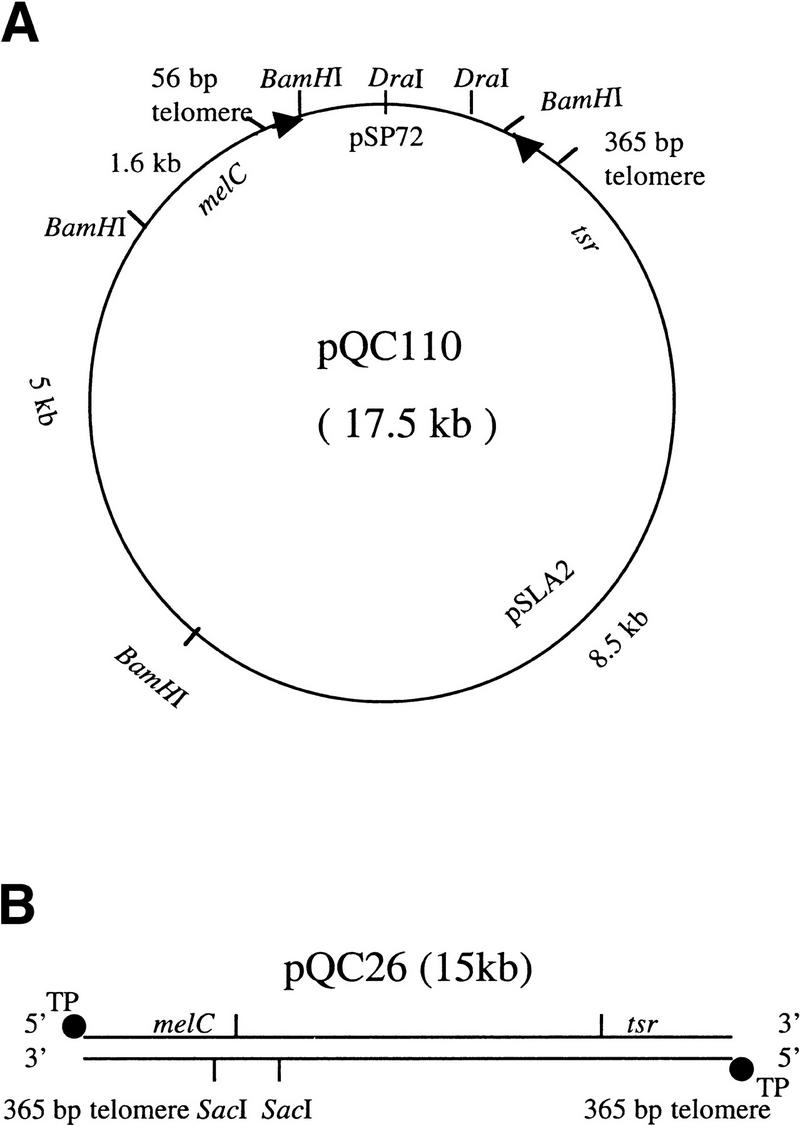
During investigations of sequence requirements for propagation of the S. rochei plasmid pSLA2 (Qin and Cohen 1998), we observed that constructs having only one intact telomere occasionally yielded viable plasmid replicons. To determine the nature of such plasmids, we constructed pSLA2 derivatives containing a functionally defective telomere that includes only the terminal 56 bp (Qin and Cohen 1998) (pQC110; Fig. 1); these were linearized with DraI endonuclease and introduced by transformation into Streptomyces lividans strain ZX7. As seen in Table 1, using >1 μg plasmid DNA and ZX7 protoplasts transformable at high efficiency, we obtained stable transformants; these were observed at 0.01× the transformation frequency seen for pSLA2 replicons containing two functional telomeres. Similar results were obtained for constructs containing a telomere damaged at other sites or lacking one telomere entirely (data not shown).
Figure 1.
Schematic map of pSLA2-derived circular and linear plasmids. (A) pQC110, showing the E. coli pSP72 plasmid-derived segment and a pSLA2-derived DNA segment containing one functional 365-bp telomere (Qin and Cohen 1998) and one defective telomere containing only the terminal 56-bp sequence. Telomere ends are indicated by arrowheads. Relevant restriction endonuclease cleavage sites are shown. (B) pQC26, a pSLA2-derived linear plasmid isolated from S. lividans ZX7. Terminal protein (TP) is attached covalently to 5′ DNA termini. The locations of the melC (melanin-producing) and tsr (thiostrepton resistance) genes are shown, as are the locations of SacI cleavage sites. The plasmid contains two functional telomeres. Plasmid DNA was cloned in E. coli, linearized by treatment with DraI enconuclease, and introduced into ZX7 by transformation, as indicated in Materials and Methods.
Table 1.
Transformation of Streptomyces lividans ZX7 using pSLA2-derived circular and linear plasmids
| Plasmids
|
Treatmenta
|
Transformation frequencyb
|
No. of transformant clones examinedc
|
|---|---|---|---|
| DraI-linearized pQC110 (from E. coli) | NT | 1 × 101 | 16 |
| λ exonuclease | 2 × 102 | 6 | |
| exonuclease III | 3 × 101 | 8 | |
| NaOH/HCl | 5 × 102 | 10 | |
| SacI-cleaved linear pQC26 (from ZX7) | NT | 8 × 101 | 8 |
| λ exonuclease | 1 × 103 | 19 | |
| exonuclease IIId | 9 × 101 | 8 | |
| NaOH/HCl | 5 × 103 | 35 |
NaOH/HCl treatment (see Materials and Methods). Partial digestion of DNA by λ exonuclease or exonuclease III. (NT) No treatment.
Relative transformation frequency obtained by comparing to transformation frequency of DraI-linearized pQC101 (5 × 103 transformants/μg DNA).
Linear plasmid DNA replicons were isolated from individual colonies by adding proteinase K/SDS or NaOH/SDS (see Materials and Methods). In all instances, 100% of clones examined contained long-palindromic DNA.
Treated with Klenow fragment of DNA polymerase I before exonuclease III treatment.
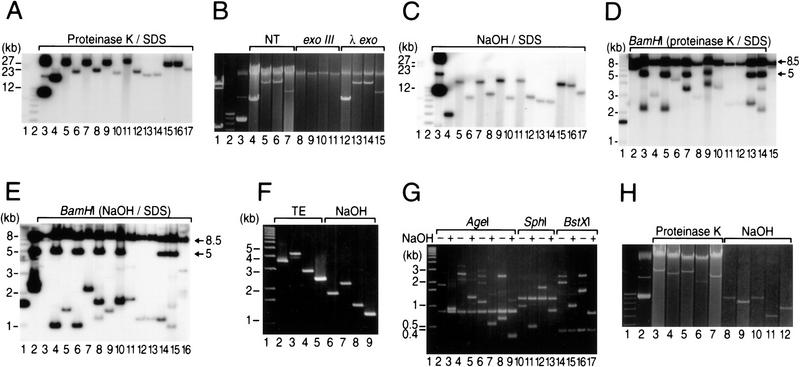
As seen in Figure 1, the distance between the telomere termini of pQC110 is ∼15 kb. However, gel analysis of plasmid DNA isolated from 14 individual pQC110-derived transformants showed bands corresponding to DNA species 14–30 kb in length (Fig. 2A); these DNAs were resistant to treatment with bacteriophage λ exonuclease but sensitive to E. coli exonuclease III (Fig. 2B), as described by Hirochika et al. (1984), Hirochika and Sakaguchi (1982), and by Chang and Cohen (1994), indicating that the rescued replicons are linear plasmids whose 5′ ends, like those of pSLA2, are protected by covalently bound terminal protein. Electrophoresis of these DNAs following treatment with 0.2 N NaOH and 1% SDS and neutralization by addition of acid-phenol-chloroform (Hopwood 1985) showed bands migrating in each instance as DNA molecules containing half the original mass (Fig. 2, cf. A with C). The conversion of the DNAs to faster migrating molecules implies that they consist of palindromic duplex linear replicons that yield single-strand hairpins on denaturation/renaturation (Ford and Fried 1986; Walton et al. 1986). Consistent with this notion, we found that transformation of ZX7 with gel-purified 14.5-kb molecules from lane 7 of Figure 2C produced 29-kb linear replicons identical in size to those seen in the corresponding lane of Figure 2A.
Figure 2.
Southern blot of pQC110 and pQC26-derived DNAs isolated from ZX7 transformants. (A) DNA isolated by treatment with proteinase K and SDS (Qin and Cohen 1998) from transformants receiving DraI-cleaved pQC110 DNA. DNAs were electrophoresed for 13 hr at 40 v in 0.5% agarose gel and probed with 32P-labled pQC110 DNA (lanes 4–17). Molecular lengths were calculated relative to HindIII-treated bacteriophage λ DNA (lane 1), a 1-kb DNA-size ladder (Life Technologies, Inc.) (lane 2), or covalently closed circular pQC110 DNA isolated from E. coli (lane 3). (B) Surviving replicons are linear plasmids. Lanes 4–7 (NT) show DNA isolated from 4 randomly selected transformants by proteinase K/SDS treatment. Aliquots of the same DNAs were treated with 100 units exonuclease III (lanes 8–11) or 10 units λ exonuclease (lanes 12–15) at 37°C for 4 hr and electrophoresed for 18 hr at 38 v in 0.5 % agarose gel. λ HindIII-treated DNA (lane 1), 1-kb DNA ladder (lane 2), and pQC110 DNA (from E. coli, lane 3) are molecular size markers. (C) Electrophoresis of pQC110-derived DNAs shown in A after treatment with NaOH and renaturation. Lane designations are as in A. (D) BamHI digestion of pQC110-derived DNAs from A. Lane 1 contains a 1-kb ladder. Lanes 2–15 correspond to DNAs in lanes 4–17 of A. The 8.5-kb and 5-kb DNA bands discussed in the text are indicated. (E) BamHI digestion of pQC110-derived DNAs following denaturation and renaturation. Lanes 2–16 correspond to DNAs in lanes 3–17 of A. (F) Effect of denaturation on migration of BamHI fragments containing putative palindrome apices of linear plasmids. Agarose gel analysis of inserts recovered from agarose gel following BamHI digestion of pQC143–pQC146. The banding position of DNAs dissolved in TE (lanes 2–5) or analyzed following treatment with NaOH and neutralization is shown in lanes 6–9. (G) Endonuclease analysis of BamHI fragments containing putative palindrome apices. DNAs were digested by the enzymes indicated and electrophoresed for 3 hr at 80 v on 1% agarose gel. Lanes 2, 4, 6, 8, and lanes 10, 12, 14, 16 correspond to lanes 2–5 from F. Lanes 3, 5, 7, 9, and lanes 11, 13, 15, 17 correspond to lanes 6–9 from F. (H) Effect of denaturation on migration of SacI-cleaved pQC26 DNA isolated from four transformants by adding proteinase K/SDS (lanes 3–7) or NaOH/SDS (lanes 8–12), and electrophoresed for 20 hr at 36 v in 0.5% agarose gel. Lane 1 (1-kb ladder) and 2 (pQC26, from E. coli) are markers.
Native and denatured/renatured linear plasmid DNA preparations isolated from 14 pQC110-derived transformants were compared by Southern blotting following digestion with BamHI endonuclease (Fig. 2D,E). No preparation from any transformant showed a DNA fragment corresponding to the distance between the end of the damaged telomere and the nearest internal BamHI site (see Fig. 1), implying that the damaged telomere was absent in every case. However, bands ∼8.5 kb in length, which is the distance from the end of the intact telomere to the proximal internal BamHI site (see Fig. 1), were observed for both types of DNA preparations in all plasmid survivors (Figs. 2D,E). Additionally, BamHI treatment of both native and denatured DNA preparations from some plasmids released an ∼5-kb band. When absent, this band was replaced by one that differed in length among different transformants and also differed in length for native versus denatured/renatured DNA. Importantly, BamHI treatment of all of the native plasmid DNA preparations also generated an additional variable-length band that dropped to half its original mass following denaturation and neutralization. One plasmid DNA (Fig. 2E, lane 3) had undergone an additional rearrangement that resembled the central deletions produced by palindrome resolution and recombination in mouse germ-line cells (Akgun et al. 1997).
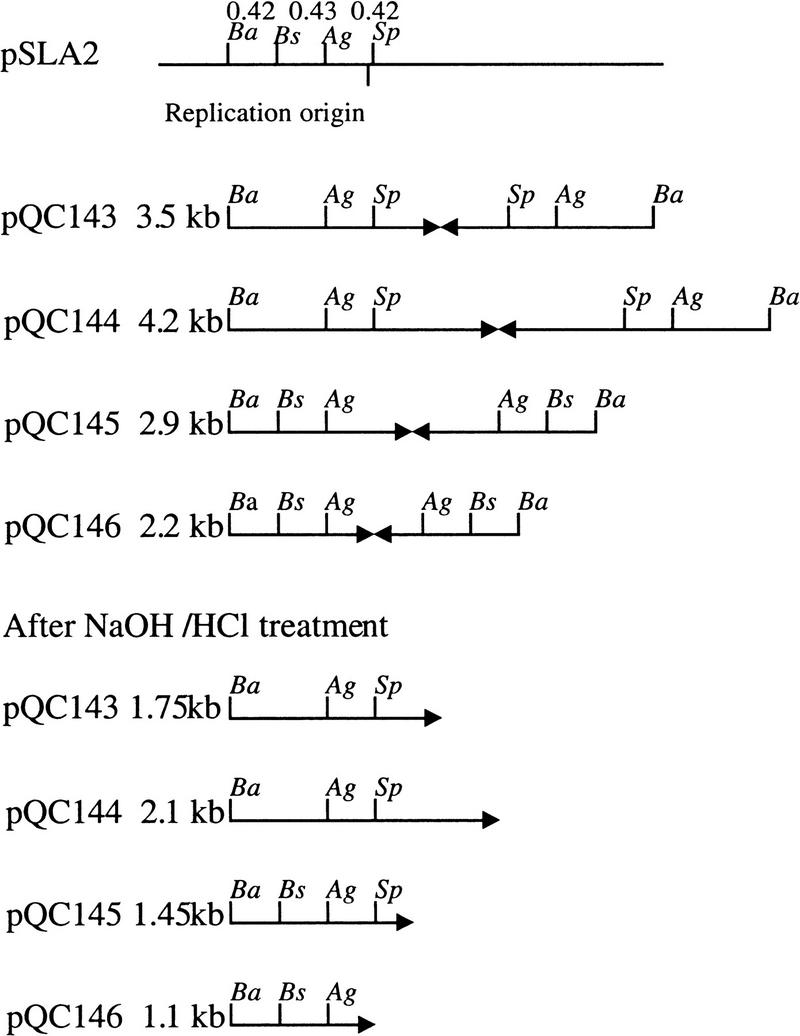
Together, the above observations suggested that deletions of different lengths had occurred in the damaged arm of pQC110 and that a process leading to the formation of palindromic replicons had duplicated the undamaged arm. To determine the correctness of this interpretation, we recovered the variable length BamHI fragments from the gel shown in Figure 2D and cloned these fragments in BamHI-cleaved pSP72, yielding the plasmids pQC143, pQC144, pQC145, and pQC146, respectively, from lanes 10, 6, 4, and 11. The BamHI pQC110-derived insert of each of these plasmids was reduced to half the original duplex DNA mass following denaturation and renaturation (Fig. 2F), indicating that the inserts are palindromic. These inserts were recovered from the Figure 2F gel, treated further with BstXI, AgeI, or SphI, and analyzed again by electrophoresis (Fig. 2G). The resulting cleavage patterns established that these fragments contain the apices of palindromes, each limb of which includes a copy of the replication origin of pSLA2 (Fig. 3).
Figure 3.
Diagrammatic interpretation of enzyme digestion pattern in Figure 2G. The apices of long palindromes are shown by pairs of arrows. Cleavage sites are (Ba) BamHI; (Ag) AgeI; (Sp) SphI; (Bs) BstXI. The sizes determined for BamHI fragments determined by gel migration prior to or following denaturation/renaturation are shown for each plasmid. A restriction map of the replication origin region is shown at the top.
The above results indicate that rescue of telomerically damaged Streptomyces linear plasmid replicons had occurred by deletion of the plasmid arm containing the defective telomere and duplication of the plasmid arm containing a functional telomere. The longest deletion in the plasmids we analyzed extended to a position just short of the pSLA2 rep2 gene (Chang et al. 1996), an essential component of the replication origin, indicating that no genes between rep2 and the damaged telomere are required for propagation of pSLA2.
Mechanism of long-palindrome formation
Butler et al. (1995, 1996) have proposed a model for the formation of long DNA palindromes in both Saccharomyces cerevisiae and Tetrahymena by intramolecular recombination when a double strand DNA break is introduced adjacent to short IRs. To better understand the mechanism of long-palindrome formation in Streptomyces linear plasmids, the pSLA2-derived plasmid pQC26 (Fig. 1; Qin and Cohen 1998) isolated from S. lividans ZX7 was treated with SacI endonuclease to intentionally produce a site-specific double-strand DNA break between one telomere and the plasmid's replication origin. As seen in Figure 2H, transformation of ZX7 by pQC26 DNA molecules that had been cleaved site-specifically by SacI yielded a series of different-sized plasmid replicons, which were reduced to half their original mass following denaturation/renaturation-as had been observed for pQC110. This finding, which is consistent with the view that double-strand breaks promote long-palindrome formation (Butler et al. 1995), also suggested that the apices of the palindromes produced in different plasmids were located at varying distances (calculated to be ≤3.6 kb) from the SacI-generated DNA break.
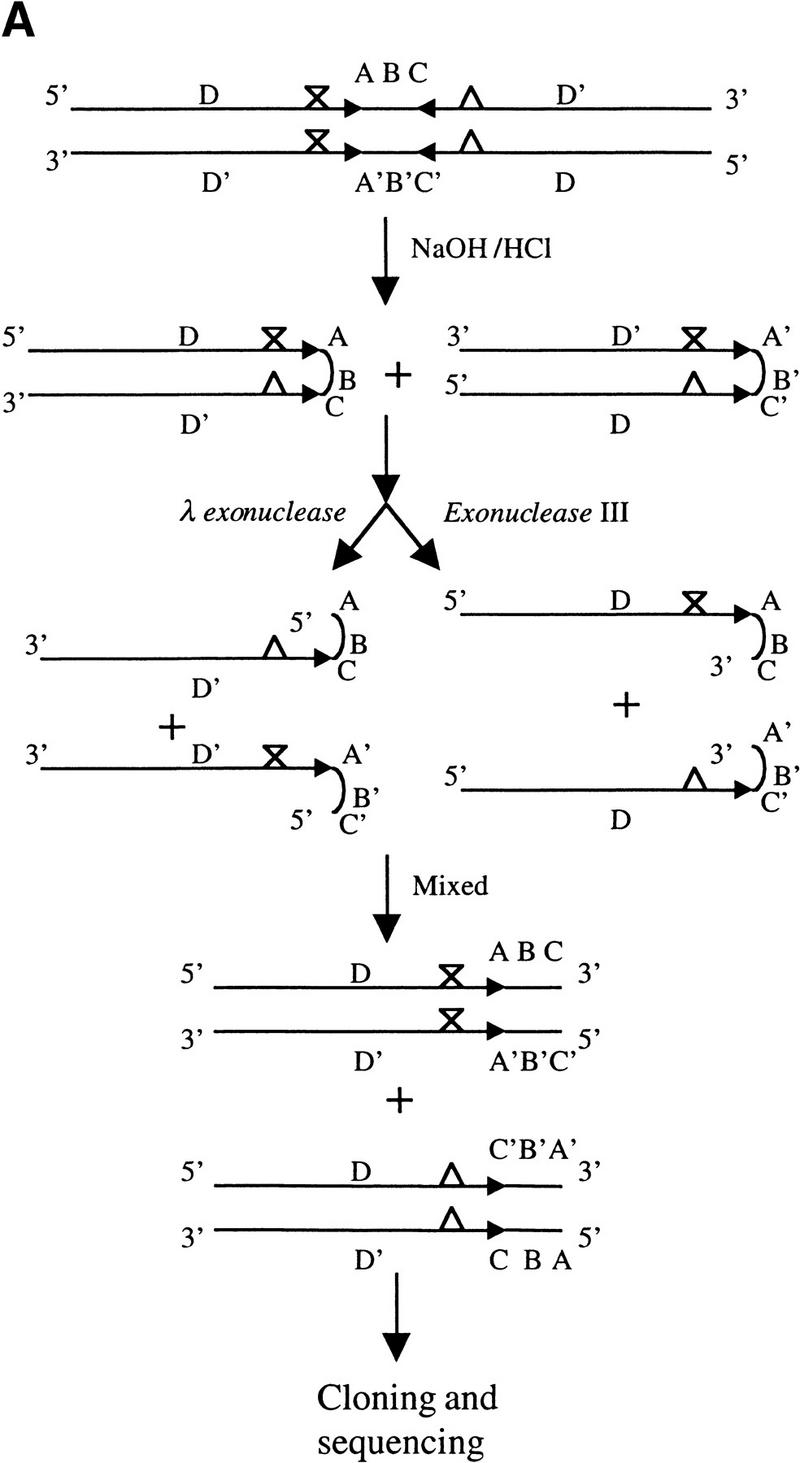
Previously, it has not been practical to directly determine the DNA sequence at the junctions of long palindromes (>100 bp) with adjoining nonpalindromic DNA (Butler et al. 1996; Devine et al. 1997), largely because rapid fold-back and base-pairing between palindrome arms prevent the binding of sequencing primers (Devine et al. 1997). To address this problem, we devised a strategy (Fig. 4A) that uses selective single-strand digestion within palindromes followed by annealing of undigested strands to produce hybrid DNAs that can be cloned and sequenced, enabling the identification of palindrome/spacer junctions and also the detection of nucleotide sequence differences between the two limbs of palindromes. We sequenced the palindrome junctions in the variable-length BamHI fragments from four independent palindromic replicons derived from pQC110 replicons (pQC143, pQC144, pQC145, and pQC146) and of two pQC26-derived replicons (pQC383 and pQC384), and compared these sequences with those of the parental pSLA2 plasmid. In all six instances, we found that a short IR sequence of pSLA2 was incorporated into the arms of the long palindrome; the results for plasmids pQC143, pQC144, and pQC384 are shown in Figure 4B. These short IRs, which differed in different palindromic replicons and ranged from 7–19 bp in length, were located at junctions of the arms of the long palindrome with a central spacer region that corresponded to the sequence present in pSLA2 between short IRs. In each palindromic plasmid, the sequence adjoining one limb of the short IR was replaced by the complement of the corresponding pSLA2 sequence, as was reported also for palindromes formed in yeast (Butler et al. 1996). The longest distances between the sequenced palindrome apices and the site of SacI cleavage that produced the double-strand DNA break removing the plasmid telomere were 2.1 kb (pQC383) and 2.6 kb (pQC384).
Figure 4.
Strategy for cloning and sequencing long-palindromic DNA and sequencing results. (A) Schematic diagram of a strategy for cloning and sequencing of the junctions of palindromes with adjacent nonpalindromic DNA. DNA fragments containing long palindromes were released from a circular plasmid DNA by restriction enzyme digestion and treated as described in Materials and Methods. X and Δ indicate the position of base-pair differences between the two arms of palindromes. (B) Sequence analysis of the junctions of long palindromes with nonpalindromic DNA. T7 or SP6-specific primers complementary to vector sequences were used. The junctions of long palindromes from pQC143, pQC144, and pQC384 with pSLA2 DNA sequences are shown along with the corresponding segment of the parental pSLA2 plasmid. Short- and long-palindromic sequences are indicated by pairs of arrows. X designates a one base-pair difference between the two arms of a pair of short IRs of pSLA2 that was harmonized in pQC143 and another plasmid, but persistent in the long palindromes of other replicons (data not shown). Pairs of short IRs are numerically designated. Sequences between the limbs of long-palindromes (i.e., spacer regions) of pSLP2-derived plasmids are shown in bold.
The presence of a melC (melanin-producing) gene near one telomere of pQC26 enabled us to also identify instances of palindrome formation by native linear plasmid DNA introduced into ZX7 by transformation. Whereas cells containing pQC26 normally produce black-colored colonies of tsr-resistant transformants, we observed rare Mel− tsr-resistant colonies (frequency <1%) after transformation by linear pQC26. Gel analysis of linear plasmids isolated from two such colonies indicated that they had deleted the telomere adjacent to melC and had become large palindromic replicons that included the tsr telomere (Fig. 1) at both ends.
The finding of pairs of short IRs at the apices of long palindromes in palindromic linear plasmid replicons of Streptomyces is consistent with previous evidence that short IR sequences are an essential ingredient for long-palindrome formation in other species (Yasuda and Yao 1991; Butler et al. 1995, 1996). These IRs were proposed to form cruciform structures that promote intramolecular recombination in yeast and Tetrahymena (Butler et al. 1995, 1996). To investigate the mechanism by which short IRs may promote palindrome formation in Streptomyces, we tested the ability of pQC26 DNA that had been cleaved by SacI and then denatured, and also of similarly-treated total DNA from pQC26-containing cells, to yield palindromic linear plasmids. We found that all of 35 randomly selected transformants receiving denatured SacI-cleaved DNA, and all 8 transformants that received undenatured SacI-cleaved DNA, contained palindromic linear plasmid replicons and that the transformation frequency increased 50-fold following denaturation (see Table 1). Additionally, trimming back the SacI-generated 5′ end of pQC26 DNA by λ exonuclease (Chang and Cohen 1994; Qin and Cohen 1998) increased the frequency of formation of palindromic linear replicons 10-fold (Table 1), whereas 3′–5′ single-strand digestion by E. coli exonuclease III had no effect on this frequency. These results indicate that the formation of long DNA palindromes is favored by conditions that promote single-strandedness of 3′ termini. Additionally, our finding that SacI-generated DNA fragments that have been denatured can generate palindromic linear replicons in S. lividans (Table 1) indicates that palindrome formation in this species does not require a cruciform substrate nor intramolecular homologous recombination.
Discussion
The investigations reported here indicate that giant palindromic replicons in Streptomyces linear plasmids can be produced by intra-strand annealing of, and turn-around replication at, short IR sequences following a double-strand DNA break telomeric to both a short IR and replication origin. The unimolecular intra-strand annealing (ISA) mechanism proposed here for the formation of long DNA palindromes in Streptomyces differs from the cruciform/recombination model proposed by Butler et al. (1995,1996) as the mechanism for palindrome formation in yeast and Tetrahymena. We show that neither cruciform formation nor recombination is essential to generate long DNA palindromes in Streptomyces: Single-stranded linear plasmid DNA molecules containing a replication origin, one functional telomere, short IRs, and SacI-generated end can form palindromic replicons. A second intramolecular recombination model based on the single-strand annealing mechanism (SSA) developed from work with mammalian cells (Lin et al. 1984; Fishman-Lobell et al. 1992; Jeggo 1998) was also considered by Butler et al. (1995) but discarded as a plausible mechanism for long-palindrome formation. Like the nonrecombinational ISA model of palindrome formation suggested by the data we have obtained for Streptomyces, the SSA mechanism of Butler et al. (1995) involves duplex formation between short IRs located on the same strand.
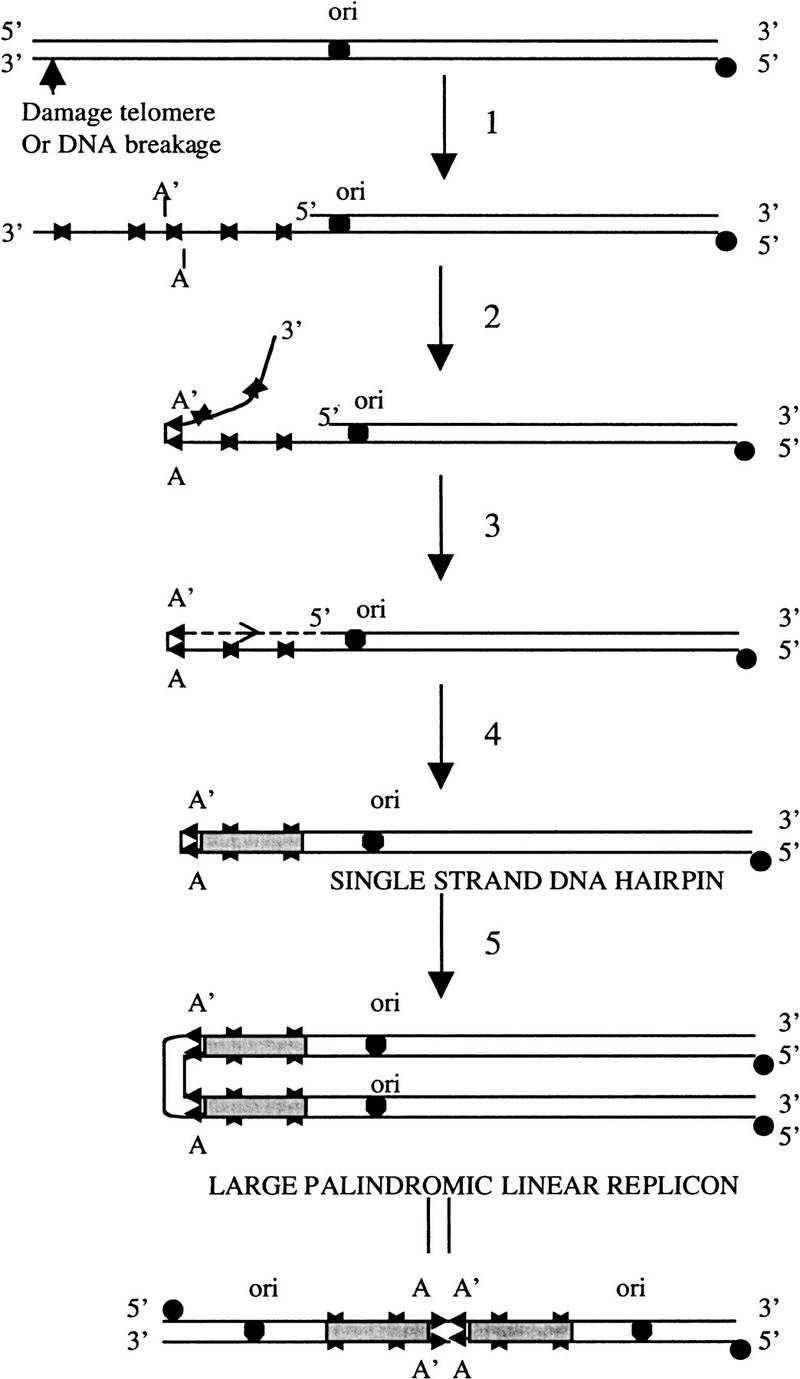
DNA breakage, which can occur at a distance of several kilobases from short IRs produced by intrastrand annealing of sequences that can be as short as 7 nucleotides in length, may result from restriction endonuclease cleavage, defective DNA replication, or environmental insult by chemical or physical agents (e.g., Jeggo 1998). We suggest that the annealing of randomly located short IR limbs can generate a substrate for long palindrome formation when present on single-strand 3′ overhangs protruding from duplex DNA or on entirely single-stranded molecules (Fig. 5). According to this model, trimming back of unpaired sequences 3′ to the DNA segment formed by IR intra-strand annealing generates a 3′ end that is extended on a template consisting of the parental DNA sequence adjacent to the IR limb. Such extension of the folded-back DNA strand duplicates the functional arm of the parental plasmid. Thus, long-palindrome formation by the ISA mechanism involves single-strand DNA digestion and replication of an intermediate hairpin structure rather than DNA fusion. As occurs in long palindromes of yeast and Tetrahymena, the limbs of the short IR form the apex of the long Streptomyces palindrome and the sequence located between the short IR becomes the central spacer of the long palindrome. We suggest that the mechanism proposed for the formation of long DNA palindromes in Streptomyces may be applicable to other organisms as well. Nevertheless, there potentially may be multiple mechanisms by which long palindromes can be formed in different species and our data do not exclude other possible mechanisms more complicated than the one we have described.
Figure 5.
Model for long-palindrome formation in Streptomyces. Following double-strand breakage of the DNA of linear replicons, the 5′ end at the site of breakage is digested by a single-strand-specific 5′–3′ exonuclease to make a long 3′ single-stranded overhang (step 1). Step 2 is fold-back annealing of 3′ single-stranded overhang at an internally located pair of short IRs (marked by arrowheads A and A′). This is followed by exonucleolytic removal of unpaired sequences distal to the paired IR and extension of the 3′ end to produce a giant hairpin molecule (steps 3 and 4). After bidirectional replication, a large-palindromic linear replicon is formed (step 5).
Replication of hairpin DNA molecules generated by extension of the 3′ DNA terminus produces giant palindromic duplex linear Streptomyces plasmids containing two copies of the replication origin. Our results indicate that on denaturation and neutralization of the DNA of such plasmids, base-pairing of complementary sequences regenerates hairpin molecules containing an intact telomere sequence at each end. When these are introduced into cells, they replicate to again produce duplex linear plasmids containing long palindromes.
Potentially, intra-strand annealing of other pairs of short IRs located in the single-strand fold-back segment of pSLA2 may assist in stabilizing annealing of the apical IR by dividing the fold-back loop into multiple duplex and single-strand segments. Consistent with this notion is our finding by analysis of GenBank sequences that the 215-bp central spacer regions of naturally occurring linear plasmid DNA molecules from the yeast Kluyveromyces lactis (Kitada and Gunge 1988) also contain multiple short IRs, which may promote stabilization of the structure as hypothesized for pSLA2. We suggest that the novel sequencing strategy that has enabled us to examine the palindrome spacer junctions of multiple molecules and consequently to arrive at these conclusions may be useful in studying long-palindromic DNA from other organisms.
Materials and methods
Strains, plasmids, and general methods
S. lividans strain ZX7 (Zhou et al. 1988) was used as the Streptomyces host strain. E. coli DH5α (Life Technologies, Inc.) and pSP72 (Promega, Inc.) were the E. coli host and cloning vector, respectively. Standard methods were used for culturing cells and DNA cloning in E. coli (Sambrook et al. 1989), and S. lividans (Hopwood 1985). Long-palindromic DNA was cloned in strain DH5α or JC7623 (Gibson et al. 1992) and DNA was extracted from agarose gel by using the Qiaquick Gel Extraction Kit (Qiagen, Inc.). Linear plasmid DNA was isolated from colonies grown on R5 plates or liquid culture as described by Qin and Cohen (1998). Southern blot hybridization used the procedure of Church and Gilbert (1984). Sequencing of DNA was done by using an Applied Biosystem (ABI, Inc.) Prism 310 Genetic Analyzer and the ABI dye terminator sequencing kit.
Cloning and sequencing the junctions of long-palindromic DNA
Cloned plasmids containing BamHI DNA fragments that included long palindromes were digested with the indicated restriction endonuclease and the projecting single-strand segment filled in using the Klenow fragment of DNA polymerase I (Life Technologies, Inc.) in the presence of dNTPs to make blunt-ended DNA. DNA fragments recovered from low-melting-point agarose gels using a Qiaquick Gel Extraction were denatured by adding 0.2 N NaOH at 37°C for 10 min, then neutralized at 65°C by adding 0.2 N HCl and 0.1 N TrisHCl (pH 8.0) and incubating for 10 min. After precipitation by addition of isopropanol and ethanol (Hopwood 1985), DNA was dissolved in 50 μl TE (10 mm TrisHCl, pH 8.0; 1 mm EDTA). Aliquots of DNA were incubated with 100 units of E. coli exonuclease III or 10 units of bacteriophage λ exonuclease (either purchased from Life Technologies, Inc. or a gift of Drs. Deb Chatterjee and Per Harbury) at 37°C for 1 hr and the completeness of their digestion was confirmed by gel electrophoresis. DNA samples digested by λ exonuclease or exonuclease III were mixed and annealed at 65°C for 2 hr following addition of 2× SSC. DNAs were precipitated by addition of isopropanol and ethanol, dissolved in TE, and treated with 0.5 μ Klenow fragment of DNA polymerase I at 37°C for 5 min. One μl 0.1 mm dNTP mix was added and samples were incubated for another 5 min. DNAs were electrophoresed in agarose gel (0.7%–1.4%, depending on expected length of annealed DNA fragments) and bands of the expected sizes were recovered and ligated into pSP72 treated with EcoRV. Ligated DNA was introduced into DH5α by transformation. Clones containing plasmids were identified and sequenced by PCR using a T7 or SP6 primer complementary to pSP72 sequences.
Acknowledgments
These studies were supported by NIH grant AI08619 to S.N.C. We thank Drs. Allan Campbell, Uta Francke and David Botstein for comments on the manuscript and Deb Chatterjee and Per Harbury for the generous gift of bacteriophage λ exonuclease. Z.Q. thanks Annie Chang, Chris Miller, Limin Li, and other members of our lab for technical help and advice.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL sncohen@stanford.edu; FAX (650) 725-1536.
References
- Akgun E, Zahn J, Baumes S, Brown G, Liang F, Romanienko PJ, Lewis S, Jasin M. Palindrome resolution and recombination in the mammalian germ line. Mol Cell Biol. 1997;17:5559–5570. doi: 10.1128/mcb.17.9.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DE, Howe MM. Mobile DNA. Washington, DC.: American Society for Microbiology; 1989. [Google Scholar]
- Beverley SM, Coderre JA, Santi DV, Schimke RT. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984;38:431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Bishop JM. Molecular themes in oncogenesis. Cell. 1991;64:235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Brison O. Gene amplification and tumor progression. Biochim Biophys Acta. 1993;1155:25–41. doi: 10.1016/0304-419x(93)90020-d. [DOI] [PubMed] [Google Scholar]
- Butler DK, Yasuda LE, Yao MC. An intramolecular recombination mechanism for the formation of the rRNA gene palindrome of Tetrahymena thermophila. Mol Cell Biol. 1995;15:7117–7126. doi: 10.1128/mcb.15.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Induction of large DNA palindrome formation in yeast: Implications for gene amplification and genome stability in eukaryotes. Cell. 1996;87:1115–1122. doi: 10.1016/s0092-8674(00)81805-x. [DOI] [PubMed] [Google Scholar]
- Chang PC, Cohen SN. Bidirectional replication from an internal origin in a linear streptomyces plasmid. Science. 1994;265:952–954. doi: 10.1126/science.8052852. [DOI] [PubMed] [Google Scholar]
- Chang PC, Kim ES, Cohen SN. Streptomyces linear plasmids that contain a phage-like, centrally located, replication origin. Mol Microbiol. 1996;22:789–800. doi: 10.1046/j.1365-2958.1996.01526.x. [DOI] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle A, Pipiras E, Toledo F, Buttin G, Debatisse M. Expression of fragile sites triggers intrachromosomal mammalian gene amplification and sets boundaries to early amplicons. Cell. 1997;89:215–225. doi: 10.1016/s0092-8674(00)80201-9. [DOI] [PubMed] [Google Scholar]
- Coyne RS, Chalker DL, Yao MC. Genome downsizing during ciliate development: Nuclear division of labor through chromosome restructuring. Annu Rev Genet. 1996;30:557–578. doi: 10.1146/annurev.genet.30.1.557. [DOI] [PubMed] [Google Scholar]
- Cromie GA, Millar CB, Schmidt KH, Leach DR. Palindromes as substrates for multiple pathways of recombination in Escherichia coli. Genetics. 2000;154:513–522. doi: 10.1093/genetics/154.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine SE, Chissoe SL, Eby Y, Wilson RK, Boeke JD. A transposon-based strategy for sequencing repetitive DNA in eukaryotic genomes. Genome Res. 1997;7:551–563. doi: 10.1101/gr.7.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis THN, Day A. A hairpin plastid genome in barley. EMBO J. 1986;5:2769–2774. doi: 10.1002/j.1460-2075.1986.tb04566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Holl AC, Volff JN, Vandewiele D, Decaris B, Leblond P. Replication of the linear chromosomal DNA from the centrally located oriC of Streptomyces ambofaciens revealed by PFGE gene dosage analysis. Res Microbiol. 1998;149:203–210. doi: 10.1016/s0923-2508(98)80080-6. [DOI] [PubMed] [Google Scholar]
- Fishman-Lobell J, Rudin N, Haber JE. Two alternative pathways of double-strand break repair that are kinetically separable and independently modulated. Mol Cell Biol. 1992;12:1292–1303. doi: 10.1128/mcb.12.3.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M, Fried M. Large inverted duplications are associated with gene amplification. Cell. 1986;45:425–430. doi: 10.1016/0092-8674(86)90328-4. [DOI] [PubMed] [Google Scholar]
- Fried M, Feo S, Heard E. The role of inverted duplication in the generation of gene amplification in mammalian cells. Biochim Biophys Acta. 1991;1090:143–155. doi: 10.1016/0167-4781(91)90095-4. [DOI] [PubMed] [Google Scholar]
- Gibson FP, Leach DR, Lloyd RG. Identification of sbcD mutations as cosuppressors of recBC that allow propagation of DNA palindromes in Escherichia coli K-12. J Bacteriol. 1992;174:1222–1228. doi: 10.1128/jb.174.4.1222-1228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch J, Tilly K. Linear plasmids and chromosomes in bacteria. Mol Microbiol. 1993;10:917–922. doi: 10.1111/j.1365-2958.1993.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Hirochika H, Sakaguchi K. Analysis of linear plasmids isolated from Streptomyces: Association of protein with the ends of the plasmid DNA. Plasmid. 1982;7:59–65. doi: 10.1016/0147-619x(82)90027-0. [DOI] [PubMed] [Google Scholar]
- Hirochika H, Nakamura K, Sakaguchi K. A linear DNA plasmid from Streptomyces rochei with an inverted terminal repetition of 614 base pairs. EMBO J. 1984;3:761–766. doi: 10.1002/j.1460-2075.1984.tb01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H. Genetics manipulation of Streptomyces: A laboratory manual. Norwich, UK: John Innes Institute; 1985. [Google Scholar]
- Huang CH, Lin YS, Yang YL, Huang SW, Chen CW. The telomeres of Streptomyces chromosomes contain conserved palindromic sequences with potential to form complex secondary structures. Mol Microbiol. 1998;28:905–916. doi: 10.1046/j.1365-2958.1998.00856.x. [DOI] [PubMed] [Google Scholar]
- Hyrien O, Debatisse M, Buttin G, de Saint Vincent BR. The multicopy appearance of a large inverted duplication and the sequence at the inversion joint suggest a new model for gene amplification. EMBO J. 1988;7:407–417. doi: 10.1002/j.1460-2075.1988.tb02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo PA. DNA breakage and repair. Adv Genet. 1998;38:185–218. doi: 10.1016/s0065-2660(08)60144-3. [DOI] [PubMed] [Google Scholar]
- Keen CL, Mendelovitz S, Cohen G, Aharonowitz Y, Roy KL. Isolation and characterization of a linear DNA plasmid from Streptomyces clavuligerus. Mol Gen Genet. 1988;212:172–176. doi: 10.1007/BF00322461. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Hirai K, Gunge N, Hishinuma F. Hairpin plasmid—A novel linear DNA of perfect hairpin structure. EMBO J. 1985;4:1881–1886. doi: 10.1002/j.1460-2075.1985.tb03864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi H, Shimaji M, Sakai A. Giant linear plasmids in Streptomyces which code for antibiotic biosynthesis genes. Nature. 1987;328:454–456. doi: 10.1038/328454a0. [DOI] [PubMed] [Google Scholar]
- Kitada K, Gunge N. Palindrome-hairpin linear plasmids possessing only a part of the ORF1 gene of the yeast killer plasmid pGKL1. Mol Gen Genet. 1988;215:46–52. doi: 10.1007/BF00331301. [DOI] [PubMed] [Google Scholar]
- Kopecko DJ, Cohen SN. Site specific recA-independent recombination between bacterial plasmids: Involvement of palindromes at the recombinational loci. Proc Natl Acad Sci. 1975;72:1373–1377. doi: 10.1073/pnas.72.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunes S, Botstein D, Fox MS. Synapsis-mediated fusion of free DNA ends forms inverted dimer plasmids in yeast. Genetics. 1990;124:67–80. doi: 10.1093/genetics/124.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CT, Lyu YL, Liu LF. A cruciform-dumbbell model for inverted dimer formation mediated by inverted repeats. Nucleic Acids Res. 1997;25:3009–3016. doi: 10.1093/nar/25.15.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FL, Sperle K, Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: Role for DNA ends in the recombination process. Mol Cell Biol. 1984;4:1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Kieser HM, Hopwood DA, Chen CW. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol Microbiol. 1993;10:923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Lyu YL, Lin CT, Liu LF. Inversion/dimerization of plasmids mediated by inverted repeats. J Mol Biol. 1999;285:1485–1501. doi: 10.1006/jmbi.1998.2419. [DOI] [PubMed] [Google Scholar]
- Ma C, Martin S, Trask B, Hamlin JL. Sister chromatid fusion initiates amplification of the dihydrofolate reductase gene in Chinese hamster cells. Genes & Dev. 1993;7:605–620. doi: 10.1101/gad.7.4.605. [DOI] [PubMed] [Google Scholar]
- Pandza S, Biukovic G, Paravic A, Dadbin A, Cullum J, Hranueli D. Recombination between the linear plasmid pPZG101 and the linear chromosome of Streptomyces rimosus can lead to exchange of ends. Mol Microbiol. 1998;28:1165–1176. doi: 10.1046/j.1365-2958.1998.00877.x. [DOI] [PubMed] [Google Scholar]
- Passananti C, Davies B, Ford M, Fried M. Structure of an inverted duplication formed as a first step in a gene amplification event: Implications for a model of gene amplification. EMBO J. 1987;6:1697–1703. doi: 10.1002/j.1460-2075.1987.tb02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Cohen SN. Replication at the telomeres of the Streptomyces linear plasmid pSLA2. Mol Microbiol. 1998;28:893–903. doi: 10.1046/j.1365-2958.1998.00838.x. [DOI] [PubMed] [Google Scholar]
- Rayko E. Organization, generation and replication of amphimeric genomes: A review. Gene. 1997;199:1–18. doi: 10.1016/s0378-1119(97)00357-0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K. Invertrons, a class of structurally and functionally related genetic elements that includes linear DNA plasmids, transposable elements, and genomes of adeno-type viruses. Microbiol Rev. 1990;54:66–74. doi: 10.1128/mr.54.1.66-74.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Stark GR. Regulation and mechanisms of mammalian gene amplification. Adv Cancer Res. 1993;61:87–113. doi: 10.1016/s0065-230x(08)60956-2. [DOI] [PubMed] [Google Scholar]
- Walton JD, Paquin CE, Kaneko K, Williamson VM. Resistance to antimycin A in yeast by amplification of ADH4 on a linear, 42-kb palindromic plasmid. Cell. 1986;46:857–863. doi: 10.1016/0092-8674(86)90067-x. [DOI] [PubMed] [Google Scholar]
- Windle B, Draper BW, Yin YX, O'Gorman S, Wahl GM. A central role for chromosome breakage in gene amplification, deletion formation, and amplicon integration. Genes & Dev. 1991;5:160–174. doi: 10.1101/gad.5.2.160. [DOI] [PubMed] [Google Scholar]
- Yasuda LF, Yao MC. Short inverted repeats at a free end signal large palindromic DNA formation in Tetrahymena. Cell. 1991;67:505–516. doi: 10.1016/0092-8674(91)90525-4. [DOI] [PubMed] [Google Scholar]
- Zhou X, Deng Z, Firmin JL, Hopwood DA, Kieser T. Site-specific degradation of Streptomyces lividans DNA during electrophoresis in buffers contaminated with ferrous iron. Nucleic Acids Res. 1988;16:4341–4352. doi: 10.1093/nar/16.10.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]