Abstract
The purpose of this study was to identify 3D kinematic and kinetic gait profiles in individuals with chronic stroke and to determine whether the magnitude or pattern (shape and direction of curve) of these profiles relate to gait performance (as measured by self-selected gait speed). More than one type of kinematic and kinetic pattern was identified in all three planes in 20 individuals with stroke (age = 61.2 ± 8.4 years). Persons in the “fast” speed group did not necessarily exhibit the gait patterns closest to the ones reported for healthy adults. For example, in the frontal plane, a variation from the typical pattern (i.e., a hip abductor pattern in swing) was more common among the “fast” group. Correlations revealed that in addition to the sagittal profiles, the magnitudes of the frontal and transverse profiles are also related to speed, particularly the frontal hip powers. The results support the importance of hip abductors, in addition to the sagittal plane muscle groups, for both the paretic and non-paretic limbs. Furthermore, profiles which resemble gait patterns of neurologically healthy adults do not necessarily result in the faster gait speeds for individuals with chronic stroke.
Keywords: Gait analysis, Biomechanics, Speed, Cerebral vascular disorders
1. Introduction
The most often stated goal by persons with stroke is improved walking function.1 Thus, it is not surprising that the retraining of walking is a major focus in the rehabilitation of persons with stroke.
The gait of persons with stroke is characterized by a decrease in self-selected speed2–3 and previous studies have reported altered kinematic and kinetic gait profiles in both magnitude (e.g., joint angle range, peak moment, peak power) and pattern (i.e., shape and direction of curves).4–7 Relative to values of neurologically healthy persons walking at their self-selected speeds, the sagittal joint magnitude has been generally reported as decreased for both lower limbs with a greater reduction on the paretic side.5,8–9 Furthermore, contrary to the gait of healthy individuals, which is fairly consistent in pattern across subjects, marked variation in gait patterns has been noted in persons with stroke. For example, Kramers de Quervain et al.4 reported four coupling patterns of sagittal knee and ankle motion during stance and Knutsson and Richards10 described three types of abnormal muscle activation patterns during gait following stroke. As to date, very little data is available on the non-sagittal gait profiles of persons with stroke. Only a few recent studies9,11–13 have presented frontal and transverse plane gait profiles in individuals with stroke and most of these studies have interpreted the findings based on a single average profile of all subjects, thus differences in patterns were not identified.
Self-selected gait speed is recognized as a valid and sensitive measure of gait performance,14 thus, identifying kinematic and kinetic profiles that relate to gait speed could provide guidance for intervention strategies aimed at improving gait performance. The magnitude of several sagittal kinematic and kinetic profiles has been identified as significant contributors to gait speed.5 In addition, Olney et al.5,15 have further suggested that treatment such as strength training of flexors and extensors at angular positions corresponding to key gait events may be beneficial in persons with stroke. The type of pattern associated with higher walking speeds, however, has not been identified in persons with stroke except in one instance. Kramers de Quervain et al.4 identified four kinematic knee patterns in persons with acute stroke and found that slow walkers showed patterns that differed from the typical pattern found in healthy gait whereas the fast walkers demonstrated this typical pattern. In stroke rehabilitation programs, gait training is often aimed at “normalizing” the patterns. However, no study has identified whether type of gait pattern is associated with performance in persons with chronic stroke. Over time, persons with stroke may develop different compensatory strategies to achieve an efficient method of walking. Thus, it is possible that gait patterns employed by neurologically healthy individuals would not necessarily correspond to optimal performance in individuals with chronic stroke.
In this study we examined individual kinematic and kinetic gait profiles of persons with chronic stroke in all three planes and identified frontal and transverse, in addition to sagittal profiles contributing to gait performance as measured by self-selected gait speed. The following questions were addressed: What are some of the types of kinematic and kinetic gait patterns (based on shape and direction of curves) in the frontal, transverse, and sagittal profiles exhibited by individuals with chronic stroke? Secondly, does the magnitude or pattern of these profiles relate to gait speed?
2. Methods
The study was approved by the local university’s Research Ethics Board and hospital Research Advisory and Review Committee. Twenty community-dwelling stroke survivors of at least six months post-stroke with no significant musculo-skeletal problems due to conditions other than stroke were recruited on a volunteer basis (Table 1). Subjects were asked to walk wearing shoes without the use of an orthosis at their “most comfortable speed” (i.e. self-selected speed) using their usual assistive device (seven subjects used a cane) along an 8-meter walkway over three force plates (Bertec, Columbus, OH). Subjects were discouraged from targeting the force plates. Five “appropriate” trials were collected for each limb. A trial was considered “appropriate” if only one foot landed on a force plate in its entirety.
Table 1.
Subject characteristics (N = 20).
| Mean | SD | Range | |
|---|---|---|---|
| Age (yr) | 61.2 | 8.4 | 52 – 82 |
| Mass (kg) | 76.2 | 13.8 | 52.0 – 99.4 |
| Height (m) | 1.71 | 0.12 | 1.43 – 1.88 |
| Time since stroke (yr) | 4.0 | 2.6 | 1.5 – 10.0 |
| Gait speed (m/sec) | 0.45 | 0.25 | 0.20 – 1.10 |
| Gait speed (s−1, normalized to leg length) | 0.59 | 0.34 | 0.24 – 1.63 |
| Cadence (steps/min) | 73.9 | 20.7 | 38.2 – 111.2 |
| Stride length (m) | 0.71 | 0.25 | 0.34 – 1.30 |
| Paretic step length (m) | 0.36 | 0.15 | 0.05–0.70 |
| Non-paretic step length (m)a | 0.35 | 0.12 | 0.14–0.61 |
| Paretic stance time (sec) | 1.14 | 0.45 | 0.68–2.32 |
| Non-paretic stance time (sec) | 1.34 | 0.51 | 0.76–2.63 |
|
| |||
| Type of stroke (Ischemic/Hemorrhagic/Unspecified) | 11/7/2 | ||
| Gender (M/F) | 14/6 | ||
| Paretic side (Right/Left) | 11/9 | ||
| Mobility aid (Cane/None) | 7/13 | ||
| Chedoke-McMasterb (stage range) | 3–6 | ||
| Modified Ashworth Scalec (grade range) | 0–2 | ||
An optoelectronic sensor (Northern Digital, Waterloo, Canada) was used to track three non-collinear infrared emitting diodes (IREDs) attached to each lower body segment (pelvis, bilateral thighs, lower legs and feet). In this camera set-up, the error of locating the coordinates of an IRED in space was 0.9 mm in the anterior/posterior (x) direction, 0.45 mm in the up/down (y) direction and 0.45 mm in the medial/lateral (z) direction. IREDs and force plate data were synchronized and sampled at 60 and 600 Hz, respectively, and then filtered using a second-order Butterworth, low-pass filter at 6 and 50 Hz, respectively. The derivation of the 3-D joint angles, moments and powers have been described by Eng & Winter.16 Joint angles were described as the motion of the distal segment relative to the proximal segment. Power bursts were labeled according to Eng & Winter;16 the first letter refers to the joint, the number refers to the sequence of the bursts in that joint and the second letter denotes the plane (e.g. A1-S refers to the first ankle burst in the sagittal plane).
To account for possible effects due to anthropometrics, gait speed was normalized to leg length and expressed in sec−1. Joint angle, moment, and power profiles were consistent in pattern (as defined below) across trials for each subject and the intra-subject variability of the profiles was within acceptable range17 (mean coefficient of variation over the stride was 27%, 30%, and 27% for frontal, transverse, and sagittal plane profiles, respectively). Therefore, profiles were ensemble-averaged for each subject (for each of the three joints of the two limbs). Ensemble-averaging across subjects was not performed in order to identify differences in motor patterns. When extracting information from the gait profiles, each phase of the gait cycle (stance and swing phases) was observed individually for each subject. However, for illustration purposes, profiles were normalized on a time base of 0–60% stance, 61–100% swing (i.e. 0%= foot contact, 60%= toe-off). Two aspects of the gait profiles were examined at each joint for each of the three planes of motion: 1) the pattern which was identified based on the shape and direction of the curves (or bursts in the case of moment and power profiles) regardless of magnitude and 2) the magnitude which was defined as the angle range, peak moments and peak powers. Peaks were extracted within the same events or phases of the gait cycle and peaks generally occurred during the stance phase.
To determine the relationship between gait speed and profiles, each aspect of the profiles was examined in the following way: 1) the frequency of the patterns was compared between two speed groups. (cluster analysis was used to separate the subjects into a “slow” and a “fast” speed group using an iterative procedure to determine the cluster centre by optimizing the distance from the clusters) and 2) the magnitude of the profiles (angle range, peak moments, and peak powers) was correlated with gait speed using Pearson product moment correlation (r). The significance level selected for this study was p<0.05, two-tailed test. Scatterplots of correlations were visually examined to ensure outliers did not compromise the results of the correlations.
3. Results
3.1 Types of gait patterns
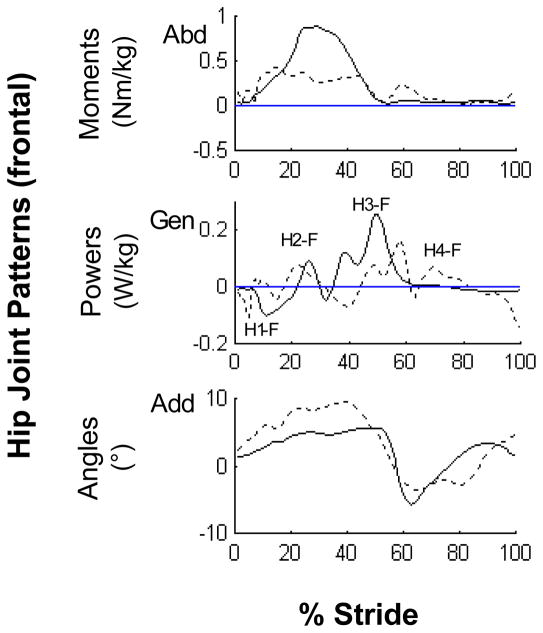
More than one type of kinematic and kinetic gait pattern was identified in all planes across subjects. In the frontal plane, two different patterns were identified at the hip joint on the paretic side. In addition to the pattern found in healthy gait,16,18–19 a second pattern exhibited by 12 subjects displayed an additional hip abductor moment associated with a positive power burst (labeled as H4-F) at toe-off into swing which resulted in a second abduction angle peak (Fig. 1, dashed line). Two common patterns were also identified at the knee in the frontal plane. As in healthy gait,16 the first pattern was an abductor moment throughout stance on both sides which countered the gravitational adductor stress caused by the center of mass medial to the ipsilateral knee. This abductor moment was associated with a K1-F generation phase resulting in knee abduction in early stance and a K2-F absorption phase resulting in knee adduction during propulsion (Fig. 2, solid line).
Figure 1.
Representative frontal paretic hip joint patterns. The profiles are intra-subject averages (n = 5 trials) which represent the two predominant patterns (solid line: normal pattern, dashed line: hip abductor/circumduction pattern) observed across subjects.
Abbreviations: Abduction (Abd), Adduction (Add), Generation (Gen)
Figure 2.
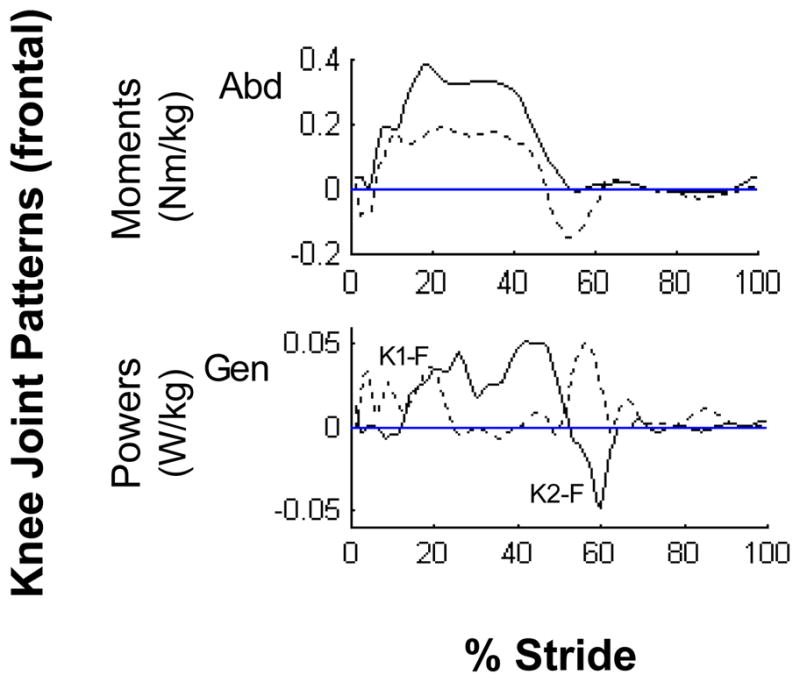
Representative frontal paretic knee joint patterns. The profiles are intra-subject averages (n = 5 trials) which represent the two predominant kinetic patterns (solid line: normal moment pattern, dashed line: propulsion adductor moment pattern) observed across subjects.
Abbreviations: Abduction (Abd), Generation (Gen)
In addition, a second pattern of knee adductor moment just prior to toe-off was found in 8 and 6 subjects on their paretic and non-paretic limbs, respectively, which was associated with a power generation phase during propulsion (Fig. 2, dashed line).
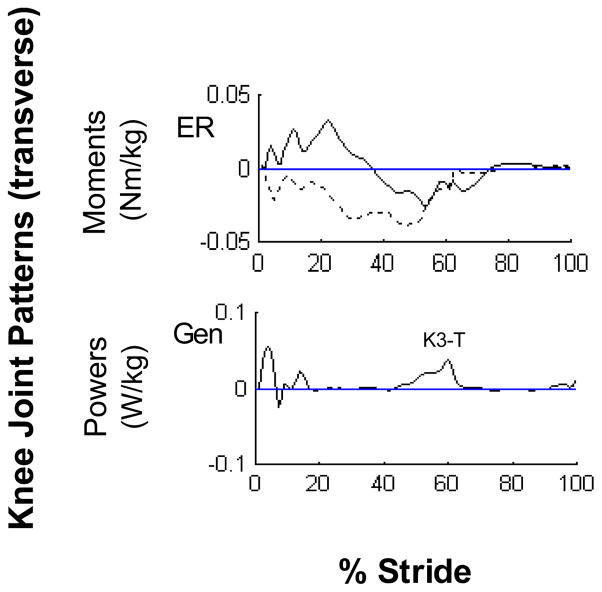
In the transverse plane, except for the hip joint, patterns on the paretic side were highly variable across subjects. At the knee, at least two distinct moment patterns were identified. One pattern consisted of an external rotator moment for the first half of stance followed by an internal rotator moment in the latter half of stance (Fig. 3, solid line). Another pattern consisted of an internal rotator moment throughout stance (Fig. 3, dashed line). The knee power phases were very low and highly variable with only one consistent characteristic: a generation burst during push-off (labeled as K3-T). Several patterns were also identified at the ankle joint. The most predominant pattern (8 paretic, 17 non-paretic sides) was abductor moment through most of stance. This moment pattern was associated with a consistent absorption burst during push-off resulting in ankle adduction as the heel lifted off from the floor and moved laterally on the planted forefoot.
Figure 3.
Representative transverse paretic knee joint patterns. The profiles are intra-subject averages (n = 5 trials) which represent the two predominant moment patterns (solid line: external-internal rotator pattern, dashed line: internal rotator pattern) observed across subjects. Power patterns were highly variable across subjects except for a consistent K3-T phase.
Abbreviations: External Rotation (ER), Generation (Gen)
Even in the sagittal plane, more than one type of gait pattern was noted. At the paretic knee, in addition to the typical extensor-flexor-extensor moment pattern found in healthy gait,16 half of the subjects displayed a flexor moment pattern (Fig. 4, dashed line). This flexor moment pattern was associated with a kinematic knee extension thrust pattern (Fig. 4, dashed line) in eight subjects. These subjects extended (or hyperextended) their knee at weight acceptance. Therefore the corresponding K1-S power phase represented the negative work done by the flexors and other soft tissues eccentrically controlling the abrupt knee extension. The K2-S phase, which occurred later in stance than commonly seen in healthy gait, represented the positive work to flex the extended knee in preparation for swing (Fig. 4, dashed line). Interestingly, although five subjects also displayed the kinematic knee extension thrust pattern at the non-paretic knee, the associated kinetic pattern was different from that of the paretic side, i.e. the knee extension was the result of the positive power (K2-S) generated by the knee extensors, not flexors. At the ankle joint, sagittal kinetic patterns differed between the paretic and non-paretic sides in that there was an absence of dorsiflexor moment at initial contact on the paretic side. The absence of the normal eccentric dorsiflexor moment resulted in a lack of plantarflexion after initial contact in 12 subjects.
Figure 4.
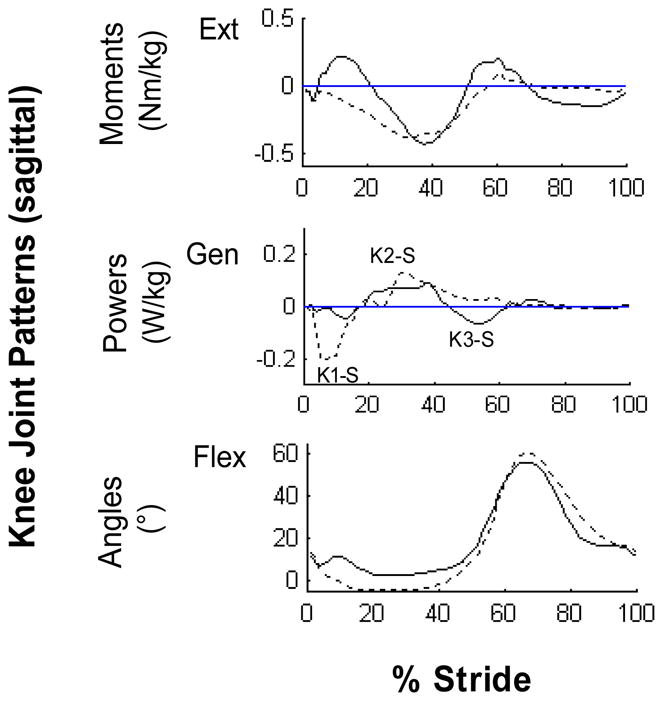
Representative sagittal paretic knee joint patterns. The profiles are intra-subject averages (n = 5 trials) which represent the two predominant patterns (solid line: normal pattern, dashed line: hyperextension pattern) observed across subjects.
Abbreviations: Extension (Ext), Generation (Gen), Flexion (Flex)
3.2 Patterns across joints
Eight of the 12 subjects that exhibited a forefoot landing (i.e., lack of plantarflexion after initial contact) on their paretic side also demonstrated the kinematic knee extension thrust (knee hyperextension). Furthermore, 6 of these subjects also demonstrated the hip circumduction (i.e., additional hip abductor moment and hip abduction angle from toe-off into swing) and frontal knee adductor moment during propulsion. The increased knee adductor moment during propulsion may act to stabilize the knee in the frontal plane to accommodate the subsequent hip circumduction which compensates for the inability to push-off on a hyperextended knee and is required for foot clearance. In contrast, subjects who had a normal ankle weight-acceptance profile (therefore, no forefoot landing) did not exhibit the knee hyperextension or hip circumduction profile, but did have greater hip sagittal range of motion.
3.3 Relationship to walking speed
Of the 13 joint angle and moment/power profiles in which more than one pattern was identified, there were three cases where type of gait pattern was related to gait speed and contrary to expectations, the “fast” group did not necessarily exhibit the patterns closest to the ones found in healthy gait. Cluster analysis resulted in a “slow” group of 12 subjects with a mean speed of 0.41 sec−1 and a “fast” group of 8 subjects with a mean speed of 0.86 sec−1. Only 2 of the 8 “fast” walkers demonstrated the typical frontal moment profile at the paretic hip while 6 of these “fast” walkers displayed the additional abductor moment profile. On the other hand, 7 of 8 subjects in the “fast” group displayed typical sagittal joint angle and frontal joint moment patterns at the non-paretic knee.
The magnitudes of kinematic and kinetic gait variables (Table 2) were significantly related to gait speed in all three planes (Table 3). Power variables were more strongly related to speed than joint angle or moment variables. On the paretic side, the sagittal hip power generation (r = 0.90) bore the strongest correlation with speed and on the non-paretic side, the sagittal ankle power generation (r = 0.95). In addition to the sagittal plane, significant correlations were also evident in the frontal and transverse planes particularly for the paretic and non-paretic hips. Correlations were as high or sometimes higher on the non-paretic limb compared to the paretic limb.
Table 2.
Meana ± standard deviation of the joint angle rangesb (°), peak moments (Nm/kg) and peak powers (W/kg) over the stride.
| Sagittal | Paretic | Non-paretic | Frontal | Paretic | Non-paretic | Transverse | Paretic | Non-paretic |
|---|---|---|---|---|---|---|---|---|
| Joint Angles | ||||||||
| Hip range | 34.6 ± 19.1 | 41.1 ± 8.1 | Hip range | 10.7 ± 5.0 | 12.0 ± 4.8 | Hip range | 17.8 ± 9.5 | 17.3 ± 8.0 |
| Knee range | 33.7 ± 21.3 | 49.8 ± 8.0 | Knee range | 7.8 ± 4.5 | 8.0 ± 3.9 | Knee range | 10.0 ± 6.2 | 12.9 ± 6.8 |
| Ankle range | 17.7 ± 5.1 | 24.6 ± 8.0 | Ankle range | 13.6 ± 6.2 | 9.0 ± 3.0 | Ankle range | 11.2 ± 5.4 | 13.1 ± 4.8 |
| Joint Moments | ||||||||
| Hip ext | 0.33 ± 0.17 | 0.60 ± 0.25 | Hip abd | 0.68 ± 0.23 | 0.93 ± 0.23 | Hip IR | 0.06 ± 0.04 | 0.10 ± 0.05 |
| Hip flex | 0.32 ± 0.21 | 0.41 ± 0.23 | Knee abd | 0.24 ± 0.17 | 0.30 ± 0.15 | Hip ER | 0.07 ± 0.03 | 0.15 ± 0.06 |
| Knee flex | 0.30 ± 0.20 | 0.20 ± 0.12 | Ankle inv | 0.17 ± 0.13 | 0.17 ± 0.11 | Knee IR | 0.05 ± 0.03 | 0.06 ± 0.04 |
| Ankle plflex | 0.64 ± 0.22 | 1.10 ± 0.25 | ||||||
| Joint Powers | ||||||||
| Hip gen | 0.50 ± 0.64 | 0.61 ± 0.27 | Hip gen | 0.22 ± 0.28 | 0.40 ± 0.32 | Hip gen | 0.05 ± 0.05 | 0.06 ± 0.05 |
| Hip abs | 0.19 ± 0.16 | 0.25 ± 0.16 | Hip abs | 0.19 ± 0.15 | 0.28 ± 0.26 | Hip abs | 0.06 ± 0.05 | 0.09 ± 0.10 |
| Knee gen | 0.19 ± 0.15 | 0.30 ± 0.21 | Knee gen | 0.07 ± 0.07 | 0.07 ± 0.06 | Knee gen | 0.01 ± 0.01 | 0.05 ± 0.07 |
| Knee abs | 0.31 ± 0.37 | 0.73 ± 0.53 | Ankle gen | 0.11 ± 0.16 | 0.13 ± 0.16 | |||
| Ankle gen | 0.40 ± 0.43 | 1.64 ± 1.19 | Ankle abs | 0.05 ± 0.04 | 0.08 ± 0.13 | |||
| Ankle abs | 0.21 ± 0.15 | 0.43 ± 0.26 | ||||||
N = 20 for joint angles and N = 19 for joint moments and powers.
Joint angle range = maximum-minimum joint angle values.
Abbreviations: Extension (ext), Flexion (flex), Plantarflexion (plflex), Abduction (Abd), Adduction (add), Inversion (inv), Internal Rotation (IR), External Rotation (ER), Generation (gen), Absorption (abs)
Table 3.
Pearson product moment correlation (r) a between gait speed (sec−1) and gait variables (joint angle range, peak moment and peak power) over the stride.
| Sagittal | Paretic | Non-paretic | Frontal | Paretic | Non-paretic | Transverse | Paretic | Non-paretic |
|---|---|---|---|---|---|---|---|---|
| Joint Angles | ||||||||
| Hip range | 0.66** | 0.14 | Hip range | 0.47* | 0.63** | Hip range | 0.61** | 0.30 |
| Knee range | 0.55* | 0.31 | Knee range | 0.36 | 0.77** | Knee range | 0.56* | 0.06 |
| Ankle range | 0.35 | 0.69** | Ankle range | −0.22 | 0.42 | Ankle range | 0.26 | 0.40 |
| Joint Moments | ||||||||
| Hip ext | 0.05 | 0.67** | Hip abd | −0.05 | 0.01 | Hip IR | 0.16 | 0.68** |
| Hip flex | 0.84** | 0.61** | Knee abd | −0.21 | 0.22 | Hip ER | 0.65** | 0.38 |
| Knee flex | 0.08 | 0.14 | Ankle inv | 0.48* | 0.47* | Knee IR | −0.14 | 0.71** |
| Ankle plflex | 0.43 | 0.80** | ||||||
| Joint Powers | ||||||||
| Hip gen | 0.90** | 0.71** | Hip gen | 0.87** | 0.84** | Hip gen | −0.01 | 0.27 |
| Hip abs | 0.64** | 0.51* | Hip abs | 0.79** | −0.17 | Hip abs | 0.63** | 0.66** |
| Knee gen | 0.64** | −0.03 | Knee gen | 0.39 | 0.38 | Knee gen | 0.58** | 0.19 |
| Knee abs | 0.88** | 0.85** | Ankle gen | 0.09 | 0.32 | |||
| Ankle gen | 0.69** | 0.95** | Ankle abs | 0.57* | 0.83** | |||
| Ankle abs | 0.81** | 0.85** | ||||||
Correlation is significant at p < 0.05.
Correlation is significant at p < 0.01.
N = 20 for correlations with joint angles and N = 19 for joint moments and powers.
4. Discussion
It is common knowledge that persons with stroke often present with abnormal motor control involving all three planes of movement. However, little is known of the gait profiles in the three planes of movement and their role in promoting gait performance in persons with chronic stroke. The purpose of this study was to identify types of kinematic and kinetic gait patterns in all three planes and to determine whether the pattern or magnitude of these profiles contribute to gait performance in persons with chronic stroke. We measured the relationship of these kinematic and kinetic gait patterns to gait speed, and not to the presence of stroke-specific impairments; however, gait speed has been shown to strongly relate to stage of stroke recovery and impairment level.14 The technique used to analyze pattern variations was limited to visual observations of the kinematic and kinetic profiles. Nevertheless, specific guidelines regarding the direction of movement (or bursts in the case of kinetic profiles) in specified phases of the gait cycle were followed to reduce the subjective nature of the analysis. Future investigations using more sophisticated techniques such as fuzzy or multivariate analyses20 based on kinematic and kinetic patterns on a larger sample size may be of benefit to further understand the strategies used in walking by persons with stroke. Another limitation of this study was the lack of comparison between our data and that of a control group of neurologically healthy individuals. On the other hand, subjects in the present study walked as slow as 0.2 m/s and forcing such a slow speed on a healthy person would result in unnatural gait patterns. It may be argued that the altered gait patterns found in this study are simply the result of the slow walking speed of these subjects. However, the changes seen in these subjects are asymmetrical which suggests that they are stroke-induced and observed characteristics such as forefoot landing, hip circumduction and knee hyperextension are not known to occur in healthy individuals. Finally, the inclusion of the seven subjects requiring the assistance of a cane to walk could potentially have influenced the results especially those of kinetic profiles. However, post-hoc sub-analyses of cane and non-cane users did not reveal any trends in pattern or magnitude of gait profiles as well as in gait speed.
More than one type of kinematic and kinetic gait pattern was identified across subjects indicating that persons with stroke use different strategies to achieve the goal of walking. Moreover, some stroke survivors may use different kinetic strategies to achieve similar kinematic movement outcomes. For example, at the knee in the sagittal plane, two different kinetic profiles on the paretic (flexor moment) and non-paretic (extensor-flexor-extensor moment) sides were associated with the same kinematic profile (knee extension thrust angle profile). Other studies have also identified variation in kinematic4 and electromyographic10 gait patterns within a group of persons with stroke. The results of the present and these previous studies indicate that the choice of therapeutic procedures should be individualized depending upon the type of disturbed motor control presented by the individual person. Furthermore, these findings suggest that the common practice of averaging gait profiles across subjects be reconsidered in light of the fact that valuable information regarding different movement patterns may be lost.
Interestingly, our results suggest that movement patterns typically employed by neurologically healthy individuals do not necessarily relate to higher performance in persons with chronic stroke. A common frontal plane variation identified in more than half of the subjects was a prolonged hip abduction pattern in swing which was associated with a fourth power burst (labeled as H4-F) not normally found in healthy gait. Most subjects in the “fast” walking group displayed this “not normal” pattern suggesting that it may be a mechanism to compensate for the lack of dorsiflexion and the insufficient flexion at the hip and knee required to clear the ground, thus improving gait speed. This finding challenges the notion that treatment approaches should be directed towards restoring “normal” patterns if the goal is to achieve optimal functional performance rather than an aesthetic one.
Although the magnitude of kinematic and kinetic profiles was generally more strongly related to speed in the sagittal plane, correlations were also significant in the frontal and transverse planes, particularly at the hip. The high correlation coefficients found in the sagittal plane concur with the results by Olney et al.6 and further support the importance of muscle function for both the paretic and non-paretic limbs in this plane, particularly of the hip flexors during pull-off and the ankle plantarflexors during stance and push-off. The maximum knee absorption power (K3-S), which represents the work of the quadriceps to eccentrically control the flexing knee during propulsion, was highly correlated with gait speed on both sides indicating that the slower walkers did not require this eccentric control as the weak ankle push-off/hip pull-off did not result in the forces that normally collapse the knee. In the frontal plane, the eccentric (H1-F) control of the lateral pelvic drop and concentric (H3-F) work to raise the pelvis on the contralateral side by the ipsilateral hip abductors is likely important in increasing the contralateral step length and consequently, the gait speed. In the transverse plane, in spite of low magnitudes, some correlations were significant at the hip and knee. However, interpretations in this plane are difficult due to the large variability in patterns. Further research with a larger sample size is warranted.
Some clinical guidance for gait training in persons with stroke may be implied from the results of this study. Although causation cannot be inferred from this type of study design, our findings could suggest that interventions aimed at increasing the frontal hip powers, e.g. concentric strengthening of the hip abductors, in addition to the sagittal hip, knee and ankle powers, may be of particular benefit during the gait training of stroke survivors. In addition, our results do not support the “normalization” of movement patterns as a goal of treatment in persons with chronic stroke. The contribution of the non-paretic limb performance in the gait of persons with stroke is noteworthy. During the rehabilitation of persons with stroke, treatment programs frequently emphasize the performance of the paretic limb alone with the function of the non-paretic limb only used as a reference point. However, the high correlations found between gait speed and selected gait variables of the non-paretic limb suggest that the performance of the non-paretic limb itself may be a contributing factor of gait performance and justify the inclusion of the non-paretic limb in training. Previous studies have shown that the muscle strength of the non-paretic limb is highly correlated with gait speed.21–22 A plausible explanation for the high correlations found on the non-paretic limb may be that motor impairments are present bilaterally following stroke.23–25 Since cause-effect relationships cannot be determined from correlation studies, future investigations need to evaluate the benefits of training programs that include non-sagittal components and the non-paretic limb on the gait of persons with stroke.
Acknowledgments
We wish to acknowledge a New Investigator salary support award to JJE from the Canadian Institute of Health Research (MSH-63617) and Michael Smith Foundation for Health Research, Grant-in-Aid from the Heart and Stroke Foundation of BC and Yukon, and a University of BC Graduate Fellowship to CMK.
References
- 1.Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res. 1988;11:181–183. [Google Scholar]
- 2.Turnbull GI, Charteris J, Wall JC. A comparison of the range of walking speeds between normal and hemiplegic subjects. Scand J Rehabil Med. 1995;27:175–182. [PubMed] [Google Scholar]
- 3.Witte US, Carlsson JY. Self-selected walking speed in patients with hemiparesis after stroke. Scand J Rehabil Med. 1997;29:161–165. [PubMed] [Google Scholar]
- 4.Kramers de Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. J Bone Joint Surg. 1996;78-A:1506–14. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Olney SJ, Griffin MP, Monga TN, McBride ID. Work and power in gait of stroke patients. Arch Phys Med Rehabil. 1991;72:309–314. [PubMed] [Google Scholar]
- 6.Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther. 1994;74:872–885. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- 7.Olney SJ, Griffin MP, McBride ID. Multivariate examination of data from gait analysis of persons with stroke. Phys Ther. 1998;78:814–828. doi: 10.1093/ptj/78.8.814. [DOI] [PubMed] [Google Scholar]
- 8.Lehman JF, Condon SM, Price R, deLateur BJ. Gait abnormalities in hemiplegia: their correction by ankle-foot orthoses. Arch Phys Med Rehabil. 1987;68:763–71. [PubMed] [Google Scholar]
- 9.Kerrigan DC, Frates EP, Rogan S, Riley PO. Spastic paretic stiff-legged gait: biomechanics of the unaffected limb. Am J Phys Med Rehabil. 1999;78:354–360. doi: 10.1097/00002060-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Knutsson E, Richards C. Different types of disturbed motor control in gait of hemiparetic patients. Brain. 1979;102:405–30. doi: 10.1093/brain/102.2.405. [DOI] [PubMed] [Google Scholar]
- 11.Kerrigan DC, Frates EP, Rogan S, Riley PO. Hip hiking and circumduction: quantitative definitions. Am J Phys Med Rehabil. 2000;79:247–252. doi: 10.1097/00002060-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kuan T-S, Tsou J-Y, Su F-C. Hemiplegic gait of stroke patients: the effect of using a cane. Arch Phys Med Rehabil. 1999;80:777–84. doi: 10.1016/s0003-9993(99)90227-7. [DOI] [PubMed] [Google Scholar]
- 13.Voigt M, Sinkjaer T. Kinematic and kinetic analysis of the walking pattern in hemiplegic patients with foot-drop using a peroneal nerve stimulator. Clin Biomech. 2000;15:340–351. doi: 10.1016/s0268-0033(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 14.Richards CL, Malouin F, Dumas F, Tardif D. Gait velocity as an outcome measure of locomotor recovery after stroke. In: Craik R, Oatis C, editors. Gait analysis: Theory and Applications. St. Louis: Mosby, Inc; 1995. pp. 355–364. [Google Scholar]
- 15.Olney SJ, Jackson VG, George SR. Gait re-education guidelines for stroke patients with hemiplegia using mechanical energy and power analyses. Physiotherapy Canada. 1988;40:242–8. [Google Scholar]
- 16.Eng JJ, Winter DA. Kinetic analysis of the lower limb during walking: what information can be gained from a three-dimensional model? J Biomech. 1995;28:753–758. doi: 10.1016/0021-9290(94)00124-m. [DOI] [PubMed] [Google Scholar]
- 17.Winter DA. Kinematic and kinetic patterns in human gait: variability and compensating effects. Hum Mov Sci. 1984;3:51–76. [Google Scholar]
- 18.Apkarian J, Naumann S, Cairns B. A three-dimensional kinematic and dynamic model of the lower limb. J Biomech. 1984;22:143–155. doi: 10.1016/0021-9290(89)90037-7. [DOI] [PubMed] [Google Scholar]
- 19.Winter DA. The Biomechanics and Motor Control of Human Gait: Normal, Elderly and Pathological. Waterloo: University of Waterloo Press; 1991. [Google Scholar]
- 20.Chau T. A review of analytical techniques for gait data. Part 1: fuzzy, statistical and fractal methods. Gait Posture. 2001;13:49–66. doi: 10.1016/s0966-6362(00)00094-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim CM, Eng JJ. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys Ther. 2003;83:49–57. [PubMed] [Google Scholar]
- 22.Suzuki K, Nakamura R, Yamada Y, Handa T. Determinants of maximum speed in hemiparetic stroke patients. Tohoku J Exp Med. 1990;162:337–344. doi: 10.1620/tjem.162.337. [DOI] [PubMed] [Google Scholar]
- 23.Bohannon RW, Andrews AW. Limb muscle strength is impaired bilaterally after stroke. J Phys Ther Sci. 1995;7:1–7. [Google Scholar]
- 24.Goldie PA, Matyas TA, Evans OM, Galea M, Bach TM. Maximum voluntary weight-bearing by the affected and unaffected legs in standing following stroke. Clin Biomech. 1996;11:333–342. doi: 10.1016/0268-0033(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 25.Sjostrom M, Fugl-Meyer AR, Nordin G, Wahlby L. Post-stroke hemiplegia; crural muscle strength and structure. Scand J Rehabil Med. 1980;(Supp 7):53–67. [PubMed] [Google Scholar]
- 26.Gowland C, VanHullenaar S, Torresin W, Moreland J, Vanspall B, Barrecca S, Ward M, Huijbregts M, Stratford P, Barclay-Goddard R. Chedoke-McMaster Stroke Assessment: Development, Validation and Administration Manual. Hamilton: Chedoke-McMaster Hospitals and McMaster University; 1995. [Google Scholar]
- 27.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]