Abstract
Objective
To determine whether aerobic exercise improves aerobic capacity in individuals with stroke.
Design
A systematic review of randomized controlled trials.
Databases searched
MEDLINE, CINAHL, EMBASE, Cochrane Database of Systematic Reviews, Physiotherapy Evidence Database were searched.
Inclusion criteria
Design: randomized controlled trials; Participants: individuals with stroke; Interventions: aerobic exercise training aimed at improving aerobic capacity;
Outcomes
Primary outcomes: aerobic capacity [peak oxygen consumption (VO2), peak workload); Secondary outcomes: walking velocity, walking endurance.
Data Analysis
The methodological quality was assessed by the PEDro scale. Meta-analyses were performed for all primary and secondary outcomes.
Results
Nine articles (seven RCTs) were identified. The exercise intensity ranged from 50% to 80% heart rate reserve. Exercise duration was 20–40 minutes for 3–5 days a week. The total number of subjects included in the studies was 480. All studies reported positive effects on aerobic capacity, regardless of the stage of stroke recovery. Meta-analysis revealed a significant homogeneous standardized effect size (SES) in favour of aerobic exercise to improve peak VO2 (SES, 0.42; 95%CI, 0.15 to 0.69; p=0.001) and peak workload (SES, 0.50; 95%CI, 0.26 to 0.73; p<0.001). There was also a significant homogeneous SES in favour of aerobic training to improve walking velocity (SES, 0.26; 95%CI, 0.05 to 0.48; p=0.008) and walking endurance (SES, 0.30; 95%CI, 0.06to 0.55; p=0.008).
Conclusions
There is good evidence that aerobic exercise is beneficial for improving aerobic capacity in people with mild and moderate stroke. Aerobic exercise should be an important component of stroke rehabilitation.
Introduction
Stroke is a major cause of chronic disability.1 Studies have indicated an increasing incidence of stroke, particularly in elderly people.2 On the other hand, the stroke mortality rate has been declining,2 which translates into a larger number of chronic stroke survivors. Many stroke survivors continue to live with residual physical impairments (i.e., reduced mobility, poor balance and muscle weakness), which may lead to physical inactivity and a sedentary lifestyle.3–5
Physical activity level is positively related to aerobic capacity,6–10 which is the product of the capacity of cardiorespiratory system to supply oxygen (i.e. cardiac output) and the capacity of the skeletal muscle to utilize oxygen (i.e. arterial-venous oxygen difference).11 Therefore, it is not surprising that sustained physical inactivity (deconditioning) induces a reduction in aerobic capacity.12 Peak oxygen consumption (VO2), the criterion measure for aerobic capacity, is poor in the stroke population. Peak VO2 in individuals with stroke has been found to be as low as 50%–70% of the age- and sex-matched value in sedentary individuals.13,14
It is common that individuals with acute stroke have low peak VO2, indicating that these individuals are unfit before they have the stroke.14 Indeed, poor aerobic fitness is an important risk factor for stroke.15 Low aerobic fitness has also been related to an increased risk of various forms of cardiovascular disease in man.16–18 Therefore, it is not surprising that a large proportion (up to 75%) of stroke patients have some form of cardiovascular disease.19 Additional decline in aerobic fitness resulting from physical inactivity in stroke survivors may further increase the risk of cardiovascular disease in these individuals above that associated with stroke itself. Moreover, low aerobic fitness is a significant determinant of poor bone health (i.e. osteoporosis) in individuals with chronic stroke.20 Therefore, improving aerobic capacity may be essential in prevention of secondary diseases due to lack of fitness in the stroke population. Prevention of secondary disabling conditions is a major component of health promotion for people with chronic disabilities and is an emerging practice in rehabilitation.21,22
Low aerobic capacity may also pose limitations on daily function of the individual. Peak VO2 has been positively related to functional performance in elderly people.23 Individuals with low peak VO2 values work at a higher relative exercise intensity to complete the same daily functional activities, when compared with their more fit counterparts.24 The reduction in fitness reserve can contribute to reduced activity endurance, which is the most striking area of difficulty for chronic stroke survivors in the community.5 Previous studies have also indicated that a critical level of aerobic capacity must be met in order to function independently.25,26 For example, Cress et al.25 found that a peak VO2 of 20ml/kg/min was needed for independent living for adults aged 65 to 97 years. The low peak VO2 values found in individuals with stroke suggest that many stroke survivors do not meet the minimum fitness level required for independent living.13,14,27 Therefore, in addition to disease prevention, enhancing aerobic capacity in individuals with stroke may also have beneficial effects on promoting functional abilities and independent living.
Given the potentially adverse health consequences of reduced aerobic capacity in individuals with stroke, there has been an increasing recognition of the importance of aerobic exercise training in this population. This increased awareness is reflected in the emergence of research studies on aerobic exercise in stroke over the past decade. However, this trend is not reflected in clinical practice as a recent study showed that the cardiovascular stress of a contemporary stroke rehabilitation program is much too low to induce a positive aerobic training effect.28
Given the mounting research evidence on the effects of aerobic exercise training in stroke and the belief that clinical practice should follow research-based evidence, we feel that it is timely to perform a systematic review on aerobic training in individuals with stroke. A couple of systematic reviews on physical therapy trials in stroke have been published recently.29,30 This systematic review, however, will provide specific information on aerobic training programs and their efficacy in improving aerobic capacity in individuals with stroke. This review is intended to aid clinicians in translating the knowledge to their daily clinical practice.
Methods
Definition of review questions
We systematically reviewed the literature regarding aerobic exercise programs in people with stroke to address the following questions: (1) Does aerobic exercise training improve aerobic capacity in individuals recovering from a stroke? (2) How much improvement in aerobic capacity can be obtained? and (3) What are the specific exercise protocols used to induce an aerobic training effect?
For the purpose of this systematic review, both peak VO2 and peak workload obtained during a graded exercise test on a cycle ergometer or treadmill were used to indicate aerobic capacity. Peak VO2 (ml/kg/min) measures the peak rate of oxygen consumption achieved and is a criterion measure for aerobic capacity.11 Peak workload (W) is also a useful indicator of aerobic capacity since it is directly 11 related to peak VO2.
Definition of inclusion and exclusion criteria
The inclusion criteria were: (1) research studies on the effects of aerobic exercise training in individuals with stroke, (2) studies which used peak VO2 or peak workload as outcome measures to indicate aerobic capacity, (3) randomized controlled trials (RCTs). Aerobic exercise training is defined as a structured exercise program that involves the use of large muscle groups for extended periods of time in activities that are rhythmic in nature, including but not limited to walking, stepping, running, swimming, cycling, and rowing.11
Exclusion criteria were: (1) doctoral dissertations, (2) reports published in books, and (3) reports published conference proceedings. These exclusion criteria were used because books are considered secondary sources and reports in doctoral dissertations/conference proceedings are often not peer-reviewed. For the purpose of this systematic review, acute and subacute stages of stroke recovery were defined as 0–1 month, and 1–6 months after the onset of stroke, respectively. A post-stroke duration of more than six months was considered chronic.
Search Strategy: Location and selection of studies
The following electronic databases were searched online through the local University’s library system: MEDLINE (1966 to July week 2, 2005), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982-July week 3, 2005), and the Excerpta Medica database (EMBASE) (1980-week 29, 2005). The specific search strategy for the MEDLINE database is described in Appendix 1. An equivalent search strategy was used for the CINAHL and EMBASE databases, following the same logic as the MEDLINE search strategy, with a few modifications due to differences in indexing and syntax in different databases. In addition, the Cochrane Library Database of Systematic Reviews (2005, issue 2) and Physiotherapy Evidence Database31 were also searched online. For these databases, the keyword “stroke” was entered to search relevant articles. All of the databases were last searched in July 2005. For further iterations, manual searches were performed on the reference list of each selected paper.
Qualitative assessment
All selected RCTs were evaluated by using the PEDro scale. The PEDro scale was originally developed to assess the methodological quality of physical therapy RCTs on the Physiotherapy Evidence Database.31 It is an 11-item scale, in which the first item relates to external validity and the other ten items assess the internal validity of a clinical trial. One point was given for each satisfied criterion (except for the first item, which was given a YES or NO), yielding a maximum score of ten. The higher the score, the better the quality of the study (9–10: excellent; 6–8: good; 4–5: fair; <4: poor).32 A point for a particular criterion was awarded only if the article explicitly reported that the criterion was met. The reliability of the PEDro scale has been demonstrated.33 For those studies that had been listed on the Physiotherapy Evidence Database website at the time of the search, the PEDro score was independently determined by two individuals, who are physical therapists and have extensive experience in physical therapy research methods and are not involved in any of the studies reviewed. A third rater was consulted if consensus was not reached.
Quantitative Analysis
Since the purpose of this systematic review was to evaluate the effects of aerobic exercise on improving aerobic capacity in individuals with stroke, the primary outcome variables of interest were peak VO2 and peak workload. For each selected study, the mean change scores (post-intervention scores – pre-intervention scores) in peak VO2 and peak workload in experimental and control groups were extracted. The baseline standard deviations (SDs) in experimental and control groups were used to calculate the pooled population SD.34 The baseline means and SDs were requested from the respective authors if they were not reported in the article.
The standardized effect size (SES) for each study was established by computing the difference between mean change scores of the experimental and control groups divided by the pooled population SD.34 Since there was a tendency for the SES to overestimate the pooled effect size in studies with a small sample size, a correction was implemented to obtain the unbiased SES.34 The 95% confidence interval (CI) for the SES was also computed. The SESs and 95%CIs were calculated using a custom spreadsheet (Microsoft Excel for Windows XP). Additionally, since many of the selected studies measured walking performance and ambulation is a key priority in stroke rehabilitation, the same quantitative analysis was also performed for walking velocity and walking endurance.
A meta-analysis was then performed for each set of SESs. Q statistic was used to determine whether there was significant heterogeneity. If significant heterogeneity was found, a random effects model was used.35 Otherwise, a fixed effects model was applied.36 The size of the pooled SES from meta-analysis was defined as small (0.2–0.5), medium (0.5–0.8), or large (≥0.8).37 The meta-analyses were performed using an online software program (Meta-analysis software Version 5.2). For all outcome variables of interest, the critical value for rejecting the null hypothesis (i.e. there is no evidence to support the use of aerobic exercise training in individuals with stroke) was set at 0.05.
Results
Based on the information in the title and abstract, 29 papers were identified as being potentially relevant for this systematic review. A total of 20 articles did not meet the criteria and were excluded from further analysis.38–57 Of these, aerobic capacity was not measured in 18 articles.38–55 One study did not use random allocation of subjects.56 Carr and Jones57 did not have a true control group which did not undergo aerobic exercise training. In their study, one group underwent aerobic exercise training whereas another group underwent aerobic and strength training, making it impossible to evaluate the effects of aerobic training.
A total of nine articles fulfilled all criteria and were selected for this review.58–66 Of these, two reported duplicate results: da Cunha Filho et al.60 used the same participants as da Cunha et al.61 but used different outcome measures. Another article64 was a six-month follow-up report of Katz-Leurer et al.63 In summary, a total of nine articles (seven RCTs) were selected for this systematic review. The main characteristics of each selected study are described in Table 1.
Table 1.
Characteristics of randomized controlled trials on aerobic exercise training in individuals with stroke
| Study | Subjects | Cardiovascular exclusion criteria | Exercise training protocol | Resultsa |
|---|---|---|---|---|
| Potempa58 |
|
|
Mode: 10 weeks of cycle ergometer exercise. Intensity: 30–50% maximal workload, ↑ as tolerated (week 1–4), maintain the highest load achieved at end of 4 weeks (week 5–10). Duration: 30 min per session. Frequency: 3×/week. Control group: Passive range of motion. |
Peak VO2: + (↑13.3%) Peak workload: + (↑44.3%) Peak minute ventilation: + Exercise time: + |
| Bateman59 |
|
|
Mode: 12 weeks of cycle ergometer exercise. Intensity: 50 rpm, 60–80% APMHR. Duration: 30 min per session. Frequency: 3×/week. Control group: Relaxation therapy. |
At week 12: Peak workload (loge): + (↑7.3%) RMI: 0 10-m walking speed: 0 BI: 0 FIM: 0 NEADL: 0 Fatigue questionnaire: 0 At week 24: Peak workload significantly declined in the experimental group. |
| da Cunha 60,61 |
|
|
Mode: 3 weeks of treadmill walking with body weight support. Intensity: unknown. Duration: 20 min/session. Frequency: 5×/week. Control group: Regular therapy (usual gait training without treadmill walking with body weight support). |
Peak VO2: + (↑34.8%) Peak workload: 0 Total cycling time: 0 FAC: 0 FIM (locomotion): 0 Gait speed: 0 Walking distance in five minutes: 0 Gait energy expenditure: 0 Gait energy cost: 0 |
| Duncan62 |
|
|
Mode: 36 sessions (12–14 weeks) of supervised home based therapy program including cycle ergometer exercise. Intensity: up to 25–30 min at 40rpm, ↑ resistance as tolerated. Duration: 90 min per session (aerobic component up to 30 min). Frequency: 3×/week. Control group: Usual care (subjects had services as prescribed by their physicians). |
Peak VO2: + (↑9.0%) Bike exercise duration: + 10-m gait speed: + (25.7%) 6MWT: + (25.9%) |
| Katz-Leurer63,64 |
|
|
Mode: 8 weeks of cycle ergometer exercise. Intensity: up to 60% of HRR. Duration: up to 20 min (week 1–2), up to 30 min (week 3–8). Frequency: 5×/week (week 1–2), 3×/week (week 3–8). Control group: treatment not mentioned. |
Peak workload: + (↑176.9%) Number of stairs climbed until fatigued: + FIM: 0 10-m gait speed: 0 Walking distance until fatigued: 0 6-month follow-up: FAI: 0 |
| Chu65 |
|
|
Mode: 8 weeks of water-based endurance exercise. Intensity: up to 80% HRR. Duration: 1 hour (30 min of aerobic activities). Frequency: 3×/week. Control group: a seated upper extremity exercise program. |
Peak VO2: + (↑22.5%) Peak workload: + (↑9.2%) Self-selected gait speed: + (9.2%) |
| Pang66 |
|
|
Mode: 19 weeks of land-based exercise (stepping, walking, repeated sit-to-stand, functional strengthening, balance activities). Intensity: up to 80% HRR. Duration: 1 hour (up to 30 min of aerobic activities). Frequency: 3×/week. Control group: a seated upper extremity exercise program with no aerobic exercise component. |
Peak VO2: + (10.7%) 6MWT: + (19.7%) PASIPD: 0 |
Outcome measures not concerning aerobic capacity, activity endurance or mobility are omitted.
‘+’ refers to a significant difference in favour of the experimental group (p<0.05).
‘0’ refers to no significant difference between groups.
APMHR, age-predicted maximal heart rate; BI, Barthel Index; CMSA, Chedoke McMaster Stroke Assessment; DBP, diastolic blood pressure; ECG, electrocardiography; FAC, Functional Ambulation Category; FAI, Frenchay Activities Index; FIM, Functional Independence Measure; FMA: Fugl-Meyer Assessment; HRR, heart rate reserve; HTN, hypertension; min, minutes; NEADL: Nottingham Extended Activities of Daily Living; PASIPD: Physical Activities Scale for Individuals with Physical Impairments; RMI, Rivermead Mobility Index; 6MWT, Six Minute Walk Test; SBP, systolic blood pressure.
Subjects
The number of subjects included in each study ranged from 13 to 157. However, in Bateman et al.,59 only 70 out of the 157 brain-injured subjects had a diagnosis of stroke. Subjects in acute,60,61,63 subacute,62 and chronic58,65,66 stages of recovery were used in two, one, and three studies, respectively. Bateman et al.,59 however, used subjects in all stages of recovery. In all selected studies, subjects had mild or moderate deficits in motor function and functional abilities. Examining the inclusion and exclusion criteria revealed that individuals in Class C (moderate-to-high risk for cardiac complications during exercise; medical supervision required for all exercise sessions) and Class D (unstable disease with activity restriction; no conditioning exercise recommended) according to the American Heart Association (AHA) Risk Stratification Criteria were excluded.11,67
Exercise Training Protocol
Cycle ergometer was the most common method used for aerobic training (4 studies).58,59,62,63 Treadmill walking was employed in one study.60,61 One study used a combination of stepping, brisk walking and repeated sit-to-stand.66 In Chu et al.,65 subjects performed aerobic exercises in the water. The exercise protocols described in the reviewed studies are similar to the guidelines recommended by American College of Sports Medicine (ACSM) in terms of improving peak VO2 (55%–90% of maximal heart rate or 40%–85% of heart rate reserve; 3–5 days, 20–30 minutes per session).11 All reviewed studies used a protocol consisting of continuous exercise from 20–40 minutes. In earlier stages of training, however, periods of short exercise bouts were used in Katz-Leurer et al..63
Stress test protocol
All reviewed studies used cycle ergometry to conduct the exercise test. The testing protocols and the criteria for termination of the exercise test differed slightly among the studies. In addition to volitional fatigue or exhaustion, many studies used additional measures (e.g. respiratory exchange ratio, heart rate, VO2) to indicate that effort had been maximal. Five studies reported reliability of the primary outcome measures for aerobic capacity (i.e. peak VO2 or workload derived from the stress test) and the values were high (0.78–0.99).58,59,62,63,65 da Cunha Filho et al.60 provided a measure of reliability of the test. However, on closer examination, it was found that they simply quoted the reliability value derived from another study using a different testing protocol.58
Adverse effects
Few adverse events were reported. Duncan et al.62 reported that three of the 50 subjects (6%) in the intervention group had a recurrent stroke during the exercise trial. Two of the strokes occurred within two weeks after randomization and one occurred at seven weeks. The incidence rate of recurrent stroke reported was comparable to the recurrent stroke risk reported in the general stroke population (8% between 1–6 months after stroke).68 Thus, the authors concluded that it is unlikely that the exercise training itself contributes to the recurrent strokes. Pang et al.66 reported five falls from four individuals in the experimental group but none of the falls resulted in any injuries.
Qualitative Assessment
The methodological quality of all selected studies was assessed by the PEDro scale and the results were summarized in Table 2. Five of the seven studies were considered “good” (score: 6–8)59,62–66 and two studies were “fair” (score: 4–5).58,60,61
Table 2.
Methodological quality (PEDro scale)
| Study | |||||||
|---|---|---|---|---|---|---|---|
| Criterion | Potempa58 | Bateman59 | da Cunha60,61 | Duncan62 | Katz-Leurer63,64 | Chu65 | Pang66 |
| 1. Eligibility criteria specified | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Random allocation to groups | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Concealed allocation | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 4. Groups similar at baseline | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5. Subject blinding | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6. Therapist blinding | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7. Assessor blinding | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| 8. Less than 15% dropouts | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| 9. Intent-to-treat analysis | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 10. Between groups statistics reported | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11. Point estimates and variability data reported | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total score | 4 | 7 | 4 | 8 | 6 | 6 | 8 |
Meta-Analysis: Aerobic capacity
Five RCTs used peak VO2 as an outcome measure.58,60,62,65,66 All of these studies reported statistically significant increase in peak VO2 in the experimental group after aerobic exercise training, ranging from 9.0%–34.8%.58,60,62,65,66 Meta-analysis revealed a significant homogeneous SES (small size) (SES, 0.42; 95%CI, 0.15 to 0.69; Z=3.06; p=0.001) in favour of aerobic exercise training (Figure 1A).
Figure 1.
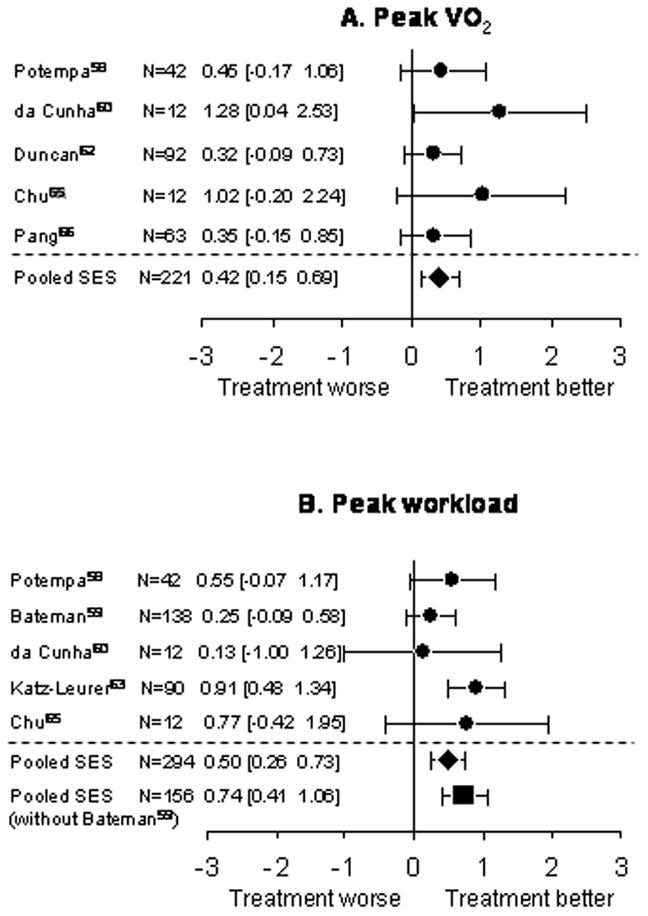
Meta-analysis: aerobic capacity. Each set of dot (●) and error bars represent the standardized effect size (SES) and its 95% confidence interval (CI), respectively, for each study. The first author, the number of subjects involved, the SES value and its 95% CI of each study were also indicated beside each respective set of dot and error bars. The pooled SES was indicated by ◆ (all studies) and ■ (without Bateman et al. 200159).
Five RCTs used peak workload as an outcome measure.58,59,60,63,65 Four of these studies reported a statistically significant increase in peak workload in the experimental group following training, ranging from 7.3%–176.9%.58,59,63,65 da Cunha Filho et al.60 reported a 50.0% increase in workload in the experimental group but it did not reach statistical significance (p=0.226), probably due to the small sample size. There was a significant homogeneous SES (medium size) (0.50; 95% CI, 0.26 to 0.73; Z=4.17; p<0.001) in favour of aerobic exercise training (Figure 1B).
Since the sample used Bateman et al.59 did not all have a diagnosis of stroke, a sensitivity analysis was performed by excluding this study in the meta-analysis. A significant and greater homogeneous SES (medium size) in favour of aerobic exercise was found (SES, 0.74; 95%CI, 0.41 to 1.06; Z=4.42; p<0.001) when Bateman et al.59 was excluded (Figure 1B).
Meta-Analysis: Walking performance
Five studies evaluated walking velocity.59,61–63,65 The distance involved in the walking test varied slightly among different studies, ranging form five to 12 meters. In Katz-Leurer et al.,63 none of the patients were able to walk independently and the baseline walking velocity was considered zero. The other four studies all reported that after aerobic exercise training, walking velocity increased from baseline values, ranging from 9.2 to 63.8%.59,61,62,65 However, statistical significance was obtained in two studies only.62,65 The results were pooled in the meta-analysis. The calculation of SES for Katz-Leurer et al.63 was based on the post-intervention SDs because both experimental and control group had a pre-test score of zero. A significant homogeneous SES (small size) in favour of aerobic exercise on walking velocity was found (0.26; 95%CI, 0.05 to 0.48; Z=2.43; p=0.008) (Figure 2A). Excluding Bateman et al.59 also resulted in a significant and greater homogeneous SES (small size) (SES, 0.44; 95%CI, 0.16 to 0.72; Z=3.11; p<0.001) in favour of aerobic training on walking velocity (Figure 2A). Four studies measured walking endurance.61–63,66 Two of the studies used the Six Minute Walk Test as an indicator of walking endurance,62,66 and one study measured the walking distance in five minutes.61 All three studies reported an increase in walking distance in the experimental group by 19.7 to 109.4%, when compared with the baseline values.61,62,66 Statistical significance was reached in two of the studies.62,66 In Katz-Leurer et al.,63 subjects were asked to cover as much distance as they could until volitional fatigue. The baseline walking endurance was zero for both groups of subjects as none of the subject could walk independently. Meta-analysis found a significant homogeneous SES (small size) (0.30; 95%CI, 0.06 to 0.55; Z=2.41; p=0.008) in favour of aerobic training on walking endurance (Figure 2B).
Figure 2.
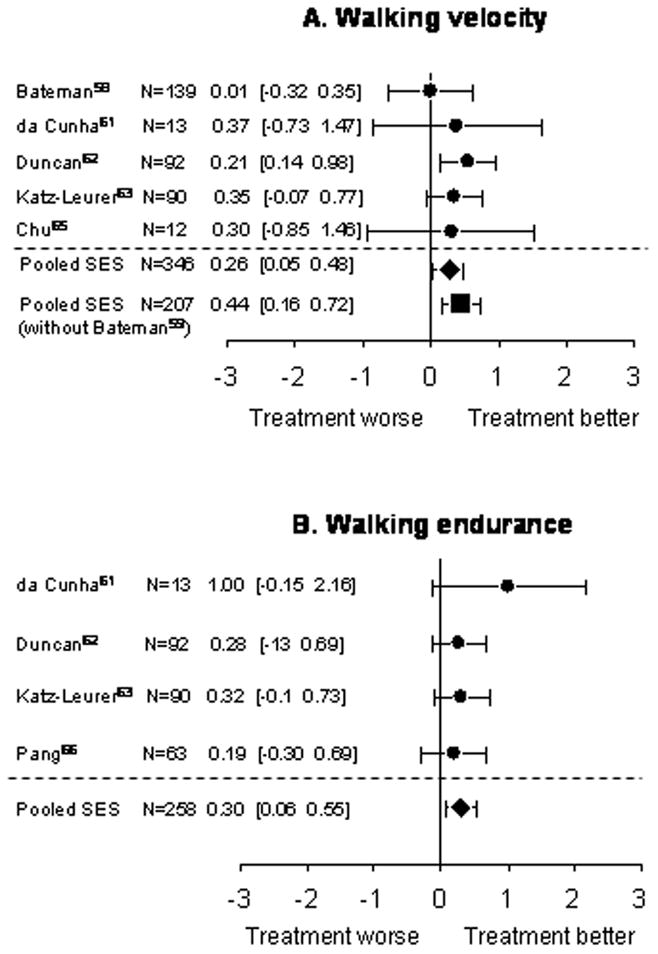
Meta-analysis: walking performance. Each set of dot (●) and error bars represent the standardized effect size (SES) and its 95% confidence interval (CI), respectively, for each study. The first author, the number of subjects involved, the SES value and its 95% CI of each study were also indicated beside each respective set of dot and error bars. The pooled SES was indicated by ◆ (all studies) and ■ (without Bateman et al. 200159).
Discussion
Mode of training
The mode of training varies in different studies, including cycle ergometer, treadmill or functional activities such as brisk stepping. Exercising on a cycle ergometer does not require as much postural control when compared to treadmill walking and thus may be a better alternative for those individuals with poor balance. A water-based program was implemented by Chu et al.65 Water may serve as an excellent exercise medium because it offers resistance and the buoyancy of water reduces joint impact loading and provides partial weight support.69 This may be relevant because individuals with stroke often have reduced ability to weightbear on the paretic leg70,71 and arthritis is also quite common in this population.62,66,72 Higher impact activities (e.g., walking/running) tend to cause more injuries than non-impact activities (e.g., cycling/exercising in water) in the elderly.73–75 This is also an important factor to consider when determining the mode of training for older individuals with stroke.
Exercise Protocol
Although the exercise protocols described in the reviewed studies are consistent with the guidelines recommended by ACSM for improving peak VO2, it is important to note that these guidelines are established for otherwise healthy individuals. It is not known whether lower exercise intensities would be sufficient to induce a positive outcome in individuals with stroke, many of whom have severe deconditioning.13,14,27 The threshold for endurance training effects may be less for individuals with lower fitness levels. In fact, Swain and Franklin76 found that aerobic training intensities as low as 30% oxygen uptake reserve were effective in improving cardiovascular fitness in less fit, but healthy individuals.
It has been established in healthy subjects that cumulative short periods of exercise bouts can achieve the same training effects as a long exercise session.77–79 Whether this principle is true in individuals with stroke is not known. In a previous study by Macko et al.,80 periods of short exercise bouts, consisting of 2–3 minutes of treadmill walking interspersed with rest periods of similar duration were used for chronic stroke patients who were more deconditioned and positive outcomes were reported. However, the study did not have a control group. Activity endurance is a major deficit in stroke survivors.5 If short bouts of activity are equally effective, it may be an excellent way to train aerobic capacity for this population.
Effect on aerobic capacity
Meta-analysis revealed a small effect size for improving peak VO2 and medium effect size for improving peak workload. Subjects in the acute stage of recovery tended to demonstrate a higher percentage increase in both peak VO260 and peak workload63 than subacute62 and chronic subjects,58,65,66 probably due to the low aerobic capacity at baseline. Katz-Leurer et al.63 showed that the control group had a 65.4% increase in maximal workload at the end of the training period, indicating that spontaneous recovery may play a role at the acute stage.
Two studies used a combination of aerobic exercise and strengthening exercise in the training regimen.62,66 However, there is no evidence to suggest that aerobic plus resistive training is better than aerobic exercise alone to improve aerobic capacity. Water-based program resulted in an impressively high percentage of improvement in peak VO2 (22.5%)65 compared with most land-based programs with chronic subjects. It may also be due to the high intensity used in the exercise protocol (80% heart rate reserve).65 It would be interesting to compare the efficacy of a land-based and a water-based program in the future.
Effect on walking performance
To rehabilitation practitioners, whether aerobic exercise actually improves functional abilities is also a topic of clinical importance. A small effect size was found in favour of aerobic exercise for improving walking velocity and walking endurance. Two studies used walking as part of the training protocol.60,61,66 It is thus possible that the improvement in walking performance reported might be partly attributable to repeated gait practice. The effects of aerobic training on other daily functions in individuals with stroke will require further investigations.
Limitations of studies reviewed
Several weaknesses in the studies reviewed can be identified. First, certain medications (i.e. beta-blockers) can alter the cardiovascular response to exercise.81–83 Only one study reported whether the subjects changed medications during the course of the study.58 Failure to report information on medications may have lowered the quality of some of the studies. Second, the scarcity of adverse events may be because the selected studies did not track or report adverse events. Third, four of the selected studies used the same task to assess and train aerobic capacity.58,59,62,63 Therefore, the possibility that the improvement of performance in the exercise test post-training was due to a practice effect rather than a true aerobic adaptation could not be ruled out. Fourth, there was a lack of long-term follow-up. Only Bateman et al.59 performed follow-up assessment of aerobic capacity 12 weeks after the termination of training and found no significant group by time interaction in peak workload. Finally, most studies used individuals with a single ischemic or hemorrhagic stroke who were mildly or moderately impaired and did not have any unstable or serious cardiovascular conditions. Therefore, the effects of aerobic exercise training in other subpopulations (i.e. individuals with severe impairments, higher cardiac risks) will require further investigations.
Limitations of the systematic review
The major limitation of this systematic review is related to the difficulty in defining what studies have investigated aerobic exercise. For example, a number of studies investigated treadmill walking for the purpose of gait training in individuals with stroke.38–47 In additional to improving gait, this training method may potentially have an aerobic training effect. However, these studies did not include any outcome measure on aerobic capacity. Since the focus of this systematic review was to investigate the effect on aerobic capacity, these studies were eventually excluded from analysis. Although walking endurance was used in some of these studies,39–41,47 it was not necessarily a good indicator of aerobic fitness in individuals with stroke because other impairments such as balance and muscle strength also impact on walking endurance.84 Moreover, it is very difficult to determine whether the improvement in walking performance reported in these studies is due to repeated gait practice or increase in aerobic capacity. Nevertheless, our methodology might have eliminated some studies that have investigated an exercise program with a potential aerobic training effect.
Conclusion
From the systematic review of the current literature, we have good evidence to suggest that aerobic exercise training (50–80% heart rate reserve, 3–5 days a week for 20–40 minutes) is beneficial in improving aerobic capacity in stroke survivors. The results could be generalized to those who are mildly or moderately impaired by stroke and who have relatively low risk of cardiac complications with exercise. Further research is needed to determine the optimal protocol to train individuals with different levels of physical impairment and cardiac risk, the long term effects of aerobic exercise training as well as the relationship between improvement in aerobic capacity and daily function.
Acknowledgments
MYCP was supported by a post-doctoral fellowship from Natural Sciences and Engineering Research Council of Canada. This study was supported by a grant-in-aid from the Heart and Stroke Foundation of New Brunswick and from career scientist awards to JJE from Canadian Institute of Health Research (MSH-63617)) and the Michael Smith Foundation for Health Research.
Appendix 1. Search Strategy (MEDLINE)
| 1. exp Cerebrovascular accident/ |
| 2. (stroke or cva$ or cerebrocasecular or cerebral vascular).tw. |
| 3. exp Brain Injuries/ |
| 4. exp Hemiplegia/ |
| 5. (hemipleg$ or hemipar$ or brain injur$).tw. |
| 6. or/1–5 |
| 7. exp Exercise Therapy/ or exp Exercise/ |
| 8. exp Physical Fitness/ |
| 9. exp Physical Endurance/ |
| 10. treadmill.tw. |
| 11. ((aerobic or endurance or cardio$ or fitness) adj5 (train$ or program$ or protocol$ or |
| 12. or/7–11 |
| 13. exp Randomized Controlled Trials/ |
| 14. Clinical trial.pt. |
| 15. exp Random Allocation/ |
| 16. Randon$.tw. |
| 17. exp Cross-Over Studies/ |
| 18. Control$.tw. |
| 19. experimental$.tw. |
| 20. exp Follow-Up Studies/ |
| 21. or/13–20 |
| 22. 6 and 12 and 21 |
Free text terms (.tw.); MESH controlled vocabulary (/); truncation ($)
adj5: adjacency operator, retrieves records that contain the search term within 5 words of each other in either direction; exp: explode
Clinical Message.
There is good evidence to support the use of aerobic exercise to improve aerobic capacity in individuals with stroke.
The results can be generalized to those who are mildly or moderately impaired by stroke and who have relatively low risk of cardiac complications with exercise.
Footnotes
Competing interests
None declared
Contributors
MYCP: data collection, designing the methodology, writing the paper.
JJE (the guarantor): initiating the study, designing the methodology, monitoring progress, revising the paper.
ASD: monitoring progress, revising the paper.
SG: data collection, writing the paper.
References
- 1.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The stroke data bank: designs, methods, and baseline characteristics. Stroke. 1988;19:547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Stroke: Neurologic and functional recovery. The Copenhagen Study. Phys Med Rehabil Clin N Am. 1999;10:887–906. [PubMed] [Google Scholar]
- 4.Gresham GE, Dawber TR. Residual disability in survivors of stroke: the Framingham study. N Engl J Med. 1975;293:954–956. doi: 10.1056/NEJM197511062931903. [DOI] [PubMed] [Google Scholar]
- 5.Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disability Rehabil. 1999;21:258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- 6.Berthouze SE, Minaire PM, Castells J, Busso T, Vico L, Lacour JR. Relationship between mean habitual daily energy expenditure and maximal oxygen uptake. Med Sci Sports Exerc. 1995;27:1170–1179. [PubMed] [Google Scholar]
- 7.Talbot LA, Metter EJ, Fleg JL. Leisure-time physical activities and their relationship to cardiopulmonary fitness in healthy men and women 18–95 years old. Med Sci Sports Exerc. 2000;32:417–425. doi: 10.1097/00005768-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Mezzani A, Corra U, Baroffio C, Bosimini E, Giannuzzi P. Habitual activities and peak aerobic capacity in patients with asymptomatic and symptomatic left ventricular dysfunction. Chest. 2000;117:1291–1299. doi: 10.1378/chest.117.5.1291. [DOI] [PubMed] [Google Scholar]
- 9.Fujitani J, Ishikawa T, Akai M, Kakurai S. Influence of daily activity on changes in physical fitness for people with post-stroke hemiplegia. Am J Phys Med Rehabil. 1999;78:540–544. doi: 10.1097/00002060-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Marchionni N, Fattirolli F, Fumagalli S, et al. Determinants of exercise tolerance after acute myocardial infarction in older persons. J Am Ger Soc. 2000;48:146–153. doi: 10.1111/j.1532-5415.2000.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 11.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 12.Raven PB, Welch-O’Connor RM, Shi X. Cardiovascular function following reduced aerobic activity. Med Sci Sports Exerc. 1998;30:1041–1052. doi: 10.1097/00005768-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85:113–118. doi: 10.1016/s0003-9993(03)00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacKay-Lyons MJ, Makrides L. Longitudinal changes in exercise capacity after stroke. Arch Phys Med Rehabil. 2004;85:1608–1612. doi: 10.1016/j.apmr.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Kurl S, Laukanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Cardiorespiratory fitness and the risk for stroke in men. Arch Intern Med. 2003;163:1682–1688. doi: 10.1001/archinte.163.14.1682. [DOI] [PubMed] [Google Scholar]
- 16.Guedes DP, Guedes JE. Physical activity, cardiorespiratory fitness, dietary content, and risk factors that cause a predisposition towards cardiovascular disease. Arq Bras Cardiol. 2001;77:243–257. doi: 10.1590/s0066-782x2001000900005. [DOI] [PubMed] [Google Scholar]
- 17.Rogers MA, Yamamoto C, Hagberg JM, Holloszy JO, Ehsani AA. The effects of 7 years of intense exercise training on patients with coronary artery disease. J Am Coll Cardiol. 1987;10:321–326. doi: 10.1016/s0735-1097(87)80014-1. [DOI] [PubMed] [Google Scholar]
- 18.Lakka TA, Laukkanen J, Rauramaa R, et al. Cardiorespiratory fitness and the progression of carotid atherosclerosis in middle-aged men. Ann Intern Med. 2001;134:12–20. doi: 10.7326/0003-4819-134-1-200101020-00008. [DOI] [PubMed] [Google Scholar]
- 19.Roth EJ. Heart disease in patients with stroke: incidence, impact, and implications for rehabilitation. Part I: classification and prevalence. Arch Phys Med Rehabil. 1993;74:752–760. doi: 10.1016/0003-9993(93)90038-c. [DOI] [PubMed] [Google Scholar]
- 20.Pang MYC, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int. doi: 10.1007/s00198-005-1925-1. published online May 19, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rimmer JH. Health promotion for people with disabilities: the emerging paradigm shift from disability prevention to prevention of secondary conditions. Phys Ther. 1999;79:495–502. [PubMed] [Google Scholar]
- 22.Rimmer JH, Braddock D. Health promotion for people with physical, cognitive, and sensory disabilities: an emerging national priority. Am J Health Promot. 2002;16:220–224. doi: 10.4278/0890-1171-16.4.220. [DOI] [PubMed] [Google Scholar]
- 23.Binder EF, Birge SJ, Spina R, et al. Peak aerobic power is an important component of physical performance in older women. J Gerontol A Biol Sci Med Sci. 1999;54:M353–356. doi: 10.1093/gerona/54.7.m353. [DOI] [PubMed] [Google Scholar]
- 24.Jeng C, Chang W, Wai PM, Chou CL. Comparison of oxygen consumption in performing daily activities between patients with chronic obstructive pulmonary disease and a healthy population. Heart Lung. 2003;32:121–130. doi: 10.1067/mhl.2003.20. [DOI] [PubMed] [Google Scholar]
- 25.Cress ME, Meyer M. Maximal voluntary and functional performance levels needed for independence in adults aged 65 to 97 years. Phys Ther. 2003;83:37–48. [PubMed] [Google Scholar]
- 26.Paterson DH, Cunningham DA, Koval JJ, St Croix CM. Aerobic fitness in a population of independently living men and women aged 55–86 years. Med Sci Sports Exerc. 1999;31:1813–1820. doi: 10.1097/00005768-199912000-00018. [DOI] [PubMed] [Google Scholar]
- 27.MacKay-Lyons MJ, Makrides L. Exercise capacity early after stroke. Arch Phys Med Rehabil. 2002;83:1697–1702. doi: 10.1053/apmr.2002.36395. [DOI] [PubMed] [Google Scholar]
- 28.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect. Arch Phys Med Rehabil. 2002;83:1378–1383. doi: 10.1053/apmr.2002.35089. [DOI] [PubMed] [Google Scholar]
- 29.Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil. 2004;18:833–862. doi: 10.1191/0269215504cr843oa. [DOI] [PubMed] [Google Scholar]
- 30.Meek C, Pollock A, Potter J, Langhorne P. A systematic review of exercise trials post stroke. Clin Rehabil. 2003;17:6–13. doi: 10.1191/0269215503cr579oa. [DOI] [PubMed] [Google Scholar]
- 31.Physiotherapy Evidence Database (online) Available at www.pedro.fhs.usyd.edu.au.
- 32.Teasell R, Foley N, Salter K, et al. Evidence-based review of stroke rehabilitation. 6. Heart and Stroke Foundation of Ontario; 2004. [Google Scholar]
- 33.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 34.Hedges L, Olkin I. Statistical methods for meta-analysis. Orlando: Academic Press; 1985. [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Hedges L. Fixed effects models. In: Cooper H, Hedges LV, editors. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. pp. 285–300. [Google Scholar]
- 37.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. The t test for means; pp. 25–26. [Google Scholar]
- 38.Richards CL, Malouin F, Bravo G, Dumas F, Wood-Dauphinee S. The role of technology in task-oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2004;18:199–211. doi: 10.1177/1545968304269397. [DOI] [PubMed] [Google Scholar]
- 39.Eich HJ, Mach H, Werner C, Hesse S. Aerobic treadmill plus Bobath walking training improves walking in subacute stroke: a randomized controlled trial. Clin Rehabil. 2004;18:640–651. doi: 10.1191/0269215504cr779oa. [DOI] [PubMed] [Google Scholar]
- 40.Ada L, dean Cm, hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003;84:1486–1491. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 41.Salbach NM, Mayo NE, Wood-Dauphinee S, Hanley JA, Richards CL, Cote R. A task-oriented intervention enhances walking distance and speed in the first year post stroke: a randomized controlled trial. Clin Rehabil. 2004;18:509–519. doi: 10.1191/0269215504cr763oa. [DOI] [PubMed] [Google Scholar]
- 42.Werner C, Von Frankenberg S, Treig T, Konrad M, Hesse S. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: a randomized crossover study. Stroke. 2002;33:2895–2901. doi: 10.1161/01.str.0000035734.61539.f6. [DOI] [PubMed] [Google Scholar]
- 43.Werner C, Bardelebern A, Mauritz KH, Kirker S, Hesse S. Treadmill training with partial body weight support and physiotherapy in stroke patients: a preliminary comparison. Eur J Neurol. 2002;9:639–644. doi: 10.1046/j.1468-1331.2002.00492.x. [DOI] [PubMed] [Google Scholar]
- 44.Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trials. Stroke. 2002;33:553–558. doi: 10.1161/hs0202.102365. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson L, Carlsson J, Danielsson A, et al. Walking training of patients with hemiparesis at an early stage after stroke: a comparison of walking training on a treadmill with body weight support and walking training on the ground. Clin Rehabil. 2001;15:515–527. doi: 10.1191/026921501680425234. [DOI] [PubMed] [Google Scholar]
- 46.Laufer Y, Dickstein R, Chefez Y, Marcovitz E. The effect of treadmill training on the ambulation of stroke survivors in the early stages of rehabilitation: a randomized study. J Rehabil Res Dev. 2001;38:69–78. [PubMed] [Google Scholar]
- 47.Kosak MC, Reding MJ. Comparison of partial body weight-supported treadmill gait training versus aggressive bracing assisted walking post stroke. Neurorehabil Neural Repair. 2000;14:13–19. doi: 10.1177/154596830001400102. [DOI] [PubMed] [Google Scholar]
- 48.Glasser L. Effects of isokinetic training on the rate of movement during ambulation in hemiparetic patients. Phys Ther. 1986;66:673–676. doi: 10.1093/ptj/66.5.673. [DOI] [PubMed] [Google Scholar]
- 49.Teixeira-Salmela LF, Olney SJ, Nadeau S, Brouwer B. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Arch Phys Med Rehabil. 1999;80:1211–1218. doi: 10.1016/s0003-9993(99)90018-7. [DOI] [PubMed] [Google Scholar]
- 50.Teixeira-Salmela LF, Nadeau S, McBride I, Olney SJ. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. J Rehabil Med. 2001;33:53–60. doi: 10.1080/165019701750098867. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira L, Nadeau S, Olney SJ, McBride I, Culham E, Zee B. The impact of a muscle strengthening and physical conditioning program on gait and stairclimbing performance in chronic stroke survivors. Gait Posture. 1998;7:144–145. [Google Scholar]
- 52.Duncan P, Richards L, Wallace D, et al. A randomized controlled pilot study of a home-based exercise program for individuals with mild and moderate stroke. Stroke. 1998;29:2055–2060. doi: 10.1161/01.str.29.10.2055. [DOI] [PubMed] [Google Scholar]
- 53.Eng JJ, Chu KS, Kim CM, Dawson AS, Carswell A, Hepburn KE. A community-based group exercise program for persons with chronic stroke. Med Sci Sports Exerc. 2003;8:1271–1278. doi: 10.1249/01.MSS.0000079079.58477.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards CL, Malouin F, Wood-Dauphinee S, Williams JI, Bouchard JP, Brunet D. Task-specific physical therapy for optimization of gait recovery in acute stroke patients. Arch Phys Med Rehabil. 1993;74:612–620. doi: 10.1016/0003-9993(93)90159-8. [DOI] [PubMed] [Google Scholar]
- 55.Malouin F, Richards CL, Wood-Dauphinee S, Williams JI. A randomized controlled trial comparing early and intensive task-specific therapy to conventional therapy in acute stroke patients. Can J Rehabil. 1993;7:27–28. [Google Scholar]
- 56.Rimmer JH, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African-American group of stroke survivors. Med Sci Sports Exerc. 2000;32:1990–1996. doi: 10.1097/00005768-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Carr M, Jones J. Physiological effects of exercise on stroke survivors. Top Stroke Rehabil. 2003;9:57–64. doi: 10.1310/0J2K-MDNX-1Q0L-8LX6. [DOI] [PubMed] [Google Scholar]
- 58.Potempa K, Lopez M, Braun LT, Szidon JP, Fogg L, Tincknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26:101–105. doi: 10.1161/01.str.26.1.101. [DOI] [PubMed] [Google Scholar]
- 59.Bateman A, Culpan J, Pickering AD, Powell JH, Scott OM, Greenwood RJ. The effect of aerobic training on rehabilitation outcomes after recent severe brain injury: a randomized controlled evaluation. Arch Phys Med Rehabil. 2001;82:174–182. doi: 10.1053/apmr.2001.19744. [DOI] [PubMed] [Google Scholar]
- 60.da Cunha Filho IT, Lim PAC. A comparison of regular rehabilitation and regular rehabilitation with supported treadmill ambulation training for acute stroke patients. J Rehabil Res Dev. 2001;38:245–255. [PubMed] [Google Scholar]
- 61.da Cunha IT, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: a randomized controlled pilot study. Arch Phys Med Rehabil. 2002;83:1258–1265. doi: 10.1053/apmr.2002.34267. [DOI] [PubMed] [Google Scholar]
- 62.Duncan P, Studenski S, Richards L, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke. 2003;34:2173–2180. doi: 10.1161/01.STR.0000083699.95351.F2. [DOI] [PubMed] [Google Scholar]
- 63.Katz-Leurer M, Shochina M, Carmeli E, Friedlander Y. The influence of early aerobic training on the functional capacity in patients with cerebrovascular accident at the subacute stage. Arch Phys Med Rehabil. 2003;84:1609–1614. doi: 10.1053/s0003-9993(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 64.Katz-Leurer M, Carmeli E, Shochina M. The effect of early aerobic training on independence six months post stroke. Clin Rehabil. 2003;17:735–741. doi: 10.1191/0269215503cr671oa. [DOI] [PubMed] [Google Scholar]
- 65.Chu KS, Eng JJ, Dawson AS, Harris JE, Ozkaplan A, Gylfadottir S. Water-based exercise for cardiovascular fitness in people with chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2004;85:870–874. doi: 10.1016/j.apmr.2003.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pang MYC, Eng JJ, Dawson AS, McKay HA, Harris JE. A community-based fitness and mobility exercise (FAME) program for older adults with chronic stroke: a randomized controlled trial. J Am Geriatr Soc. 2005 doi: 10.1111/j.1532-5415.2005.53521.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for healthcare professionals from the American Heart Association. Circulation. 1995;91:580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 68.Hardie K, Hankey GJ, Jamrozik K, Broadhurst RJ, Anderson C. Ten-year risk of first recurrent stroke and disability after first-ever stroke in the Perth community stroke study. Stroke. 2004;35:731–735. doi: 10.1161/01.STR.0000116183.50167.D9. [DOI] [PubMed] [Google Scholar]
- 69.Moening D, Scheidt A, Shepardson L, Davies GJ. Biomechanical comparison of water running and treadmill running. Isokinet Exerc Sci. 1993;3:207–215. [Google Scholar]
- 70.Kim CM, Eng JJ. Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait Posture. 2003;8:23–28. doi: 10.1016/s0966-6362(02)00122-4. [DOI] [PubMed] [Google Scholar]
- 71.Hsu A-L, Tang P-F, Jan M-H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild and moderate stroke. Arch Phys Med Rehabil. 2003;84:1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 72.Andrews K, Brocklehurst JC, Richards C, Laycock PJ. The influence of age on the clinical presentation and outcome of stroke. Int Rehab Med. 1984;6:49–53. doi: 10.3109/03790798409166756. [DOI] [PubMed] [Google Scholar]
- 73.Blair SN, Kohl HW, Goodyear NN. Rates and risks for running and exercise injuries: studies in three populations. Res Q Exerc Sports. 1987;58:221–228. [Google Scholar]
- 74.Carroll JF, Pollock ML, Graves JE, Leggett SH, Spitler DL, Lowenthal DT. Incidence of injury during moderate- and high-intensity walking training in the elderly. J Gerontol. 1992;47:M61–M66. doi: 10.1093/geronj/47.3.m61. [DOI] [PubMed] [Google Scholar]
- 75.Pollock ML, Carroll JF, Graves JE, et al. Injuries and adherence to walk/jog and resistance programs in the elderly. Med Sci Sports Exerc. 1991;23:1194–1200. [PubMed] [Google Scholar]
- 76.Swain DP, Franklin BA. VO2 reserve and the minimal intensity for improving cardiorespiratory fitness. Med Sci Sports Exerc. 2002;34:152–157. doi: 10.1097/00005768-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 77.Debusk RF, Stenestrand U, Sheehan M, Haskell WL. Training effects of long versus short bouts of exercise in healthy subjects. Am J Cardiol. 1990;65:1010–1013. doi: 10.1016/0002-9149(90)91005-q. [DOI] [PubMed] [Google Scholar]
- 78.Ebisu T. Splitting the distance of endurance training: on cardiovascular endurance and blood lipids. Jpn J Physiol Educ. 1985;30:37–43. [Google Scholar]
- 79.Jakicic JM, Wing RR, Butler BA, Robertson RJ. Prescribing exercise in multiple short bouts versus one continuous bout: effect on adherence, cardiorespiratory fitness, and weight loss in overweight women. Int J Obes. 1995;19:893–901. [PubMed] [Google Scholar]
- 80.Macko RF, Smith GV, Dobrovolny L, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 81.Cohen-Solal A, Baleynaud S, Laperche T, Sebag C, Gourgon R. Cardiopulmonary response during exercise of a B1-selective β-blocker (atenolol) and a calcium-channel blocker (diltiazem) in untrained subjects with hypertension. J Cardiovasc Pharmacol. 1993;22:33–8. doi: 10.1097/00005344-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 82.Pollock ML, Lowenthal DT, Foster C, et al. Acute and chronic responses to exercise in patients treated with beta blockers. J Cardiopulm Rehabil. 1991;11:132–144. [Google Scholar]
- 83.Wilmore JH, Ewy GA, Freund BJ, et al. Cardiorespiratory alterations consequent to endurance exercise training during beta-adrenergic blockade with atenolol and propranolol. Am J Cardiol. 1985;55:142D–148D. doi: 10.1016/0002-9149(85)91071-9. [DOI] [PubMed] [Google Scholar]
- 84.Pang MYC, Eng JJ, Dawson AS. Relationship between ambulatory capacity and cardiorespiratory fitness in chronic stroke: influence of stroke-specific impairments. Chest. 2005;127:495–501. doi: 10.1378/chest.127.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]


