Abstract
Leanyer virus (LEAV), currently classified as a member of the genus Orthobunyavirus, in the family Bunyaviridae, was originally isolated from a pool of Anopheles meraukensis mosquitoes, collected at Leanyer, Northern Territory, Australia in 1974. When it failed to react in serological tests with antisera from other known viruses, full-length genomic sequencing was pursued to determine the relationship of LEAV to other orthobunyavirus species. Genetic and serological characterization confirmed its antigenic distance from other orthobunyaviruses, including to its closest genetic neighbours, the Simbu group viruses, suggesting that it may represent a new antigenic complex.
Introduction
The family Bunyaviridae constitutes one of the largest taxonomic groupings of RNA viruses, containing more than 350 viruses. The family Bunyaviridae (commonly known as bunyaviruses) comprises five genera: Hantavirus, Nairovirus, Orthobunyavirus, Phlebovirus and Tospovirus, based on serological and molecular characteristics (http://www.ictvonline.org/virusTaxonomy.asp?version=2008). All members of the family share certain common characteristics including: (i) a tri-segmented genome; (ii) a negative or ambisense coding strategy; (iii) four structural proteins; (iv) cytoplasmic replication; and (v) assembly and maturation at the Golgi apparatus. Viruses within each genus share similar segment and structural protein sizes, and nucleotide sequences at the 3′ and 5′ termini of each segment.
Viruses in the genera Orthobunyavirus, Nairovirus, Phlebovirus and Tospovirus are transmitted by arthropods (mosquitoes, midges, sandflies, ticks or thrips). In contrast, viruses in the genus Hantavirus are not transmitted by arthropods, but are acquired by aerosol exposure to virus-contaminated excreta or by a bite from their rodent or shrew hosts (Nichol et al., 2005; Ramsden et al., 2009). Viruses that impinge on human health, either directly by causing illness, or indirectly by causing disease and economic loss in domestic animals or crop plants, are found in each of the five genera (Elliott, 1997; Nichol et al., 2000).
The largest genus in the family is the Orthobunyavirus, which currently contains more than 170 viruses, assigned to 48 distinct species based on serological relatedness by complement fixation (CF) test (mediated by the N protein) or haemagglutination inhibition (HI) and neutralization tests (mediated by glycoproteins) (Nichol et al., 2005). Despite the association of many of the orthobunyaviruses with human and animal disease, molecular characterizations of the entire genus has been limited to four serological groups, namely California encephalitis, Bunyamwera, Group C and Simbu (Bowen et al., 1995; Dunn et al., 1994; Nunes et al., 2005b; Saeed et al., 2000). These antigenic complexes bring together several viruses (e.g. Guaroa, Kairi and Bunyamwera species are all grouped in the Bunyamwera complex). Because of the paucity of genetic data for many of the orthobunyaviruses, taxonomic placements must be regarded as fluid, since antigenic relatedness among segmented viruses may vary depending on the particular serological test used or because of natural reassortment among closely related viruses.
Leanyer virus (LEAV) was initially believed to be a member of the family Togaviridae based on virion size (Doherty et al., 1977), but was later shown to be morphologically consistent with a bunyavirus (Stuckly & Wright, 1983). Initial analysis of partial sequences showed LEAV to be divergent compared with other orthobunyaviruses, making it an interesting target for further characterization. Here, we describe rapid full-length genome sequencing of LEAV, and provide evidence that it represents a new orthobunyavirus species in a previously unidentified antigenic complex.
Results
Serology
A list of antibodies specific to each viruses' proteins used for serological analysis of LEAV is given in Table 1. In summary, LEAV mouse brain antigen (haemagglutinin titre 1 : 640 at pH 5.75–6.0) reacted in HI tests with a hyperimmune mouse LEAV antibody (homologous titre 1 : 5120), but it failed to react with antibodies to any of the other viruses shown in Table 1. Furthermore, LEAV antibodies failed to react with Oropouche virus (OROV), Aino virus (AINOV), Akabane virus (AKAV), Koongol virus (KOOV), Wongal virus (WONV) and Tete virus (TETEV) antigens in CF tests (Supplementary Table S1, available in JGV Online) and failed to react with OROV and WONV in HI tests (Supplementary Table S2, available in JGV Online). LEAV antibodies also failed to detect proteins from AINOV and OROV in Western blot (data not shown).
Table 1. Antisera tested by serology (HI and/or neutralization test) against LEAV at the Queensland Institute of Medical Research and University of Texas Medical Branch.
| Alphavirus | Flavivirus | Bunyaviridae | Orthobunyavirus | Orbivirus | Orthomyxovirus | Rhabdoviridae | Lyssavirus | Nairovirus |
| Getah | Alfuy | Belmont | AINOV | Corriparta | Dhori | Alpimwar | Bovine ephemeral fever | Kao Shuan |
| Ross River | Edge Hill | Gan Gan | AKAV | D'Aguillar | Johnston Atoll | Charleville | Taggert | |
| Sindbis | Kokobera | Kowanyama | Facey's Paddock (Ch 16129) | Eubenangee | Upolu | Ngaingan | ||
| Murweh (Ch 16313) | Kunjin | Maprik | KOOV | Mitchell River | ||||
| Murray Valley encephalitis | Mapputta | OROV | Mudjinbarry | |||||
| Saumarez Reef | Trubanaman | PEAV | Nugget | |||||
| Stratford | TETEV | Tilligerry | ||||||
| WONV | Wallal | |||||||
| Yacaaba | Warrego |
Sequence acquisition and analysis
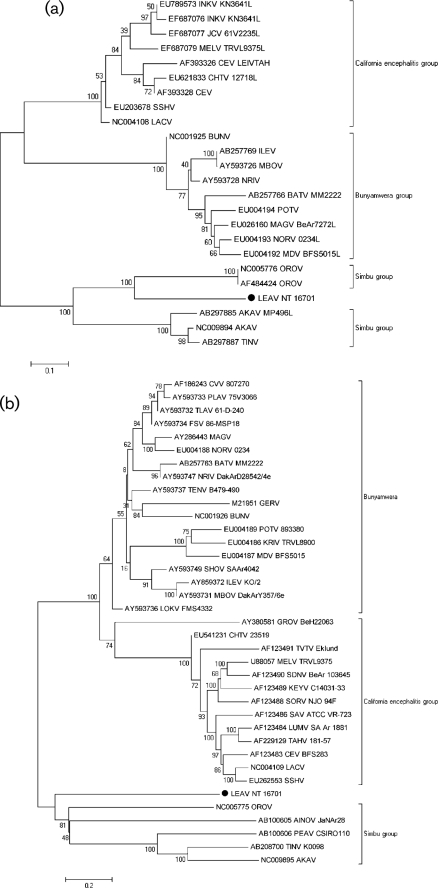
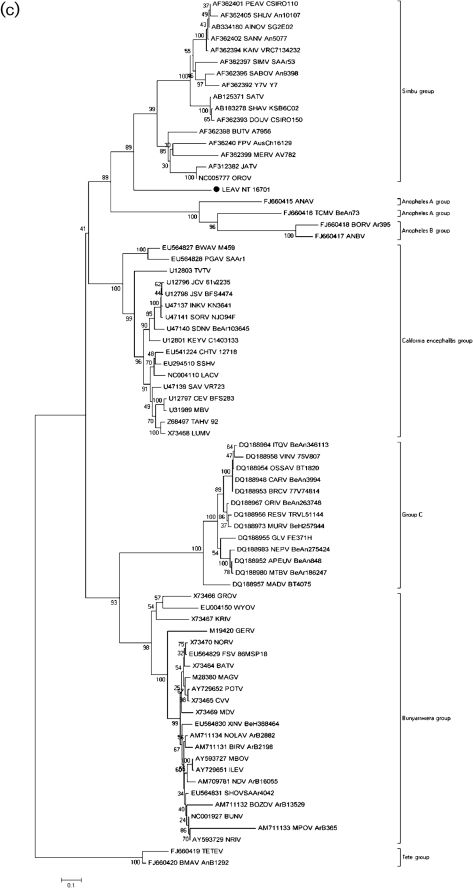
Consistent with the genomic organization of orthobunyaviruses (Gentsch et al., 1977; Gentsch & Bishop, 1978; Obijeski et al., 1976a, b), the genome of LEAV comprises three RNA segments: a large (L) segment that encodes a large (L-) RNA-dependent RNA polymerase (RdRp)-related ORF in the negative-sense orientation (GenBank accession no. HM627178); a medium (M) segment that encodes the polyprotein (M) in the negative-sense orientation (GenBank accession no. HM627176); and a small (S) segment that encodes a nucleocapsid protein (NP) and a non-structural protein (NSs), both in the negative-sense orientation, but in different ORFs (GenBank accession no. HM627177). The results of the phylogenetic analyses of the L, M and NP ORFs indicate that LEAV is distantly related to members of the Simbu serogroup. It is most closely related to OROV in all three amino acid trees (Fig. 1a–c, respectively). Nucleotide trees show similar topology, though interpretation is limited given sequence divergence (Supplementary Fig. S1). No evidence for reassortment was detected.
Fig. 1.
Phylogenetic analysis of the (a) polymerase, (b) polyprotein and (c) nucleoprotein of orthobunyaviruses. A set of all complete and partial sequences from GenBank were aligned using the clustal algorithm (as implemented in the mega package version 3) at the amino acid level for the L, M and S segments with additional manual editing to ensure the highest possible quality of alignment. A set of these sequences representing different serogroups were used for analysis, with partial sequences removed for correct tree topology. Neighbour-joining (NJ) analysis at the amino acid level was performed given the high observed variability of the nucleotide sequences. Statistical significance of the tree topology was evaluated by bootstrap resampling of the sequences 1000 times (bootstrap values are shown next to the branches). Phylogenetic analyses were performed using mega software (Kumar et al., 2004). LEAV is marked with a black dot. Bars indicate amino acid substitutions per site.
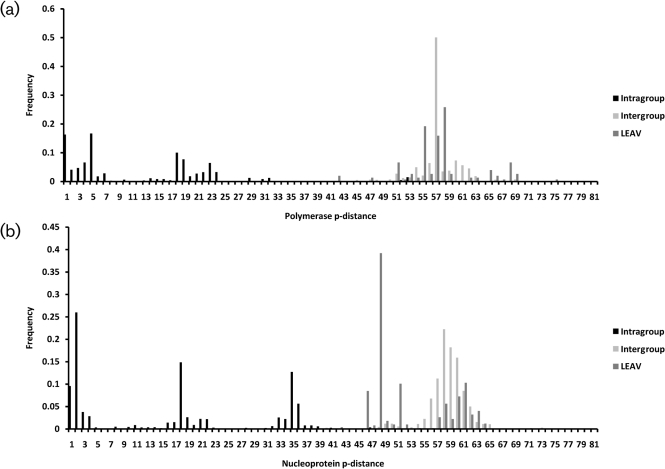
LEAV sequences were compared with published orthobunyavirus sequences for members of Bunyamwera, California encephalitis, Simbu and Group C complexes to determine mean intragroup and intergroup distances, using strategies accepted for other virus groups (Collao et al., 2009; Ward et al., 1992). The intragroup and intergroup p-distances were clearly distinguishable for the polymerase and the nucleoprotein (Fig. 2a, b, respectively). Cut-offs of 59 and 60 % similarity were established, respectively, at the amino acid level. However, some overlap was observed in the polyprotein. The mean similarity between LEAV and Simbu viruses was 47.6±3.5 % and 53.7±3.1 % in the polymerase and the nucleoprotein, respectively. Comparison between LEAV and OROV and AINOV showed 59 and 36.4 % similarity in the polymerase and polyprotein, respectively, with OROV and 30.9 % with the AINOV polyprotein. These values are in the range of similarity values observed between species belonging to different antigenic groups (Table 2).
Fig. 2.
Pairwise sequence analysis for polymerase (a) and nucleoprotein (b). The sequence of LEAV was compared with published orthobunyavirus sequences to determine intergroup and intragroup mean p-distances and to classify LEAV. Calculations were performed using p-distance at the amino acid level. P-distance values were grouped into three groups: intragroup, distances among different viruses belonging to the same serogroup (black); intergroup, distances between members of different viruses belonging to different serogroups (pale grey); and distance between LEAV and other viruses in the genus (dark grey).
Table 2. Nucleotide (and amino acid) sequence difference values (%) between LEAV and viruses of recognized orthobunyavirus serogroups.
na, Not applicable.
| Comparison | L segment | M segment | S segment |
| Within Simbu | 13.0±0.6 (9.3±0.8) | 21.0±0.3 (20.7±0.4) | 16.9±0.7 (15.0±1.2) |
| Within Bunyamwera | 24.1±1.1 (17.0±1.7) | 31.4±0.8 (30.2±1.5) | 22.9±0.9 (20.8±1.3) |
| Within Group C | na | 26.0±1.6 (22.6±2.6) | 16.4±0.9 (9.3±1.2) |
| Within California encephalitis | 16.9±1.3 (10.9±1.7) | 22.3±0.9 (16.9±1.4) | 14.7±0.7 (10.8±1.2) |
| Between LEAV and Bunyamwera | 50.5±1.3 (61.8±3.0) | 50.0±1.2 (64.1±2.4) | 49.6±1.6 (59.1±2.9) |
| Between LEAV and California encephalitis | 46.6±1.7 (57.2±3.4) | 50.5±1.8 (61.7±3.4) | 46.0±1.6 (58.2±3.0) |
| Between LEAV and Group C | na | 47.2±2.4 (60.0±4.1) | 50.0±1.7 (61.4±3.0) |
| Between LEAV and Simbu | 45.3±1.8 (52.4±3.5) | 54.1±0.6 (67.8±1.2) | 40.8±1.5 (46.7±3.1) |
| Between Bunyamwera and California encephalitis | 45.2±1.8 (55.0±3.4) | 43.7±1.2 (50.0±2.6) | 46.0±1.4 (54.6±2.7) |
| Between Bunyamwera and Group C | na | 42.7±1.9 (51.7±3.6) | 45.5±1.4 (55.1±2.8) |
| Between Bunyamwera and Simbu | 49.0±1.4 (57.3±3.3) | 53.9±0.8 (68.4±1.7) | 49.9±1.4 (59.3±2.8) |
| Between California encephalitis and Group C | na | 46.0±2.0 (56.5±3.9) | 44.8±1.5 (54.8±2.9) |
| Between California encephalitis and Simbu | 48.9±2.0 (55.9±3.8) | 55.5±1.2 (69.7±2.3) | 47.2±1.4 (57.1±2.8) |
| Between Group C and Simbu | na | 46.2±2.0 (54.7±3.7) | 45.8±1.4 (56.2±2.9) |
ORFs
Large RdRp (L).
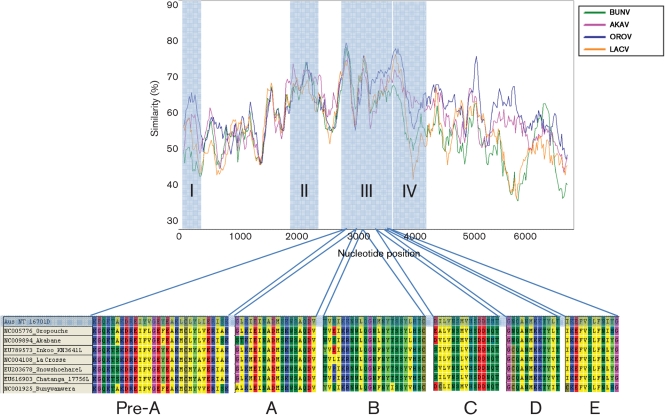
The 2260 aa LEAV viral RdRp (264 kDa, pI = 6.4) is similar in size to other orthobunyavirus reference strains falling between the 2238 aa Bunyamwera virus (BUNV) and the 2263 aa La Crosse virus (LACV) L proteins. Certain areas overlap conserved regions among all orthobunyaviruses, suggesting an association with function; region I is located in the amino terminus and is centred on aa P75D; region II, also located in the amino terminus, is centred on aa R651Y. Regions I and II have been found to also be conserved among all bunyaviruses (Müller et al., 1994). Region III (948–1239) is located in the centre of the protein and contains the polymerase motifs that comprise the polymerase module [A (1045–1062), B (1129–1151), C (1170–1184), D (1214–1225)] found in all RNA-dependent polymerases ranging from the RdRp encoded by retroid elements to the RdRp encoded by the positive, negative and dsRNA viruses (Poch et al., 1989; Xiong & Eickbush, 1990). The pre-A (948–977) and E motifs (1228–1239) identified in region III by Müller et al. (1994) were also found in LEAV. The fourth conserved region (1240–1343), identified by Aquino et al. was also conserved in LEAV (Fig. 3) (Aquino et al., 2003).
Fig. 3.
Large RdRp (L) conserved regions. Simplot analysis identifies regions I–IV with the pre-A, A, B, C, D and E motifs shown from region III.
Polyprotein (M).
The 1419 aa LEAV polyprotein (161.8 kDa, pI = 8.4) is cotranslationally cleaved into the 285 aa Gn (32.7 kDa, pI = 8.9), 947 aa Gc (107.9 kDa, pI = 6.7) and 173 aa NSm (19.7 kDa, pI = 9.2). Stuckely and Wright determined the Gn and Gc to be 35 and115 kDa, respectively, both within 10 % of our predictions and similar in size to the BUNV (Stuckly & Wright, 1983). When compared with other orthobunyaviruses reference strains and AINOV, the M proteins of LEAV are comparably sized (Table 3) (Wang et al., 2001; Yanase et al., 2003).
Table 3. M segment protein sizes.
| Virus name | Gn size (aa) | NSm size (aa) | Gc size (aa) |
| LEAV | 285 | 173 | 947 |
| LACV | 277 | 173 | 967 |
| BUNV | 292 | 174 | 955 |
| OROV | 290 | 175 | 939 |
| AKAV | 292 | 156 | 936 |
| AINOV | 291 | 155 | 941 |
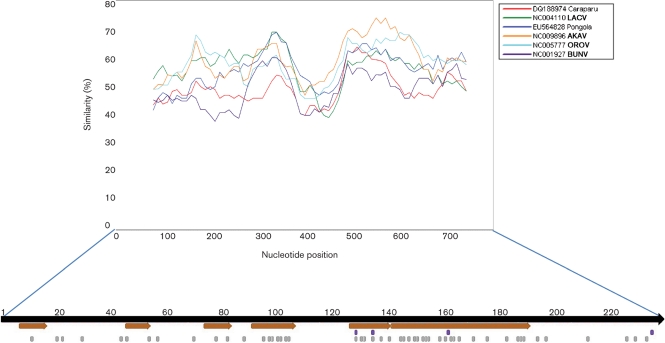
Since the Gn is poorly conserved among bunyaviruses, this is the area that is least conserved of the whole polyprotein. The N-terminal sequence is consistent with a functional signal peptide for membrane translocation (Blobel & Dobberstein, 1975; Lingappa et al., 1978; von Heijne, 1988) similar to those of other viruses in the genus (Fazakerley et al., 1988). Cleavage of the signal peptide between aa 14 and 15 with respect to the first methionine is compatible with conservation of terminal amino acid tripeptides (Lees et al., 1986). Prediction of signallase cleavage by SignalP 3.0 between aa 14 and 15 supports this view. The Gn contains the conserved arginine in position 299 suggesting cleavage of the mature Gn from the downstream NSm at the carboxy terminus of the Gn is probably mediated by an enzyme that has specificity for basic residues (Fazakerley et al., 1988). There is little conservation near the NSm/Gc junction, so a potential cleavage site, possibly executed by signalase (Fazakerley et al., 1988), is not obvious. Cleavage after a conserved alanine residue (A472) analogous to the termination of NSm in the California serogroup viruses (Campbell & Huang, 1999) is possible. SignalP predicts cleavage at VVA472-EI, which would result in −3 = V and −1 = A, one of the most frequent combinations in signallase sites. Although close to a potential glycosylation site, it is greater than the ‘minimum glycosylation distance’ of 13 aa that has been determined for cleaved internal signals (Nilsson et al., 1994).
Six potential glycosylation sites were identified using NetNGlyc 1.0 (http://www.cbs.dtu.dk/services), two in the Gn, one in the NSm and three in the Gc. Two in the Gn and one in each of the NSm and Gc are unique. The glycosylation site in the amino terminus of the Gc is conserved among Peaton virus (PEAV), Tinaroo virus (TINV) and AKAV and one in the Gc is conserved among AKAV and AINOV. Only one glycosylation site in the carboxy terminus of the Gc is conserved with OROV. PEAV, TINV, AKAV, AINOV and OROV are all Simbu serogroup viruses (Fig. 4).
Fig. 4.
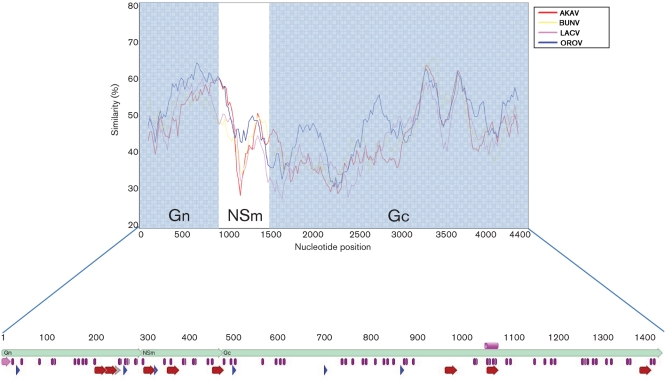
Polyprotein functional regions. Transmembrane regions (red arrows), glycosylation sites (blue arrows), signal peptide (pink), charged amino acids (grey arrow) and cysteines (purple) are all indicated. The pink cylinder represents the conserved fusion peptide sequence.
Prediction of transmembrane regions using the transmembrane hidden Markov model in the program TopPred0.01 (http://mobyle.pasteur.fr/cgi-bin/portal.py?#forms::toppred), predicts eight transmembrane regions: two in the Gn (200–220 and 223–243), three in the NSm (306–326, 357–377 and 454–474) and three in the Gc (956–976, 1046–1066 and 1375–1395). The two transmembrane regions in the Gn together form a very long hydrophobic sequence (200–243), which are subsequently followed by charged amino acids (244–252) similar to the stop-transfer sequences seen in the transmembrane domains of other viral envelope proteins (Garoff et al., 1980; Jou et al., 1980; Rose et al., 1980). The transmembrane region predicted at position 1375–1395 in the Gc acts as a potential membrane anchor (Fazakerley et al., 1988; Pekosz et al., 1995) (Fig. 4).
The LEAV Gn protein consists of 299 aa with a predicted cytoplasmic tail (CT) of 69 residues. The Gc consists of 961 aa and has a CT of 24 aa. These values are consistent with predictions of the CT for BUNV, the prototype of the genus Orthobunyavirus (Elliott, 1990; Lees et al., 1986).
The fusion peptide identified in LACV from aa 1066–1087 is conserved in LEAV from aa 1043–1064, suggesting that the Gc of LEAV acts as a class II fusion protein, similar to the E1 fusion peptide of the alphaviruses, Sindbis virus and Semliki Forest virus (Plassmeyer et al., 2007). None of the six epitopes identified in California encephalitis group viruses by Cheng et al. (2000) are conserved in LEAV. The overall topology of the virus appears to be well conserved, as indicated by the conservation of 71 cysteines with all other orthobunyaviruses (Grady et al., 1987; Lees et al., 1986; Pardigon et al., 1988), 17 in the Gn, 10 in the NsM, and 44 in the Gc (Fig. 4).
NP and NSs proteins.
The LEAV 235 aa NP (26.3 kDa, pI = 8.9) is consistent with Stuckley and Wright's NP of 29 kDa (Stuckly & Wright, 1983). It shows between 24 (TETEV) and 60 % (AINOV) conservation at the amino acid level and between 40 (TETEV) and 63 % (AINOV) at the nucleotide level to other orthobunyaviruses, thus satisfying the minimum 10 % divergence requirement of the International Committee for Taxonomy of Viruses (ICTV) in the NP for a novel species. Interestingly, of the six conserved regions identified in the Simbu serogroup viruses by Saeed et al. (2001), regions two through to six are well conserved with NT 16701 (the prototype strain of LEAV), whereas region one is only somewhat conserved. There are also several individual amino acids that have been identified as being globally conserved in the NP among the four major serogroups: Buyamwera, California, Group C and Simbu. There are 46 positions that are strictly conserved in all 51 viruses in these four groups, while a further 14 are conserved in at least 45 (90 %) of the N protein sequences (Eifan & Elliott, 2009). These residues are presumably critical for the N protein function. Of the 60 conserved amino acids, 50 are conserved in LEAV. The four residues (P125, G131, Y158 and I231) involved in formation of ribonucleoprotein complexes (Eifan & Elliott, 2009) are all conserved in LEAV (Fig. 5). Of the 10 residues identified as involved in RNA synthesis, eight are conserved in NT 16701. LEAV encodes a NSs, as do most other orthobunyaviruses, in a second ORF of the S segment. The 93 aa (10.8 kDa, pI = 10.8) falls within the range of NSs sizes of 83–109 residues (Dunn et al., 1994) and may correspond to the p8 protein identified by Stuckly & Wright (1983). The NSs is poorly conserved when compared to Simbu group viruses, showing between 28 and 37 % similarity at the amino acid level (55–60 % at the nucleotide level).
Fig. 5.
Nucleocapsid conserved regions. Conserved amino acids among all orthobunyaviruses, which are also conserved in LEAV, are indicated by grey blocks. The orange arrows indicate regions identified by Saeed et al. (2001) as being conserved among all Simbu group viruses. The purple blocks represent the four residues involved in the formation of ribonucleoprotein complexes.
Discussion
According to the ICTV, a virus belongs to a serogroup if it cross-reacts with members of that group by one or more serological tests (Nichol et al., 2005). Previous studies of Simbu group viruses have demonstrated extensive cross-reactivity through CF tests (Kinney & Calisher, 1981). LEAV does not show cross-reactivity with other orthobunyaviruses and phylogenetic analyses of the M and S segments of LEAV show it to be only distantly related to Simbu serogroup viruses. Furthermore, p-distance frequency calculations demonstrate that differences in amino acids specified by the L and S segments of LEAV and other orthobunyaviruses are consistent with intergroup distances. Although this investigation is based on the limited number of viruses tested, genetic and serological evidence indicate that LEAV represents a new species in the genus Orthobunyavirus and may represent a new antigenic complex.
Many other Simbu group viruses have been isolated from sentinel cattle and from insects in northern Australia, including AINOV, AKAV, Douglas virus, PEAV and TINV (Gard et al., 1988). AKAV and AINOV have economic and veterinary importance. AKAV causes periodic outbreaks of abortions, stillbirths and congenital malformations in cattle, sheep and goats in Australia, the Middle East and in sub-Saharan Africa (Schmaljohn & Nichol, 2007). AINOV has been associated with abortions, stillbirths and congenital defects in cattle, sheep and goats in Australia and Japan. As the second closest phylogenetic relative in the M, high relative sequence similarity in the N, and geographical overlap, AINOV and LEAV may have evolved from a similar, but distant, ancestor.
Although distantly related, LEAV appears consistently paired with OROV, which has only been isolated in Central and South America (Pinheiro et al., 2004). OROV is recognized as an important cause of acute febrile illness, known as Oropouche fever, among people living in rural and urban communities in tropical South America (Nunes et al., 2005a).
Antibodies to LEAV were detected in nine out of 30 (30 %) cattle initially tested by Doherty et al. (1977) in the Northern Territory of Australia. Neutralizing antibodies were not detected in a limited survey of humans in northern Australia, but were detected in cattle in Queensland, suggesting a geographical distribution beyond the Northern Territory (Doherty et al., 1977). Thus, a more extensive survey, with regard to sample size and geography is necessary to better understand the distribution of this virus and its role in human, livestock and wildlife diseases.
Methods
Virus isolation and antigenic characterization.
The prototype strain of LEAV (NT 16701) was originally isolated in newborn mice inoculated intracranially with a clarified homogenate of 100 Anopheles meraukensis mosquitoes collected at Leanyer, Northern Territory, Australia in April 1974 (Doherty et al., 1977). It was subsequently reisolated from Culicoides marksi at Beatrice Hill, also in northern Australia (Standfast et al., 1984). Initial characterization of NT 16701 was done at the Queensland Institute of Medical Research, Brisbane, Queensland, Australia at the time of isolation. Methods used to prepare antigens for the complement fixation (CF) tests and for making immune ascitic fluids have been described previously (Beaty et al., 1989; Travassos da Rosa et al., 1983; Xu et al., 2007). Antigens and antibodies were both prepared in mice. CF tests were performed by the microtitre technique (Beaty et al., 1989; Xu et al., 2007), using two units of guinea pig complement and overnight incubation of the antigen and antibody at 4 °C. CF titres were recorded as the highest dilutions giving 3+ or 4+ fixation of complement. Titres of 1 : 8 were considered positive. Haemagglutination inhibition (HI) testing was done in microtitre plates as described previously (Travassos da Rosa et al., 1983). HI tests were performed with 4 haemagglutination units of virus at the optimal pH (5.75) against serial twofold antiserum dilutions starting at 1 : 20. HI titres of 1 : 20 were considered positive.
By CF, HI and mouse neutralization tests, NT 16701 was found to be antigenically distinct from 40 suspected arboviruses known from Australia and New Guinea at that time (Doherty, 1977; Doherty et al., 1977). Based on these initial studies, NT 16701 was designated a new virus, and named ‘Leanyer virus’ (LEAV). Negative contrast electron microscopy of LEAV-infected mouse brain showed poorly defined spherical 50 nm diameter particles with dense cores (Doherty et al., 1977). It was later discovered that LEAV virions were approximately 110 nm in diameter, a size consistent with other bunyaviruses (Stuckly & Wright, 1983). A limited survey done by neutralization test with sera from humans and other vertebrates from Australia revealed neutralizing antibodies to LEAV in cattle, wallabies and dogs but not in humans (Doherty et al., 1977). Additional HI tests were done at the University of Texas Medical Branch, Galveston with other orthobunyaviruses and ungrouped bunyaviruses, including OROV and AKAV (Table 1).
Genome sequencing.
LEAV was extracted using TRIzol LS (Invitrogen). Total RNA extracts were treated with DNase I (DNA-Free; Ambion) and cDNA was generated using the Superscript II system (Invitrogen) using random hexamers that were linked to an arbitrary 17-mer primer sequence (Palacios et al., 2007). Resulting cDNA was treated with RNase H and then amplified by random PCR (Palacios et al., 2007). Products greater than 70 bp were selected by column purification (MinElute; Qiagen) and ligated to specific adapters for sequencing on the 454 Genome Sequencer FLX (454 Life Sciences) without fragmentation of the cDNA (Cox-Foster et al., 2007; Margulies et al., 2005; Palacios et al., 2008). Software programs accessible through the analysis applications at the GreenePortal website (http://tako.cpmc.columbia.edu/Tools/) were used for the removal of primer sequences, redundancy filtering and sequence assembly. Primers were designed using pyrosequencing data to fill gaps in the sequence (Supplementary Table S3, available in JGV Online). Conventional PCRs were performed with BIO-X-ACT polymerase (Bioline) on PTC-200 thermocyclers (Bio-Rad): an enzyme activation step of 5 min at 95 °C was followed by 45 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, and extension at 68 °C for 1–3 min depending on the expected amplicon size. PCR products were run on 1 % agarose gels, gel extracted and purified (MiniElute; Qiagen), and directly sequenced in both directions with ABI Prism Big Dye Terminator 1.1 Cycle Sequencing kits on ABI Prism 3700 DNA Analysers (Perkin-Elmer Applied Biosystems). Terminal sequences were generated using a universal orthobunyavirus primer, targeting the conserved viral termini (5′-AGTAGTGTRCTCCAC-3′). Sequences of the genomes were verified by classical Sanger sequencing using primers designed to create amplicons of ∼1000 bp with 500 bp overlap. The assembled data revealed a classical orthobunyavirus genome (GenBank accession nos HM627176, HM627177 and HM627178).
Phylogenetic analysis.
A set of orthobunyavirus sequences (151 for the L segment; 243 for the polyprotein M segment and 502 for the nucleocapsid gene) comprising all sequences from GenBank were used to determine the phylogenic history of LEAV strain NT 16701. All orthobunyavirus sequences were aligned using the clustal algorithm (as implemented in the mega package version 4) at the amino acid level, with additional manual editing to ensure the highest possible quality of the alignment. UPGMA (Unweighted Pair Group Method with Arithmetic Mean) analysis at the amino acid level was performed due to the observed high variability of the underlying nucleotide sequences. Nucleotide phylogenic trees were also investigated using the neighbour-joining algorithm and the Kimura two-parameter model. The statistical significance of the tree topology was evaluated by bootstrap resampling of the sequences 1000 times. Phylogenetic analyses were performed by using mega software (Kumar et al., 2004).
Sequence analysis.
Geneious 4.7.5 (Biomatters) was used for sequence assembly and analysis. Topology and targeting predictions were generated by employing SignalP, NetNGlyc, TMHMM (http://www.cbs.dtu.dk/services), the web-based version of TopPred0.01 (http://mobyle.pasteur.fr/cgi-bin/portal.py?#forms::toppred), and integrated predictions in Geneious (Bendtsen et al., 2004; Claros & von Heijne, 1994; Kahsay et al., 2005; Käll et al., 2004; Krogh et al., 2001).
Pairwise sequence analysis.
To establish a potential cut-off for classification of LEAV, we used pairwise sequence comparison to compare its sequences with all published orthobunyavirus sequences. Calculations were performed using mega software (Kumar et al., 2004) to calculate the p-distance of the S segment, which is used by the ICTV for demarcation of species, at both the nucleotide and amino acid level using pairwise deletion. Calculations were performed using mega software (Kumar et al., 2004) to calculate the p-distance of each segment at the nucleotide level.
Acknowledgements
We thank Vishal Kapoor for technical assistance. This work was supported by Google.org, NIH award AI57158 (Northeast Biodefense Center – Lipkin), USAID Predict funding source code 07-301-7119-52258 (Center for Infection and Immunity) and the Department of Defense. Robert Tesh and Amelia Travassos da Rosa were supported by NIH contract HHSN272201000040I/HHSN27200004/D04.
Footnotes
These authors contributed equally to this work.
Present address: School of Pharmacy and Pharmaceutical Sciences, University of Buffalo, Buffalo, NY, USA.
The GenBank/EMBL/DDBJ accession numbers for the sequences determined in this study are HM627176–HM627178.
Three supplementary tables and a supplementary figure are available with the online version of this paper.
References
- Aquino V. H., Moreli M. L., Moraes Figueiredo L. T. (2003). Analysis of oropouche virus L protein amino acid sequence showed the presence of an additional conserved region that could harbour an important role for the polymerase activity. Arch Virol 148, 19–28 10.1007/s00705-002-0913-4 [DOI] [PubMed] [Google Scholar]
- Beaty B. J., Calisher C. H., Shope R. E. (1989). Arboviruses. In Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections, pp. 797–855 Edited by Schmidt N. J., Emmons R. W. Washington, DC: American Public Health Association [Google Scholar]
- Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004). Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340, 783–795 10.1016/j.jmb.2004.05.028 [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. (1975). Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol 67, 852–862 10.1083/jcb.67.3.852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen M. D., Jackson A. O., Bruns T. D., Hacker D. L., Hardy J. L. (1995). Determination and comparative analysis of the small RNA genomic sequences of California encephalitis, Jamestown Canyon, Jerry Slough, Melao, Keystone and Trivittatus viruses (Bunyaviridae, genus Bunyavirus, California serogroup). J Gen Virol 76, 559–572 10.1099/0022-1317-76-3-559 [DOI] [PubMed] [Google Scholar]
- Campbell W. P., Huang C. (1999). Sequence comparisons of medium RNA segment among 15 California serogroup viruses. Virus Res 61, 137–144 10.1016/S0168-1702(99)00033-7 [DOI] [PubMed] [Google Scholar]
- Cheng L. L., Schultz K. T., Yuill T. M., Israel B. A. (2000). Identification and localization of conserved antigenic epitopes on the G2 proteins of California serogroup bunyaviruses. Viral Immunol 13, 201–213 10.1089/vim.2000.13.201 [DOI] [PubMed] [Google Scholar]
- Claros M. G., von Heijne G. (1994). TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci 10, 685–686 [DOI] [PubMed] [Google Scholar]
- Collao X., Palacios G., Sanbonmatsu-Gámez S., Pérez-Ruiz M., Negredo A. I., Navarro-Marí J. M., Grandadam M., Aransay A. M., Lipkin W. I., et al. (2009). Genetic diversity of Toscana virus. Emerg Infect Dis 15, 574–577 10.3201/eid1504.081111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Foster D. L., Conlan S., Holmes E. C., Palacios G., Evans J. D., Moran N. A., Quan P. L., Briese T., Hornig M., et al. (2007). A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318, 283–287 10.1126/science.1146498 [DOI] [PubMed] [Google Scholar]
- Doherty R. L. (1977). Arthropod-borne viruses in Australia, 1973–1976. Aust J Exp Biol Med Sci 55, 103–130 10.1038/icb.1977.9 [DOI] [PubMed] [Google Scholar]
- Doherty R. L., Carley J. G., Filippich C., Kay B. H., Gorman B. M., Rajapaksa N. (1977). Isolation of Sindbis (alphavirus) and Leanyer viruses from mosquitoes collected in the Northern Territory of Australia, 1974. Aust J Exp Biol Med Sci 55, 485–489 10.1038/icb.1977.47 [DOI] [PubMed] [Google Scholar]
- Dunn E. F., Pritlove D. C., Elliott R. M. (1994). The S RNA genome segments of Batai, Cache Valley, Guaroa, Kairi, Lumbo, Main Drain and Northway bunyaviruses: sequence determination and analysis. J Gen Virol 75, 597–608 10.1099/0022-1317-75-3-597 [DOI] [PubMed] [Google Scholar]
- Eifan S. A., Elliott R. M. (2009). Mutational analysis of the Bunyamwera orthobunyavirus nucleocapsid protein gene. J Virol 83, 11307–11317 10.1128/JVI.01460-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R. M. (1990). Molecular biology of the Bunyaviridae. J Gen Virol 71, 501–522 10.1099/0022-1317-71-3-501 [DOI] [PubMed] [Google Scholar]
- Elliott R. M. (1997). Emerging viruses: the Bunyaviridae. Mol Med 3, 572–577 [PMC free article] [PubMed] [Google Scholar]
- Fazakerley J. K., Gonzalez-Scarano F., Strickler J., Dietzschold B., Karush F., Nathanson N. (1988). Organization of the middle RNA segment of snowshoe hare Bunyavirus. Virology 167, 422–432 [PubMed] [Google Scholar]
- Gard G. P., Shorthose J. E., Weir R. P., Walsh S. J., Melville L. F. (1988). Arboviruses recovered from sentinel livestock in northern Australia. Vet Microbiol 18, 109–118 10.1016/0378-1135(88)90056-9 [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. (1980). Nucleotide sequence of cDNA coding for Semliki Forest virus membrane glycoproteins. Nature 288, 236–241 10.1038/288236a0 [DOI] [PubMed] [Google Scholar]
- Gentsch J. R., Bishop D. H. (1978). Small viral RNA segment of bunyaviruses codes for viral nucleocapsid protein. J Virol 28, 417–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J., Wynne L. R., Clewley J. P., Shope R. E., Bishop D. H. (1977). Formation of recombinants between snowshoe hare and La Crosse bunyaviruses. J Virol 24, 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady L. J., Sanders M. L., Campbell W. P. (1987). The sequence of the M RNA of an isolate of La Crosse virus. J Gen Virol 68, 3057–3071 10.1099/0022-1317-68-12-3057 [DOI] [PubMed] [Google Scholar]
- Jou W. M., Verhoeyen M., Devos R., Saman E., Fang R., Huylebroeck D., Fiers W., Threlfall G., Barber C., Carey N. (1980). Complete structure of the hemagglutinin gene from the human influenza A/Victoria/3/75 (H3N2) strain as determined from cloned DNA. Cell 19, 683–696 10.1016/S0092-8674(80)80045-6 [DOI] [PubMed] [Google Scholar]
- Kahsay R. Y., Gao G., Liao L. (2005). An improved hidden Markov model for transmembrane protein detection and topology prediction and its applications to complete genomes. Bioinformatics 21, 1853–1858 10.1093/bioinformatics/bti303 [DOI] [PubMed] [Google Scholar]
- Käll L., Krogh A., Sonnhammer E. L. (2004). A combined transmembrane topology and signal peptide prediction method. J Mol Biol 338, 1027–1036 10.1016/j.jmb.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Kinney R. M., Calisher C. H. (1981). Antigenic relationships among Simbu serogroup (Bunyaviridae) viruses. Am J Trop Med Hyg 30, 1307–1318 [DOI] [PubMed] [Google Scholar]
- Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001). Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305, 567–580 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- Kumar S., Tamura K., Nei M. (2004). MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5, 150–163 10.1093/bib/5.2.150 [DOI] [PubMed] [Google Scholar]
- Lees J. F., Pringle C. R., Elliott R. M. (1986). Nucleotide sequence of the Bunyamwera virus M RNA segment: conservation of structural features in the Bunyavirus glycoprotein gene product. Virology 148, 1–14 10.1016/0042-6822(86)90398-3 [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. (1978). A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem 253, 8667–8670 [PubMed] [Google Scholar]
- Margulies M., Egholm M., Altman W. E., Attiya S., Bader J. S., Bemben L. A., Berka J., Braverman M. S., Chen Y. J., et al. (2005). Genome sequencing in microfabricated high-density picolitre reactors. Nature 437, 376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Poch O., Delarue M., Bishop D. H., Bouloy M. (1994). Rift Valley fever virus L segment: correction of the sequence and possible functional role of newly identified regions conserved in RNA-dependent polymerases. J Gen Virol 75, 1345–1352 10.1099/0022-1317-75-6-1345 [DOI] [PubMed] [Google Scholar]
- Nichol S. T., Arikawa J., Kawaoka Y. (2000). Emerging viral diseases. Proc Natl Acad Sci U S A 97, 12411–12412 10.1073/pnas.210382297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol S. T., Beaty B. J., Elliot R. M., Goldbach R., Plyusnin A., Schmaljohn C. S., Tesh R. B. (2005). Family Bunyaviridae. pp. 695–716 Edited by Fauquet C. M., Mayo J. A., Maniloff J., Desselberger U., Ball L. A. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier [Google Scholar]
- Nilsson I., Whitley P., von Heijne G. (1994). The COOH-terminal ends of internal signal and signal-anchor sequences are positioned differently in the ER translocase. J Cell Biol 126, 1127–1132 10.1083/jcb.126.5.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M. R., Martins L. C., Rodrigues S. G., Chiang J. O., Azevedo Rdo. S., da Rosa A. P., Vasconcelos P. F. (2005a). Oropouche virus isolation, southeast Brazil. Emerg Infect Dis 11, 1610–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes M. R., Travassos da Rosa A. P., Weaver S. C., Tesh R. B., Vasconcelos P. F. (2005b). Molecular epidemiology of group C viruses (Bunyaviridae, Orthobunyavirus) isolated in the Americas. J Virol 79, 10561–10570 10.1128/JVI.79.16.10561-10570.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Murphy F. A., Palmer E. L. (1976a). Structural proteins of La Crosse virus. J Virol 19, 985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Palmer E. L., Murphy F. A. (1976b). Segmented genome and nucleocapsid of La Crosse virus. J Virol 20, 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G., Quan P. L., Jabado O. J., Conlan S., Hirschberg D. L., Liu Y., Zhai J., Renwick N., Hui J., et al. (2007). Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg Infect Dis 13, 73–81 10.3201/eid1301.060837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios G., Druce J., Du L., Tran T., Birch C., Briese T., Conlan S., Quan P. L., Hui J., et al. (2008). A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med 358, 991–998 10.1056/NEJMoa073785 [DOI] [PubMed] [Google Scholar]
- Pardigon N., Vialat P., Gerbaud S., Girard M., Bouloy M. (1988). Nucleotide sequence of the M segment of Germiston virus: comparison of the M gene product of several bunyaviruses. Virus Res 11, 73–85 10.1016/0168-1702(88)90068-8 [DOI] [PubMed] [Google Scholar]
- Pekosz A., Griot C., Stillmock K., Nathanson N., Gonzalez-Scarano F. (1995). Protection from La Crosse virus encephalitis with recombinant glycoproteins: role of neutralizing anti-G1 antibodies. J Virol 69, 3475–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro F. P., Travassos da Rosa A. P. A., Vasconcelos P. F. C. (2004). Oropouche fever. In Textbook of Pediatric Infectious Diseases, 5th edn, pp. 2418–2423 Edited by Feigin, R. D., Cherry J. D., Demmler G. J., Kaplan S. L. Philadelphia: Saunders [Google Scholar]
- Plassmeyer M. L., Soldan S. S., Stachelek K. M., Roth S. M., Martín-García J., González-Scarano F. (2007). Mutagenesis of the La Crosse Virus glycoprotein supports a role for Gc (1066–1087) as the fusion peptide. Virology 358, 273–282 10.1016/j.virol.2006.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. (1989). Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J 8, 3867–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden C., Holmes E. C., Charleston M. A. (2009). Hantavirus evolution in relation to its rodent and insectivore hosts: no evidence for codivergence. Mol Biol Evol 26, 143–153 10.1093/molbev/msn234 [DOI] [PubMed] [Google Scholar]
- Rose J. K., Welch W. J., Sefton B. M., Esch F. S., Ling N. C. (1980). Vesicular stomatitis virus glycoprotein is anchored in the viral membrane by a hydrophobic domain near the COOH terminus. Proc Natl Acad Sci U S A 77, 3884–3888 10.1073/pnas.77.7.3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M. F., Wang H., Nunes M., Vasconcelos P. F., Weaver S. C., Shope R. E., Watts D. M., Tesh R. B., Barrett A. D. (2000). Nucleotide sequences and phylogeny of the nucleocapsid gene of Oropouche virus. J Gen Virol 81, 743–748 [DOI] [PubMed] [Google Scholar]
- Saeed M. F., Li L., Wang H., Weaver S. C., Barrett A. D. (2001). Phylogeny of the Simbu serogroup of the genus Bunyavirus. J Gen Virol 82, 2173–2181 [DOI] [PubMed] [Google Scholar]
- Schmaljohn C. S., Nichol S. T. (2007). Bunyaviridae. In Fields Virology, 5th edn, pp. 1741–1789 Edited by Knipe D. M., Howley P. M. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins [Google Scholar]
- Standfast H. A., Dyce A. L., St George T. D., Muller M. J., Doherty R. L., Carley J. G., Filippich C. (1984). Isolation of arboviruses from insects collected at Beatrice Hill, Northern Territory of Australia, 1974–1976. Aust J Biol Sci 37, 351–366 [DOI] [PubMed] [Google Scholar]
- Stuckly K. G., Wright P. J. (1983). Characterization of Leanyer virus: resemblance to Bunyavirus. Aust J Exp Biol Med Sci 61, 193–200 10.1038/icb.1983.18 [DOI] [PubMed] [Google Scholar]
- Travassos da Rosa A. P., Tesh R. B., Pinheiro F. P., Travassos da Rosa J. F., Peterson N. E. (1983). Characterization of eight new phlebotomus fever serogroup arboviruses (Bunyaviridae: Phlebovirus) from the Amazon region of Brazil. Am J Trop Med Hyg 32, 1164–1171 [DOI] [PubMed] [Google Scholar]
- von Heijne G. (1988). Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta 947, 307–333 [DOI] [PubMed] [Google Scholar]
- Wang H., Beasley D. W., Li L., Holbrook M. R., Barrett A. D. (2001). Nucleotide sequence and deduced amino acid sequence of the medium RNA segment of Oropouche, a Simbu serogroup virus: comparison with the middle RNA of Bunyamwera and California serogroup viruses. Virus Res 73, 153–162 10.1016/S0168-1702(00)00234-3 [DOI] [PubMed] [Google Scholar]
- Ward C. W., McKern N. M., Frenkel M. J., Shukla D. D. (1992). Sequence data as the major criterion for potyvirus classification. Arch Virol Suppl 5, 283–297 [DOI] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. (1990). Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J 9, 3353–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Liu D., Nunes M. R., Da Rosa A. P., Tesh R. B., Xiao S. Y. (2007). Antigenic and genetic relationships among Rift Valley fever virus and other selected members of the genus Phlebovirus (Bunyaviridae). Am J Trop Med Hyg 76, 1194–1200 [PubMed] [Google Scholar]
- Yanase T., Yoshida K., Ohashi S., Kato T., Tsuda T. (2003). Sequence analysis of the medium RNA segment of three Simbu serogroup viruses, Akabane, Aino, and Peaton viruses. Virus Res 93, 63–69 10.1016/S0168-1702(03)00066-2 [DOI] [PubMed] [Google Scholar]