Abstract
Dysfunction of inhibitory synaptic transmission can destroy the balance between excitatory and inhibitory synaptic inputs in neurons, thereby inducing epileptic activity. The aim of the paper is to investigate the effects of successive excitatory inputs on the epileptic activity induced in the absence of inhibitions. Paired-pulse orthodromic and antidromic stimulations were used to test the changes in the evoked responses in the hippocampus. Picrotoxin (PTX), γ-aminobutyric acid (GABA) type A (GABAA) receptor antagonist, was added to block the inhibitory synaptic transmission and to establish the epileptic model. Extracellular evoked population spike (PS) was recorded in the CA1 region of the hippocampus. The results showed that the application of PTX induced a biphasic change in the paired-pulse ratio of PS amplitude. A short latency increase of the second PS (PS2) was later followed by a reappearance of PS2 depression. This type of depression was observed in both orthodromic and antidromic paired-pulse responses, whereas the GABAergic PS2 depression [called paired-pulse depression (PPD)] during baseline recordings only appeared in orthodromic-evoked responses. In addition, the depression duration at approximately 100 ms was consistent with a relative silent period observed within spontaneous burst discharges induced by prolonged application of PTX. In conclusion, the neurons may ignore the excitatory inputs and intrinsically generate bursts during epileptic activity. The depolarization block could be the mechanisms underlying the PPD in the absence of GABAA inhibitions. The distinct neuronal responses to stimulations during different epileptic stages may implicate the different antiepileptic effects of electrical stimulation.
Keywords: Epilepsy, Paired-pulse depression, γ-Aminobutyric acid (GABA), Picrotoxin (PTX), CA1 region, In-vivo
1. Introduction
The imbalance between excitatory and inhibitory synaptic inputs in neurons can result in brain diseases such as epilepsy (Badawy et al., 2009). For example, in the hippocampus, besides excitatory synaptic connections among the principal cells in main regions, there exist local inhibitory connections formed by GABAergic interneurons, including recurrent (feedback) connections and feedforward connections (Freund and Buzsaki, 1996; Wierenga and Wadman, 2003). Under normal physiological circumstances, the excitation and inhibition acting on the principal cells are balanced. If the GABAergic inhibition decreases, then epileptic activity may appear (Hablitz, 2004). In addition, there are different types of epileptic activity, such as interical and ictal (i.e., seizures), during different epileptic stages.
However, it is not clear whether the persistence of epileptic activity induced in the absence of inhibitions needs continuous excitatory inputs. Because previous investigations have showed nonsynaptic mechanisms of epilepsy (de Almeida et al., 2008), we hypothesize that the responses of neurons to the excitatory inputs could change during different epileptic stages, and the epileptic activity could be sustained by intrinsic features and not by external inputs. Therefore, by using paired-pulse orthodromic and antidromic stimulations (Albertson et al., 1996; Cunha-Reis et al., 2004), we investigated the changes of evoked responses in the hippocampal CA1 region in vivo when the γ-aminobutyric acid (GABA) receptor antagonist picrotoxin (PTX) was used to block the inhibitory synaptic transmission. The results of the investigation can provide important information for the development of new clinical treatments for epilepsy disease.
2. Materials and methods
2.1. Surgical procedures
All procedures used in this study were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Health, China). Adult Sprague-Dawley rats [body weight (BW) 250–350 g] were anesthetized with urethane [1.25–1.50 g/kg BW, intraperitoneal] and placed in a stereotaxic apparatus (Stoelting Co.). The surgical procedure was same as described in Feng et al. (2009). Briefly, part of the left skull was opened, and the cortex overlying the dorsal hippocampus was removed to expose the left hippocampus. Immediately after exposure of the hippocampus, normal artificial cerebrospinal fluid (ACSF) was put over the surface of the dorsal hippocampus.
2.2. Solutions and drugs
Normal ACSF consisted of 124 mmol/L NaCl, 5 mmol/L KCl, 1.25 mmol/L KH2PO4, 2 mmol/L CaCl2, 1.5 mmol/L MgSO4, 26 mmol/L NaHCO3, and 2 g/L d-glucose. GABA antagonist PTX 0.1–0.2 mmol/L was used to block the GABA type A (GABAA) receptor by adding PTX into an ACSF solution. PTX was obtained from Sigma. The other chemicals were obtained from China National Pharmaceutical Group. During the so-called PTX periods below, the PTX solution was used throughout.
2.3. Recording of spontaneous and evoked potentials
Two electrode contacts located on a silicon probe (NeuroNexus Technologies), separated by a distance of 400 μm, were used to record extracellular field potentials in the CA1 pyramidal stratum (Pyr.) and in the CA1 stratum radiatum (S. rad.), simultaneously. They recorded the evoked population spike (PS) and the excitatory post-synaptic potential (EPSP), respectively. The recording probe was positioned in the left hippocampal CA1 area [anteroposterior (AP), −3.0; mediolateral (ML), 2.6]. Stimulating electrodes were made from pairs of insulated nichrome wires (80 μm in diameter) with a vertical tip separation about 0.5 mm. One stimulating electrode was inserted into the Schaffer collaterals (AP, −2.0; ML, 2.3) for orthodromic stimulation of the CA1 pyramidal neurons. Another stimulating electrode was put on the alveus of the hippocampus (AP, −4.0; ML, 2.5) for antidromic stimulation of the CA1 neurons. Two separate stainless steel screws fixed in the nasal bones served as reference and ground electrodes. Patterns of the evoked potentials guided vertical positioning of the recording probe and the stimulating electrodes (Kloosterman et al., 2001).
Both evoked and spontaneous CA1 field potential signals were amplified by two-channel amplifiers (ML135, ADInstruments Pty Ltd.) with filter frequency range of 1 to 5 000 Hz. Signals were then sampled at a rate of 20 kHz by using a PowerLab data acquisition system (ADInstruments) and were stored in a hard disk for off-line analysis.
Stimulus pulses were produced by ML180 Stimulator Isolators (ADInstruments). The duration of stimulus pulse was 0.1 ms with a stimulation current intensity of 0.30–0.35 mA to induce a PS with amplitude greater than 80% of maximal PS amplitude in the CA1 region. The inter-pulse interval (IPI) of paired-pulse stimulation was set in the region of 25–400 ms.
The amplitude of orthodromic-evoked PS was measured as the average of the potential differences of the negative PS peak to the preceding and following positive peaks. The amplitude of antidromic-evoked PS was measured by the potential difference between the onset of the spike and the negative PS peak. Paired-pulse ratio (PPR) was calculated as the ratio of the second PS (PS2) amplitude to the first PS (PS1) amplitude. Data are expressed as mean±standard deviation (SD). One-way analysis of variance (ANOVA) and Bonferroni’s post-hoc test, as well as Student’s t-test were used to evaluate the differences of mean values between different experimental results.
The spontaneous bursts induced by PTX showed similar properties as stimulation evoked burst. To avoid the impact of spontaneous bursts, we applied the stimulation during a relatively-silent period, not during spontaneous bursts, and made sure that there were no obvious spontaneous bursts in a period of at least 2 s before the used evoked data.
3. Results
3.1. Changes in the orthodromic-evoked paired-pulse responses following the application of PTX
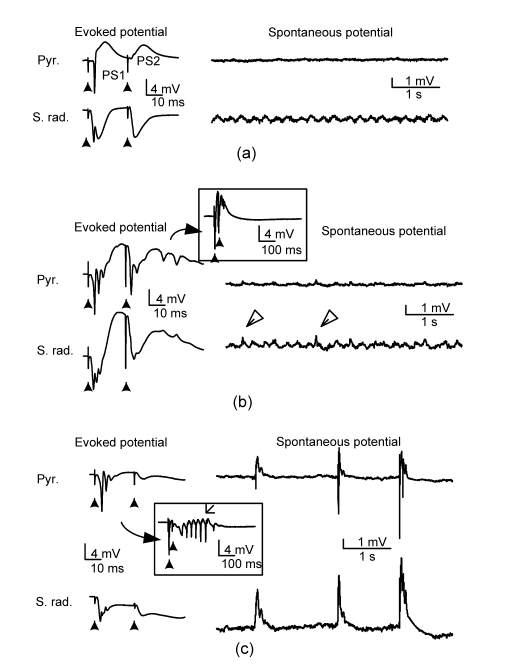
With normal excitatory and inhibitory synaptic transmissions, the first orthodromic stimulus in the Schaffer collateral with enough current intensity evoked a large-amplitude PS1 with single spike in the CA1 pyramidal layer and a large EPSP in the CA1 stratum radiatum, respectively. However, the second stimulus in the paired-pulse stimulation (IPI=25 ms) evoked no obvious PS2 (Fig. 1a), indicating a small PPR value (i.e., PS2/PS1) due to the GABAergic inhibition activity from inhibitory interneurons. Spontaneous theta activity was observed in the stratum radiatum without significant potential events in the pyramidal layer. Ten minutes after the use of ACSF solution with PTX, both evoked PS1 and PS2 changed into multiple spikes, indicating a significant decrease of GABAergic inhibition by the PTX (Fig. 1b). The appearance of a large-amplitude PS2 suggested a significant increase of PPR. The plot with an enlarged time scale showed a short after-discharge event following the stimuli (in the box, Fig. 1b). Small spike events also appeared in the spontaneous potentials (empty arrows, Fig. 1b). However, after a prolonged period application of PTX, paried-pulse stimuli only evoked multiple spikes in the PS1 but without inducing obvious PS2 (Fig. 1c), indicating a return of small PPR. At the same time, the duration of after-discharge following the stimuli increased (in the box, Fig. 1c). The field EPSP at stratum radiatum still existed, indicating that the neurons ignored the excitatory inputs. And, instead of theta activity, large amplitude bursts appeared in the spontaneous potentials (Fig. 1c).
Fig. 1.
Changes in the paired-pulse orthodromic-evoked potentials in CA1 region induced by PTX
(a) Baseline recording; (b) Ten minutes following PTX 0.1 mmol/L; (c) Twenty-five minutes following PTX 0.1 mmol/L. In (a)–(c), the left column shows paired-pulse (IPI=25 ms) evoked potentials and the right column shows spontaneous potentials from the pyramidal layer (Pyr.) and the stratum radiatum (S. rad.). Solid arrows indicate stimulating artifacts
Based on such a biphasic PPR change, we divided the period following the application of PTX into a short latency period with a larger PPR (PTX period I, <20 min) and a long latency period with a smaller PPR (PTX period II, >20 min). There was a transition duration between the two periods in which data were ignored. Only small or even unobvious epileptic bursts were observed in the spontaneous potentials during PTX period I, whereas large amplitude epileptic bursts appeared during PTX period II.
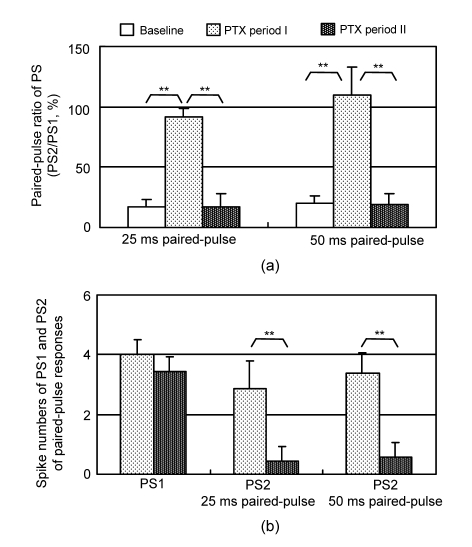
The PPRs of the orthodromic responses with 25 and 50 ms IPIs were calculated from the pyramidal layer recordings to evaluate the changes in PSs. There were significant differences among the PPRs of baseline, PTX periods I and II for both IPIs [F(2,20)>80, P<0.001, ANOVA, n=8]. The PPR significantly increased during PTX period I (P<0.001, Bonferroni’s post-hoc test). However, it returned to the baseline level during PTX period II (Fig. 2a). There was no significant difference in the spike number of the PS1 between the two PTX periods. However, the spike number of the PS2 decreased significantly during PTX period II for both IPIs (P<0.001, n=8, Student’s t-test; Fig. 2b).
Fig. 2.
Statistical data of the paired-pulse orthodromic-evoked population spikes during PTX periods I and II
(a) Changes in the paired-pulse ratio of PS amplitude for 25 and 50 ms IPIs (** P<0.001, n=8, ANOVA); (b) The spike numbers of the PS1 and PS2 in the paired-pulse responses (** P<0.001, n=8, Student’s t-test)
These data suggested that during the short latency period of PTX application, PTX eliminated GABAergic inhibition thereby inducing epileptic activity in the spontaneous potentials, and increasing the PS2 amplitude. The prolonged application of PTX increased the epileptic activity in the spontaneous potentials but resumed the depression of PS2 in the evoked responses. To further investigate the cause of PS2 disappearance, we used antidromic paired-pulse paradigms with different IPIs.
3.2. Changes in the antidromic-evoked paired-pulse responses following a prolonged application of PTX
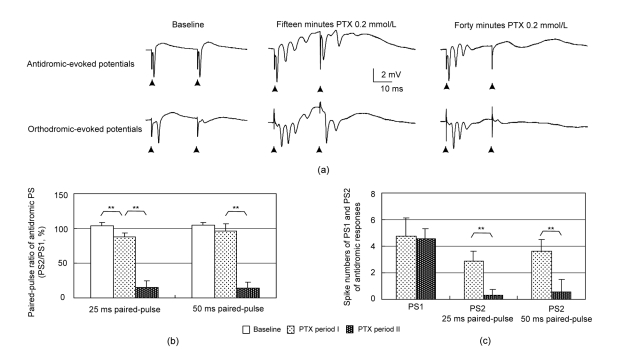
During baseline recordings, paired-pulse stimuli (IPI=25 ms) evoked antidromic PS1 and PS2 with similar amplitude but orthodromic PS responses with very small PS2 in the same preparation (Fig. 3a). This indicated that under normal physiological conditions, GABAergic inhibitions affected the development of orthodromic-evoked PS, but not antidromic-evoked PS. After a 15-min application of PTX, PS1 and PS2 in both the antidromic and orthodromic responses appeared as multiple spikes. However, after a 40-min application of PTX, PS2 disappeared in both antidromic and orthodromic paired-pulse responses. There were significant differences among the antidromic PPRs of baseline, PTX period I and PTX period II for the IPI of both 25 and 50 ms (F(2,20)>290, P<0.001, ANOVA, n=8). During PTX period II, the antidromic PPRs for both IPIs were significantly smaller than those during PTX period I (P<0.001, Bonferroni’s post-hoc test; Fig. 3b). There was no significant spike number difference in the antidromic PS1 between the two PTX periods. However, the spike numbers of the antidromic PS2 decreased significantly during PTX period II for both IPIs (P<0.001, n=8, Student’s t-test; Fig. 3c).
Fig. 3.
Changes in the antidromic paired-pulse evoked potentials in the CA1 pyramidal layer induced by PTX
(a) Examples of antidromic (top) and orthodromic (bottom) responses during different PTX application periods. Solid arrows indicate stimulating artifacts; (b) Changes in PPR of antidromic-evoked PS amplitude; (c) The spike numbers of the PS1 and PS2 in the antidromic paired-pulse responses. ** P<0.001, n=8, ANOVA
These data showed that the depression of PS2, caused by prolonged PTX application, was presented in both antidromic- and orthodromic-evoked responses. Because large spontaneous bursts appeared during PTX period II, it could be presumed that this type of depression also occurred within a spontaneous burst. Therefore, the patterns of spontaneous bursts were analyzed and the temporal features of the depression were investigated by using antidromic and orthodromic paired-pulse paradigms with different IPIs.
3.3. Temporal features of the PS2 depression during prolonged PTX application
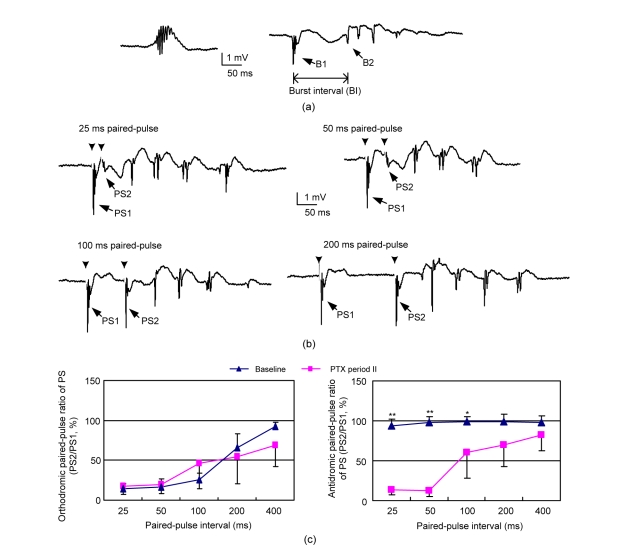
After a 50-min application of PTX, two types of epileptic bursts appeared in the spontaneous potentials (Fig. 4a). One was a short burst with a duration around tens of millisecond. The other was a long lasting event, consisting of a primary burst followed by an after-discharge. The primary burst (B1) and the after-discharge (B2) were separated by a burst interval (BI) without obvious spikes (Fig. 4a). The mean duration of the interval BI was (97±26) ms (n=73) that was calculated from spontaneous bursts recorded in six experimental preparations.
Fig. 4.
Temporal features of paired-pulse depression during PTX period II
(a) After a 50-min application of PTX, spontaneous epileptiform activity appeared as a single burst or a short burst (B1) followed by an after-discharge (B2); (b) At the same period, paired-pulse stimuli with 25 and 50 ms IPIs evoked smaller PS2, while those with 100 and 200 ms IPIs evoked larger PS2. Solid arrows indicate the locations of stimulus artifacts that were erased to clarify the spike activity; (c) Comparisons of PPRs between baseline and PTX period II. * P<0.05, ** P<0.001, n=6, Student’s t-test
Orthodromic and antidromic paired-pulse stimulations with different intervals were used to test the temporal features of the depressions following the primary burst (Fig. 4b). Orthodromic paired-pulse stimuli with 25 and 50 ms intervals evoked smaller PS2, while those with 100 and 200 ms intervals evoked larger PS2 with similar amplitude as PS1. All of the first stimuli in the paired-pulse stimulations evoked large amplitude multiple spike PS similar to the primary burst in the spontaneous events. Comparison between the baseline responses and the PTX period II responses showed that there was no significant difference in the orthodromic PPR with different IPIs. Under both of the conditions, the depression in orthodromic PS2 was significant when IPI<100 ms, and the depression decreased with the increase of IPI indicated by the increase of PPRs (Fig. 4c left, n=6). However, with short IPI of 25, 50 and 100 ms, the antidromic PPRs during the PTX period II were significantly smaller than those during the baseline responses (Fig. 4c right, P<0.05, n=6, Student’s t-test).
These data indicated that after a prolonged application of PTX, significant PS2 depression appeared in both orthodromic and antidromic paired-pulse stimulations when IPI<100 ms. This depression period following the first evoked PSs was consistent with the burst interval following the primary burst in the spontaneous epileptic events [(97±26) ms]. The results suggested that there was a similar strong inhibitory period following primary bursts either induced by a stimulus or those which appeared spontaneously. Presumably, the epileptic state of CA1 neurons with the presence of PTX may be caused by both the blockage of GABAA receptor in CA1 region and the increase of excitatory inputs coming from CA3. The spontaneous bursts in CA1 region were quite likely induced by firings from CA3 (Dzhala and Staley, 2003). The orthodromic stimulation in the Schaffer collaterals just simulated the spontaneous firing from CA3. Therefore, the appearances of both spontaneous and evoked bursts were very similar.
4. Discussion and conclusions
The main finding of the study is that the depression of PS2 can reappear following prolonged application of GABAA antagonist PTX in the CA1 region. Different from the PS2 depression only present in orthodromic-evoked responses under normal synaptic condition, this type of depression was observed in both orthodromic and antidromic paired-pulse responses. In addition, the depression duration of approximately 100 ms was consistent with a relative silent period observed in spontaneous burst discharges induced by PTX. The results suggested that the neurons may ignore the excitatory inputs and generate bursts intrinsically during epileptic activity.
Usually, the phenomenon of PS2 depression is called paired-pulse depression (PPD). The fast PPD of the population spikes, recorded extracellularly in the hippocampus with normal synaptic transmissions, is considered to be caused by the actions of post-synaptic GABAA receptors (Margineanu and Wulfert, 2000; Leung et al., 2008). In CA1 region, the first stimulus together with the first PS activates local inhibitory circuits (including feedforward and feedback circuits) formed by GABAergic interneurons, thereby suppressing the firing of second PS (Alger and Nicoll, 1982; Karnup and Stelzer, 1999). However, as shown in Fig. 3, this type of suppression does not occur in the antidromic-evoked paradigm (Papatheodoropoulos and Kostopoulos, 1998; Margineanu and Wulfert, 2000). When GABAA receptors are blocked by antagonists, the PPD decreases (Uruno et al., 1995), just as what was observed in the present study during the early period application of PTX when the PS2 increased significantly. However, after a prolonged application of PTX, the PS2 was suppressed again; whereas the first PS response remained as multiple spikes and the spontaneous bursts increased. Taken together, the application of PTX in the CA1 region in-vivo resulted in a type of biphasic change in the PPD of PS amplitude as a short latency disappearance followed later by a reappearance of PPD.
Three possible mechanisms may be related to the reappearance of PPD in the absence of GABAergic inhibition. Firstly, previous intracellular studies have shown that a burst discharge, induced by using PTX to block the synaptic inhibition, can initiate an intrinsic after-hyperpolarization (AHP), i.e., calcium-dependent potassium potential (Alger and Nicoll, 1980; Muller and Misgeld, 1991). This type of AHP has been considered to cause a refractory period during which the second just-suprathreshold stimulus of paired-pulse stimulation fails to evoke action potentials in the hippocampal neurons (Hablitz, 1984). However, the orthodromic and antidromic stimulation strengths we used here were much higher than thresholds. They were presumably strong enough to overcome AHP.
Secondly, a type of so called “depolarization block” could be responsible for the PPD induced by prolonged PTX application. In the present of the GABAA antagonist, extracellular bursts are found to correspond with paroxysmal depolarizing shifts (PDS) in intracellular recordings (Hablitz, 1984; de Curtis and Avanzini, 2001). The PDS is commonly formed by a brief primary burst firing followed by a secondary discharge. There is a depolarization plateau without action potential firings between the primary and the secondary bursts. The depolarization plateau can be assumed to account for the silent interval without obvious spikes in the PTX-induced spontaneous bursts in this study. Presumably, in both orthodromic and antidromic paired-pulse stimulations, the first stimulus evokes a PDS with a primary burst corresponding to the first multiple spike PS in the extracellular recordings, and a sequent depolarization plateau during which a second stimulus cannot evoke any PS. Similar depolarization plateau without neuronal firings was termed as “depolarization block” in low-calcium epileptiform bursts (Bikson et al., 2003). It has been shown in hippocampal slices that antidromic stimulation could not induce action potentials in CA1 pyramidal cells when the membrane potential was depolarized more than −45 mV (Liu and Leung, 2004). The burst activity evoked by the first stimulus in the presence of PTX may readily induce such depolarization. However, direct verification of the underlying depolarization mechanism requires further intracellular recording data.
Thirdly, in previous studies, GABAA receptor antagonist gabazine has been found to enhance the orthodromic-orthodromic and antidromic-antidromic PPD at IPIs<100 ms in the hippocampal CA3 region (Margineanu and Wulfert, 2000). The authors proposed an explanation that the decrease of the inhibitory input to the inhibitory interneurons might increase the inhibitory actions from the interneurons to the pyramidal neurons. However, it is unlikely that the increase of PPD in the present of GABAA receptor antagonists is still caused by the GABAergic inhibition from interneurons to pyramidal neurons. The change of PS1 from a single spike into multiple spikes already indicates a decrease of GABAergic inhibition on the pyramidal neurons. In addition, the occurrence of PPD in the antidromic evoked responses is not likely due to GABAergic inhibition (Papatheodoropoulos and Kostopoulos, 1998).
Presumably, the biphasic change following the use of PTX was caused by the penetration extent of PTX. Because the solution was just put over the surface of exposed part of the intact hippocampus in the in vivo preparation, it took time for PTX to penetrate deep into hippocampal tissues. If the PTX solution was switched back to the normal ACSF quickly, instead of persistent use of PTX, the reappearance of PPD would not occur. Obviously, there is no any exact transfer time point between the early period and late period of PTX application. We chose 20 min as the time to separate the two periods upon the fact that the PPD generally already reappeared following a 20-min application of PTX and it persisted as the PTX remained. The data of early period may be taken at any time around 5–15 min following the use of PTX.
In conclusion, the depolarization block could be the mechanisms underlying the PPD in the absence of GABAA inhibitions. A prolonged application of PTX increases the amplitude of bursts and the paroxysmal depolarizing shifts thereby enhancing the depolarization block and resulting in the reappearance of PPD. In addition, before the reappearance of PPD, when the PTX had not yet penetrated the tissue well, a period occurred where the neurons were sensitive to every stimulus. The distinct neuronal responses to stimulations in different epileptic stages may implicate the different antiepileptic effects of electrical stimulation (Schiller and Bankirer, 2007; Su et al., 2008) and provide important information for the development of new clinical treatments for epilepsy disease.
Footnotes
Project (Nos. 30770548 and 30970753) supported by the National Natural Science Foundation of China
References
- 1.Albertson TE, Walby WF, Stark LG, Joy RM. The effect of propofol on CA1 pyramidal cell excitability and GABAA-mediated inhibition in the rat hippocampal slice. Life Sci. 1996;58(26):2397–2407. doi: 10.1016/0024-3205(96)00243-3. [DOI] [PubMed] [Google Scholar]
- 2.Alger BE, Nicoll RA. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- 3.Alger BE, Nicoll RA. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badawy RA, Harvey AS, Macdonell RA. Cortical hyperexcitability and epileptogenesis: understanding the mechanisms of epilepsy–Part 1. J Clin Neurosci. 2009;16(3):355–365. doi: 10.1016/j.jocn.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Bikson M, Hahn PJ, Fox JE, Jefferys JG. Depolarization block of neurons during maintenance of electrographic seizures. J Neurophysiol. 2003;90(4):2402–2408. doi: 10.1152/jn.00467.2003. [DOI] [PubMed] [Google Scholar]
- 6.Cunha-Reis D, Sebastiao AM, Wirkner K, Illes P, Ribeiro JA. VIP enhances both pre- and postsynaptic GABAergic transmission to hippocampal interneurones leading to increased excitatory synaptic transmission to CA1 pyramidal cells. Br J Pharmacol. 2004;143(6):733–744. doi: 10.1038/sj.bjp.0705989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Almeida AC, Rodrigues AM, Scorza FA, Cavalheiro EA, Teixeira HZ, Duarte MA, Silveira GA, Arruda EZ. Mechanistic hypotheses for nonsynaptic epileptiform activity induction and its transition from the interictal to ictal state–computational simulation. Epilepsia. 2008;49(11):1908–1924. doi: 10.1111/j.1528-1167.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 8.de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63(5):541–567. doi: 10.1016/S0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 9.Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J Neurosci. 2003;23(21):7873–7880. doi: 10.1523/JNEUROSCI.23-21-07873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng ZY, Zheng XJ, Wang J. Effects of carnosine on the evoked potentials in hippocampal CA1 region. J Zhejiang Univ Sci-B. 2009;10(7):505–511. doi: 10.1631/jzus.B0820370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Hablitz JJ. Picrotoxin-induced epileptiform activity in hippocampus: role of endogenous versus synaptic factors. J Neurophysiol. 1984;51(5):1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- 13.Hablitz JJ. Regulation of circuits and excitability: implications for epileptogenesis. Epilepsy Curr. 2004;4(4):151–153. doi: 10.1111/j.1535-7597.2004.44011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karnup S, Stelzer A. Temporal overlap of excitatory and inhibitory afferent input in guinea-pig CA1 pyramidal cells. J Physiol. 1999;516(P2):485–504. doi: 10.1111/j.1469-7793.1999.0485v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloosterman F, Peloquin P, Leung LS. Apical and basal orthodromic population spikes in hippocampal CA1 in vivo show different origins and patterns of propagation. J Neurophysiol. 2001;86(5):2435–2444. doi: 10.1152/jn.2001.86.5.2435. [DOI] [PubMed] [Google Scholar]
- 16.Leung LS, Peloquin P, Canning KJ. Paired-pulse depression of excitatory postsynaptic current sinks in hippocampal CA1 in vivo. Hippocampus. 2008;18(10):1008–1020. doi: 10.1002/hipo.20458. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Leung LS. Sodium-activated potassium conductance participates in the depolarizing afterpotential following a single action potential in rat hippocampal CA1 pyramidal cells. Brain Res. 2004;1023(2):185–192. doi: 10.1016/j.brainres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Margineanu DG, Wulfert E. Differential paired-pulse effects of gabazine and bicuculline in rat hippocampal CA3 area. Brain Res Bull. 2000;51(1):69–74. doi: 10.1016/S0361-9230(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 19.Muller W, Misgeld U. Picrotoxin- and 4-amino-pyridine-induced activity in hilar neurons in the guinea pig hippocampal slice. J Neurophysiol. 1991;65(1):141–147. doi: 10.1152/jn.1991.65.1.141. [DOI] [PubMed] [Google Scholar]
- 20.Papatheodoropoulos C, Kostopoulos G. Development of a transient increase in recurrent inhibition and paired-pulse facilitation in hippocampal CA1 region. Brain Res Dev. 1998;108(1-2):273–285. doi: 10.1016/S0165-3806(98)00061-3. [DOI] [PubMed] [Google Scholar]
- 21.Schiller Y, Bankirer Y. Cellular mechanisms underlying antiepileptic effects of low- and high-frequency electrical stimulation in acute epilepsy in neocortical brain slices in vitro. J Neurophysiol. 2007;97(3):1887–1902. doi: 10.1152/jn.00514.2006. [DOI] [PubMed] [Google Scholar]
- 22.Su Y, Radman T, Vaynshteyn J, Parra LC, Bikson M. Effects of high-frequency stimulation on epileptiform activity in vitro: ON/OFF control paradigm. Epilepsia. 2008;49(9):1586–1593. doi: 10.1111/j.1528-1167.2008.01592.x. [DOI] [PubMed] [Google Scholar]
- 23.Uruno K, O′Connor MJ, Masukawa LM. Effects of bicuculline and baclofen on paired-pulse depression in the dentate gyrus of epileptic patients. Brain Res. 1995;695(2):163–172. doi: 10.1016/0006-8993(95)00652-7. [DOI] [PubMed] [Google Scholar]
- 24.Wierenga CJ, Wadman WJ. Functional relation between interneuron input and population activity in the rat hippocampal cornu ammonis 1 area. Neuroscience. 2003;118(4):1129–1139. doi: 10.1016/S0306-4522(03)00060-5. [DOI] [PubMed] [Google Scholar]