Abstract
We hypothesized whether systemic administration of high-molecular-weight hyaluronic acid (HMW HA) could rescue trinitrobenzene sulfonic acid (TNBS)-induced colitis through Toll-like receptor 4 (TLR4) signal. C3H/HeN mice and C3H/HeJ mice were used. Mice were divided into four groups: control, 50% ethanol treatment group, TNBS treatment group, and TNBS plus HA treatment group. The weight changes, clinical scores, macroscopic scores, and histological scores were recorded. Cyclooxygenase 2 (Cox-2) and prostaglandin E2 (PGE2) expressions were measured both in colons and peritoneal macrophages from these mice. HA was a rescue therapy for the colitis induced by TNBS only in C3H/HeN mice. The clinical score, macroscopic score, and histological score were much lower in C3H/HeN mice receiving TNBS plus HA treatment. Cox-2 and PGE2 expressions only increased in C3H/HeN mice. These Cox-2 expressing cells were macrophages. HA can also promote the production of Cox-2 and PGE2 in peritoneal macrophages from C3H/HeN mice. Our data demonstrated that HMW HA can rescue TNBS-induced colitis through inducing Cox-2 and PGE2 expressions in a TLR4-dependent way. Macrophages may be the effector cells of HMW HA.
Keywords: Trinitrobenzene sulfonic acid (TNBS) colitis, Therapy, Hyaluronic acid, Toll-like receptor
1. Introduction
Hyaluronic acid (HA), an important component of the extracellular matrix, is composed of repeating disaccharide units containing alternating D-glucuronic acid and N-acetyl glucosamine. Two kinds of HA have been found: high-molecular-weight (HMW HA, >500 kDa) and low-molecular-weight (LMW HA, <500 kDa) (Spicer and McDonald, 1998; Itano et al., 1999). These two kinds of HA have different functions. LMW HA exists mainly in the inflammatory conditions (Horton et al., 1998). It can act as an intracellular signaling molecule and enhance productions of many cytokines, such as interleukin-8 (IL-8) (Bai et al., 2005; Mascarenhas et al., 2004; Boodoo et al., 2006). HMW HA exists predominantly in the physiological conditions and maintains the structural integrity of the extracellular matrix. It has been shown to be protective in many inflammation models including acute liver injury, bleomycin-induced lung injury, and sepsis-induced lung injury, etc. (Jiang et al., 2005; Liu et al., 2008).
Studies showed that HA may play an important role in inflammatory bowel disease. HA expression increased in inflamed tissue sections from patients with ulcerative colitis or Crohn’s disease compared to non-inflamed and less inflamed areas (de la Motte et al., 1999). Majors et al. (2003) incubated human colonic smooth muscle cells with dextran sulfate sodium (DSS) in vitro, and the results showed that the production of HA in smooth muscle cells increased after DSS stimulation. In a genetic model of ileitis in which tumor necrosis factor (TNF)-α is over-expressed, the production of HA also significantly increased (Collins et al., 2008).
Toll like receptors (TLRs) are widely distributed in the gastrointestinal tract. For example, TLR4 and myeloid differential protein-2 (MD-2) are highly expressed in the normal colonic epithelium (Janeway and Medzhitov, 1999). A deficiency in TLR4 and Myd88 leads to more severe symptoms in DSS colitis model, which suggests that TLR4 is important in colonic defence response. HA is one of the endogenous receptors of TLR4. HA binding to TLR4 can promote the protective component of the host response in sterile skin and lung injury models (Jiang et al., 2005; Taylor et al., 2007). Whether HA can work through TLR4 to participate in the host response to gastrointestinal injury is not widely explored. In this paper, we used C3H/HeN and C3H/HeJ mice to confirm this hypothesis. These two substrains are similar genetically, but there is a spontaneous mutation in C3H/HeJ mice at lipopolysaccharide response locus (mutation in TLR4 gene, Tlr4lps).
2. Materials and methods
2.1. Animals
Adult (6 weeks old) female C3H/HeN and C3H/HeJ mice were bought from Charles River Laboratories (Wilmington, MA, USA) and Jackson Laboratories (Bar Harbor, ME, USA), separately. All mice were fed standard laboratory food and tap water ad libitum. All the experimental procedures affecting the mice were approved by the Animal Studies Committee of West China Hospital of Sichuan University, China.
2.2. Experimental protocol
The colitis was induced by trinitrobenzene sulfonic acid (TNBS) as previously described (Fukata et al., 2006). Briefly, 25 mg/ml TNBS solution (100 μl) in 50% (v/v) ethanol was administrated into the colon via a thin round-tip needle equipped with a 1-ml syringe. The needle was inserted so that the tip was 4 cm proximal to the anal verge. Mice were held in a vertical position for 30 s after the injection.
All the mice were weighed and randomized into four groups: control, 50% ethanol treatment, TNBS plus HA treatment, and TNBS plus phosphate-buffered saline (PBS) treatment. Control mice received 0.1 ml of saline using the same technique. For mice treated with TNBS plus HA (30 mg/kg), HA was given by intraperitoneal injection at 2 h, 3 d, and 5 d after beginning the TNBS treatment. For mice receiving TNBS plus PBS treatment, PBS was given by intraperitoneal injection at the same time as HA injection. The weight changes were recorded at 1, 3, 5, and 7 d after TNBS treatment. Mice were sacrificed at 7 d after TNBS administration. At least eight mice were used for each group. The clinical-grade HMW HA (M w 2×103 kDa; Healon) was from Physician Sales and Service (St. Louis, MO, USA).
Clinical severity was scored based on the following standards: (1) appearance of diarrhea, (2) signs of fecal blood (macroscopic signs of fecal blood), and (3) perfuse bleeding from anus.
Colons were cut longitudinally and the macroscopic damage score was assessed following Majors et al. (2003): 0, no damage; 1, localized hyperemia without ulceration; 2, linear ulceration without significant inflammation; 3, linear ulceration with inflammation in one site; 4, two or more major sites of inflammation and ulceration extending 1 cm; 5–8, one point added for each centimeter of ulceration beyond an initial 2 cm.
2.3. Histological assessment of colitis
After opening the colons, a 2-cm segment of the distal colon was fixed in 40 g/L formalin and embedded in paraffin. Hematoxylin and eosin (H & E)-stained sections were evaluated by an expert gastrointestinal tract pathologist who was blinded to the treatment. Each sample was scored according to Lahat et al. (2007) with slight modification: percentage of area involved, crypt loss, erosions, number of follicle aggregates, edema, and infiltration of mononuclear and polymorphonuclear cells (Table 1).
Table 1.
Histological scoring system*
| Parameter | Scoring (0–20) |
| Percentage of area involved, crypt loss | 0: Normal |
| 1: <10% | |
| 2: 10% | |
| 3: 10%–15% | |
| 4: 15%–50% | |
| Erosions | 0: Intact epithelium |
| 1: Involvement of the lamina propria | |
| 2: Involvement of the submucosa | |
| 3: Transmural ulceration | |
| No. of follicle aggregates, edema, infiltration of mononuclear and polymorphonuclear cells | 0: Absent |
| 1: Weak | |
| 2: Moderate | |
| 3: Severe | |
* Lahat et al. (2007) with slight modification
2.4. Isolation of peritoneal macrophages
Ice-cold PBS was injected into the peritoneal cavity of each mouse. This fluid was carefully collected and centrifuged at 1 000 r/min for 10 min. The supernatant was then withdrawn, and the cell pellet was resuspended in RPMI 1640 medium to obtain 105 cells/ml. The cells were allowed to adhere for 3 h, and then washed three times with pre-warmed PBS to remove nonadherent cells. The medium was then replaced with fresh RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum. A total of 2×106 to 3×106 cells were obtained from each mouse.
2.5. Immunofluorescent analysis for double staining
Horseradish peroxidase (HRP)-catalyzing immunohistochemical staining method was used as described as our previous study (Zhang et al., 2010). Non-specific bindings were blocked with 3% bovine serum albumin (BSA) and avidin-biotin blocking kit (Vector). Antigen retrieval was performed by immersing the slides in 10 mmol/L citrate buffer for 15 min in microwave. Primary antibodies included mouse monoclonal anti-cyclooxygenase 2 (anti-Cox-2) (BD Pharmingen; 1:50), rat anti-mouse F4/80 (for macrophage staining, AbD Serotec, Raleigh, NC; 1:50), rabbit monoclonal anti-TLR4 (Santa Cruz; 1:100), and goat anti-mouse macrophage inflammatory protein (MIP)-2 (R & D Systems, Minneapolis, MN; 1:100). Secondary antibodies included Cy3-labeled donkey anti-rat IgG (Jackson ImmunoResearch; 1:500), fluorescein isothiocyanate (FITC)-labeled goat anti-mouse (Jackson ImmunoResearch; 1:200), and FITC-labeled donkey anti-S-rabbit/goat IgG (Jackson ImmunoResearch; 1:250).
For hyaluronan staining, distal colon sections were stained with biotinylated hyaluronan-binding proteins (North Star; 1:100) and then detected with Alexa Fluor® 488 conjugate streptavidin (Invitrogen; 1:500).
2.6. Western blot analysis
Proteins were extracted from the colon via homogenization in ice-cold lysis buffer. The concentrations were measured using the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA). Protein samples were separated on 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. Then the membranes were incubated with the primary antibody (mouse Cox-2, Cayman Chemical; 1:600) overnight at 4 °C, followed by the secondary antibodies (HRP-conjugated donkey anti-mouse/rabbit immunoglobulin; Amersham, GE Healthcare; 1:3000) at 24 °C for 1 h. The blots were detected by an enhanced chemiluminescence (ECL) system (Amersham, GE Healthcare, UK). Bands were scanned and semi-quantified by densitometry.
2.7. Prostaglandin E2 assay
Prostaglandin E2 (PGE2) concentration was measured by enzyme-linked immunosorbent assay (ELISA) using a PGE2 kit (Cayman Chemical) according to the manufacturer’s directions. Triplicate aliquots of supernatant were measured for each sample.
2.8. Statistic analysis
All the data were expressed as mean±standard deviation (SD). The difference between groups was analyzed by Student’s t-test. All the calculations were performed using Microsoft Excel 2003.
3. Results
3.1. HA expression only increased in C3H/HeN mice
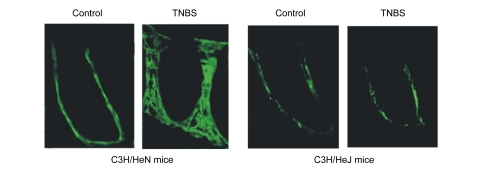
HA expression was located in the lamina propria. After TNBS administration for 7 d, HA expression increased only in C3H/HeN mice, while no change was observed in C3H/HeJ mice (Fig. 1).
Fig. 1.
HA expression in C3H/HeN and C3H/HeJ mice
After TNBS administration for 7 d, HA expression increased in C3H/HeN mice, but did not change in C3H/HeJ mice
3.2. HA rescued the colitis induced by TNBS only in C3H/HeN mice
TNBS-treated mice developed a severe illness characterized by diarrhea, hematochezia, and weight loss. The mortality in mice only receiving TNBS was as high as 40%, while the mortality was 0% in the other three groups. TNBS administration in C3H/HeN mice resulted in a 12% loss of body weight over 7 d, whereas C3H/HeN mice receiving TNBS plus HA maintained their weight. There was no difference in body weight between TNBS and TNBS plus HA groups in C3H/HeJ mice (Figs. 2a and 2b).
Fig. 2.
Effect of HA on TNBS colitis in C3H/HeN and C3H/HeJ mice
(a, b) Body weight. HA maintained the body weight in C3H/HeN mice (a), but not in C3H/HeJ mice (b). (c, d) Clinical score. The clinical score was decreased by HA in C3H/HeN mice (c), but not in C3H/HeJ mice (d). (e, f) Macroscopic damage score. The macroscopic damage score was decreased by HA in C3H/HeN mice (e), but not in C3H/HeJ mice (f). (g, h) Histological score. The histological score was much lower in C3H/HeN mice (g) receiving HA administration, but remained unchanged in C3H/HeJ mice receiving HA (h). (i, j) Microscopic view of the colon. HA can alleviate the damage caused by TNBS only in C3H/HeN mice (i), not in C3H/HeJ mice (j). E: 50% ethanol; TNBS+HA: TNBS plus HA; * P<0.05, TNBS plus HA group compared to TNBS group
The severity of colitis was decreased by HA in C3H/HeN mice. Seven days after TNBS or TNBS plus HA administration, the clinical score and the macroscopic damage score were much lower in TNBS plus HA treatment group (2.8±0.4 vs. 1.3±0.5; 4.2±1.2 vs. 2.2±1.3; P<0.05; Figs. 2c and 2e). Microscopic examination showed hyperemia, inflammation, and ulcer in mice receiving TNBS administration. The histological score was much lower in TNBS plus HA treatment group (9.5±1.5 vs. 4.5±1.1, P<0.05; Figs. 2i and 2g).
There were no differences in the clinical score, macroscopic damage score, or histological score between TNBS and TNBS plus HA groups in C3H/HeJ mice 7 d after administration (3.6±0.8 vs. 3.4±0.6; 4.2±1.2 vs. 4.1±1.3; 11.2±2.1 vs. 10.6±1.9; P>0.05; Figs. 2d, 2f, 2h and 2j).
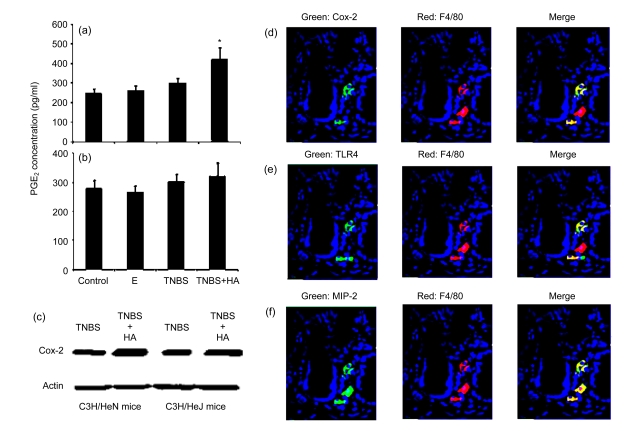
3.3. Cox-2 and PGE2 expressions only increased in C3H/HeN mice
In C3H/HeN mice receiving TNBS plus HA treatment, the expression of PGE2 significantly increased compared to mice receiving TNBS administration ((423±35) pg/ml vs. (250±26) pg/ml, P<0.05, Fig. 3a). On the contrary, the expression of PGE2 was not affected by HA administration in C3H/HeJ mice (Fig. 3b). Cox-2 expression increased approximately three-fold in the distal colon in C3H/HeN mice, but remained unchanged in C3H/HeJ mice after TNBS plus HA treatment (Fig. 3c). These Cox-2 expressing cells were located in the lamina propria. Most of them were double stained with macrophages (Fig. 3d). The latter also expressed TLR4 and MIP-2 (Figs. 3e and 3f).
Fig. 3.
Cox-2 and PGE2 expressions in C3H/HeN and C3H/HeJ mice
(a, b) PGE2 expression significantly increased in C3H/HeN mice (a), but not in C3H/HeJ mice (b); (c) Cox-2 expression increased three times in C3H/HeN mice, but not in C3H/HeJ mice; (d) Most of the Cox-2 expressing cells were macrophages (F4/80); (e) Most of the macrophages expressed TLR4; (f) All of the macrophages expressed MIP-2. E: 50% ethanol; TNBS+HA: TNBS plus HA. * P<0.05, TNBS plus HA group compared to TNBS group
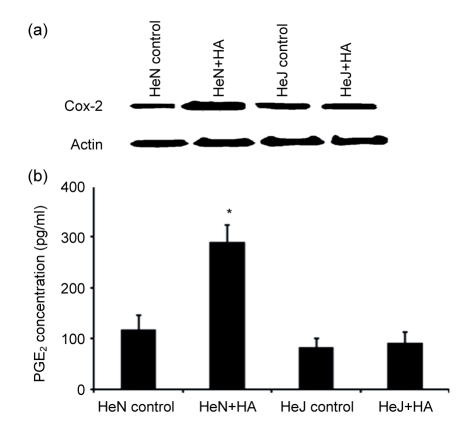
3.4. HA increased Cox-2 and PGE2 expressions in peritoneal macrophages
Incubation of C3H/HeN mice peritoneal macrophages with HA (100 g/ml) induced two-to three-fold increases in Cox-2 and PGE2 expressions (Fig. 4). These increases were not seen in peritoneal macrophages from C3H/HeJ mice.
Fig. 4.
Effect of HA on Cox-2 and PGE2 expressions in peritoneal macrophages of C3H/HeN and C3H/HeJ mice
(a) Cox-2 expression increased about three times in C3H/HeN mice peritoneal macrophages, but not in C3H/HeJ mice; (b) PGE2 expression increased about two times in C3H/HeN mice peritoneal macrophages. HeN: C3H/HeN mice; HeJ: C3H/HeN mice. HeN+HA: C3H/HeN mice treated with HA. HeJ+HA: C3H/HeJ mice treated with HA. * P<0.05, HA group compared to control group
4. Discussion
Our results showed that HMW HA can rescue TNBS-induced colitis, and this protective effect occurred only in C3H/HeN mice. This result is consistent with the study of Zheng et al. (2009). Their study showed HA can rescue DSS colitis in wild type mice, but not in TLR4−/− mice. These data suggested HA may work through TLR4 signal to participate in the host response to gastrointestinal injury.
Except highly conserved pathogen-associated molecular patterns (PAMPs), TLRs also act to some endogenous molecules, such as heat shock proteins, which are capable of signaling the presence of danger to surrounding cells and tissues (Deng et al., 2007; Földes et al., 2008; Zhang et al., 2005). Studies have showed that HA is one of the endogenous receptors of TLR4. HMW HA can reduce bleomycin-induced apoptosis in alveolar epithelial cells from wild type mice and the protective effect was not seen in TLR4−/− mice (Jiang et al., 2005). Using TLR4-specific small interfering RNA (siRNA), the expressions of matrix metalloprotease 2 (MMP2) and IL-8 induced by HA were blocked (Wallet et al., 2010). Taylor et al. (2007) also found that recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on TLR4, CD44, and MD-2. These results showed that the interaction between TLR4 and HA may be crucial in host defense against noninfectious injury.
The expression of HA increased not only in patients with DSS colitis but also in patients with inflammatory bowel disease (Majors et al., 2003). Our results showed HA expression also increased in TNBS colitis and this occurred only in C3H/HeN mice, which suggested that the expression of endogenous HA also relied on TLR4 signaling. There may be a positive feedback between endogenous HA expression and TLR4 signaling. The consequence is most likely related to the interaction of the repair process and the mobilization of antimicrobial defense at the site of tissue disruption.
The protective effect of HWM HA has been confirmed in many inflammatory diseases, such as emphysema, acute liver injury, and bleomycin-induced lung injury (Połubinska et al., 2000; Breborowicz et al., 2001; Jiang et al., 2005). The anti-inflammatory activity of HA is not well understood. It depends not only on direct interaction with inflammatory cells, but also on the physical properties of the molecule itself. It can decrease many cytokines production, such as TNF-α and Interferon-γ (IFN-γ) (Wang C.T. et al., 2006). Our study showed that after HMW HA injection, the expressions of Cox-2 and PGE2 in colon mucosa increased only in C3H/HeN mice. This may suggest that HMW HA may work through Cox-2 and PGE2 production to protect the colon from injury. Cox-2 and PGE2 are very important for mucosa protection. Deficiency in Cox-2 results in the colitis induced by DSS becoming more severe. PGE2 can increase the blood flow and activate the epidermal growth factor receptor. Supplementation of PGE2 in TLR4−/− mice resulted in improvement in clinical signs of colitis (Fukata et al., 2006).
Our results showed that Cox-2 expressing cells were double stained with macrophages and most of macrophages were TLR4 expressing cells. We also isolated peritoneal macrophages and found that HA can promote the production of Cox-2 and PGE2 in C3H/HeN mice macrophages. These data suggested that macrophages may be the effector cells of HMW HA. The interaction between HA and macrophage has been confirmed in many studies. For example, HA fragments can induce the expression of many chemokines in macrophages, such as the T helper 1 (TH1) cytokine IL-12p70 (Wang M.J. et al., 2006; Wallet et al., 2010). In a study using macrophages from bleomycin-injured rat lungs, HA fragments can induce metalloelastase and inducible nitric oxide synthase (iNOS) production in macrophages (McKee et al., 1997; Horton et al., 1999). Noble et al. (1996) also demonstrated that HA fragments can induce the nuclear factor-kappa B (NF-κB) activation in murine macrophages. Our results together with these data suggested that HMW HA could activate macrophages to improve Cox-2 and PGE2 production.
In summary, our data demonstrated that HMW HA can rescue the TNBS colitis through inducing Cox-2 and PGE2 production in a TLR4-dependent way. The interactions between HA and macrophages may have an important role in determining how inflammatory responses are resolved. This may help to find a new therapy for human inflammatory bowel disease.
Footnotes
Project (No. C140404) supported by National Natural Science Foundation of China
References
- 1.Bai KJ, Spicer AP, Mascarenhas MM, Yu L, Ochoa CD, Garg HG, Quinn DA. The role of hyaluronan synthase 3 in ventilator induced lung injury. Am J Respir Crit Care Med. 2005;172(1):92–98. doi: 10.1164/rccm.200405-652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boodoo S, Spannhake EW, Powell JD, Horton MR. Differential regulation of hyaluronan-induced IL-8 and IP-10 in airway epithelial cells. AJP Lung Cell Mol Physiol. 2006;291(3):L479–L486. doi: 10.1152/ajplung.00518.2005. [DOI] [PubMed] [Google Scholar]
- 3.Breborowicz A, Połubinska A, Moberly J, Ogle K, Martis L, Oreopoulos D. Hyaluronan modifies inflammatory response and peritoneal permeability during peritonitis in rats. Am J Kidney Dis. 2001;37(3):594–600. doi: 10.1016/S0272-6386(01)80018-4. [DOI] [PubMed] [Google Scholar]
- 4.Collins CB, Ho J, Wilson TE, Wermers JD, Tlaxca JL, Lawrence MB, Solga M, Lannigan J, Rivera-Nieves J. CD44 deficiency attenuates chronic murine ileitis. Gastroenterology. 2008;135(6):1993–2002. doi: 10.1053/j.gastro.2008.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C) J Biol Chem. 1999;274(43):30747–30755. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- 6.Deng JF, Geng L, Qian YG, Li H, Wang Y, Xie HY, Feng XW, Zheng SS. The role of Toll-like receptors 2 and 4 in acute allograft rejection after liver transplantation. Transplant Proc. 2007;39(10):3222–3224. doi: 10.1016/j.transproceed.2007.02.102. [DOI] [PubMed] [Google Scholar]
- 7.Földes G, von Haehling S, Okonko DO, Jankowska EA, Poole-Wilson PA, Anker SD. Fluvastatin reduces increased blood monocyte Toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int J Cardiol. 2008;124(1):80–85. doi: 10.1016/j.ijcard.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131(3):862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton MR, McKee CM, Bao C, Liao F, Farber JM, Hodge-DuFour J, Puré E, Oliver BL, Wright TM, Noble PW. Hyaluronan fragments synergize with interferon-γ to induce the C–X–C chemokines Mig and interferon-inducible protein-10 in mouse macrophages. J Biol Chem. 1998;273(52):35088–35094. doi: 10.1074/jbc.273.52.35088. [DOI] [PubMed] [Google Scholar]
- 10.Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol. 1999;162(7):4171–4176. [PubMed] [Google Scholar]
- 11.Itano N, Sawani T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274(35):25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 12.Janeway CA, Medzhitov R. Lipoproteins take their toll on the host. Curr Biol. 1999;9(23):R879–R882. doi: 10.1016/S0960-9822(00)80073-1. [DOI] [PubMed] [Google Scholar]
- 13.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11(11):1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 14.Lahat G, Halperin D, Barazovsky EL, Shalit I, Rabau M, Klausner J, Fabian I. Immunomodulatory effects of ciprofloxacin in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2007;13(5):557–565. doi: 10.1002/ibd.20077. [DOI] [PubMed] [Google Scholar]
- 15.Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA, et al. High-molecular-weight hyaluronan—a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care. 2008;12(4):R102. doi: 10.1186/cc6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majors AK, Austin RC, de la Motte CA, Pyeritz RE, Hascall VC, Kessler SP, Sen G, Strong SA. Endoplasmic reticulum stress induces hyaluronan deposition and leukocyte adhesion. J Biol Chem. 2003;278(47):47223–47231. doi: 10.1074/jbc.M304871200. [DOI] [PubMed] [Google Scholar]
- 17.Mascarenhas MM, Day RM, Ochoa CD, Choi WI, Yu L, Ouyang B, Garg HG, Hales CA, Quinn DA. Low molecular weight hyaluronan from stretched lung enhances IL-8 expression. Am J Respir Cell Mol Biol. 2004;30(1):51–60. doi: 10.1165/rcmb.2002-0167OC. [DOI] [PubMed] [Google Scholar]
- 18.McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, Choi AM, Noble PW. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor κB-dependent mechanism. J Biol Chem. 1997;272(12):8013–8018. doi: 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- 19.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-κB/I-κBα autoregulatory loop in murine macrophages. J Exp Med. 1996;183(5):2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Połubinska A, Pawlaczyk K, Kużlan-Pawlaczyk M, Wieczorowska-Tobis K, Chen C, Moberly JB, Martis L, Breborowicz A, Oreopoulos DG. Dialysis solution containing hyaluronan: effect on peritoneal permeability and inflammation in rats. Kidney Int. 2000;57(3):1182–1189. doi: 10.1046/j.1523-1755.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- 21.Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273(4):1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- 22.Taylor KR, Yamasaki K, Radek KA, di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. Recognition of hayluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282(25):18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 23.Wallet MA, Wallet SM, Guiulfo G, Sleasman JW, Goodenow MM. IFN-γ primes macrophages for inflammatory activation by high molecular weight hyaluronan. Cell Immunol. 2010;262(2):84–88. doi: 10.1016/j.cellimm.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. Weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis and Cartilage. 2006;14(12):1237–1247. doi: 10.1016/j.joca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Wang MJ, Kuo JS, Lee WW, Huang HY, Chen WF, Lin SZ. Translational event mediates differential production of tumor necrosis factor-α in hyaluronan-stimulated microglia and macrophages. J Neurochem. 2006;97(3):857–871. doi: 10.1111/j.1471-4159.2006.03776.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, Lee PJ. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175(8):4834–4838. doi: 10.4049/jimmunol.175.8.4834. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Chen H, Yang L. Toll-like receptor 4 participates in gastric mucosal protection through Cox-2 and PGE2 . Dig Liver Dis. 2010;42(7):472–476. doi: 10.1016/j.dld.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Zheng L, Riehl TE, Stenson WF. Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology. 2009;137(6):2041–2051. doi: 10.1053/j.gastro.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]