Abstract
The O subfamily of forkhead box (FoxO) proteins is the downstream effector of the insulin-like growth factor-1/phosphoinositide 3-kinase/protein kinase B (IGF-1/PI3K/PKB) signal pathway. The objective of the present study was to examine the expressions of three members of FoxO proteins, FoxO1, FoxO3a, and FoxO4 in the duodenum of Sprague-Dawley rats at different ages. The result demonstrated that the expression of FoxO4 in rat duodenum showed an age-dependent manner. At Day 21, there were no detectable localization and expression of FoxO4 in the duodenum, while, at Months 2 and 6, localization and expression of FoxO4 were distinct. In addition, FoxO4 staining was primarily concentrated in the cell nuclei of the lamina propria around the intestinal gland of the duodenum in 2-month-old rats, but was not detectable in the same area in 6-month-old rats. Our results showed also that although FoxO3a existed in the cytoplasm of the lamina propria at a low level at the 2- and 6-month marks, it was still not detectable at Day 21. Besides, FoxO1 was not detectable in all parts and stages. Taken together, our findings suggested that the cell-specific and age-dependent expressional patterns of FoxO4 and FoxO3a proteins in the duodenum play some roles in the development and growth performance of the rat duodenum.
Keywords: O subfamily of forkhead box (FoxO), Age-dependence, Duodenum, Rat
1. Introduction
The O subfamily of forkhead box (FoxO) proteins consists of FoxO3a, FoxO1, and FoxO4, all downstream effectors of the insulin-like growth factor-1/phosphoinositide 3-kinase/protein kinase B (IGF-1/PI3K/PKB) signal pathway (Sengupta et al., 2009). Moreover, there has been a novel member of Foxos found in the mouse brain, which is denoted as Foxo6 (Hoekman et al., 2006). Phosphorylation of FoxO proteins by PKB results in cytoplasmic retention and inactivation, and then inhibits the expression of FoxO-regulated genes which control the cell cycle, cell death, and cell metabolism. The shuttling of FoxOs between the cytoplasm and nucleus is a key step of apoptosis (Burgering and Kops, 2002). Our previous works have already demonstrated that FoxO4 is a primary forkhead transcriptional factor localized in the gastrointestinal tracts of the pig (Zhou et al., 2007), particularly in the duodenum (the most critical nutrient-absorbing organ). Besides, Liu et al. (2008) have found that FoxO1 mRNA is expressed at a lesser level in the duodenum, subcutaneous adipose tissue, and pancreas when compared with other tissues of pigs. Also, the expression level of the FoxO1 gene was the lowest in the pigs at 20 kg. Although many early researchers are concentrated on PI3K/PKB signal pathway in oesophagus and colorectal cancer cells (Saito et al., 2003; Bao et al., 2004), few studied FoxOs. In addition, growth hormone (GH) and IGF-1 are recognized to decline with age, while the transcriptional activity of FoxOs is increased as a result of the reduction of insulin or IGF-1 level (Brunet et al., 1999; Tomita et al., 2001). Similarly, Roubenoff and Hughes (2000) suggest that the higher expression of FoxO genes may be required to prevent certain adverse aging processes from occurring. In addition, there are only antisera against FoxO1, FoxO3a, and FoxO4 readily available to date. Accordingly, our present study focused on the expressions and localizations of FoxO1, FoxO3a, and FoxO4 in the rat duodenum at different ages.
2. Materials and methods
2.1. Animals and sample collection
A total of 90 intact male Sprague-Dawley rats at 21 d (weanling date), 2 and 6 months old, obtained from Qinglongshan Experimental Animal Breeding Farm (Nanjing, China), were used in the current experiment. Uniform commercial diets used in the experiment were also purchased from the same company. Regular rat chow and tap water were allowed ad libitum. Rats were housed individually at room temperature (25 °C) with a 12 h:12 h light/dark cycle and humidity of 65%‒70%. In order to examine the expressions of FoxO1, FoxO3a, and FoxO4 by immunohistochemical analysis in the rat duodenum at different ages, duodenal samples were immediately removed from anesthetized rats and fixed in 4% (v/v) paraformaldehyde at room temperature overnight. In addition, the duodenal mucosas were also sampled and stored in −70 °C for immunoblot analysis of FoxO levels. All procedures were designed in accordance with the generally-accepted ethical standards for animal experimentation and the guidelines established by the Institutional Animal Care and Use Committee, Nanjing Agricultural University, China.
2.2. Reagents
Antibodies for FoxO1/FKHR (Cat. No. 9462, Lot 2), FoxO3a/FKHRL1 (Cat. No. 9467, Lot 4), and FoxO4/AFX (Cat. No. 9472, Lot 1) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies of α-tubulin (Cat. No. T6074, Lot 125k4790) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Streptavidin-biotin complex (SABC) kit was obtained from BioGenex (San Ramon, CA, USA), and 3,3′-diaminobenzidine tetrachloride (DAB) was purchased from Sigma Chemical Co. Radio immunoprecipitation assay (RIPA) lysis buffer, a bicinchoninic acid (BCA) protein assay kit, was obtained from Beyotime Institute of Biotechnology (Nantong, Jiangsu, China). All other chemicals were commercially purchased and were of reagent grade.
2.3. Immunohistochemical analysis
After transferring through a graded series of alcohol and xylene, duodenal samples were embedded in paraffin sectioned at 8 µm thickness. The sample sections were mounted on slides and processed for immunohistochemical analysis which was conducted in a protocol similar to the method used in our previous reports (Meng et al., 2007; Ding et al., 2010; Zhang et al., 2011). Briefly, sections were incubated overnight at room temperature with a polyclonal rabbit immunoaffinity-purified antiserum directed against the FoxO1 (1:400), FoxO3a (1:400), and FoxO4 proteins (1:500). The specific protein immunoreactivity was visualized with an SABC kit elite and 0.1 g/ml DAB in 10 mmol/L PBS-buffered saline containing 0.01% (v/v) H2O2 for 5 min. Specificity of the antibody was examined using normal rabbit serum (NRS) instead of the primary antibody. In order to identify structural components and cell morphology, the sections were counter-stained with hematoxylin and mounted with coverslips. Relative levels of immunostaining between animals and cell types were repeated at least four times and evaluated by three independent observers.
2.4. Immunoblot analysis
To examine the expression levels of FoxO3a and FoxO4 in the rat duodenum by immunoblot analysis, a method similar to our previous works (Shi et al., 2004; Ding et al., 2010) was conducted. Proteins were extracted from frozen duodenal mucosa with RIPA lysis buffer (50 mmol/L Tris-HCl (pH=8.0), 150 mmol/L NaCl, 0.5% (v/v) NP-40, 20% (v/v) glycerol, 25 mmol/L benzamidine, 0.5 µg/ml of leupeptin, 0.7 µg/ml of pepstatin A, 3 µg/ml of aprotinin, and 10 µg/ml of trypsin inhibitor). The duodenal mucosa protein was extracted using RIPA lysis buffer in a Dounce homogenizer (Fluko Shanghai Co., Ltd., China). After homogenization, samples were incubated for 30 min on ice, vortexed, and centrifuged at 12 000×g for 10 min at 4 °C. The supernatant was separated and the protein concentration was determined by a modification of the Bradford method (BioRad) using bovine serum albumin (BSA) standards and microplate absorbance readings at 595 nm. Determination of FoxO3a and FoxO4 concentrations was performed by immunoblot analysis. Briefly, proteins were resolved using 0.1 g/ml polyacrylamide sodium dodecyl sulfate (SDS) gel electrophoresis under reducing conditions. In each experiment, equal quantities of proteins (40 µg homogenized duodenum mucosa) were loaded for each sample. The gel-separated proteins were then transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were then incubated with a blocking buffer (TBST with 0.05 g/ml milk, 0.1% (v/v) Tween-20, pH 7.5) at room temperature for 1 h and incubated overnight at 4 °C with diluted antibodies specific to FoxO3a (1:1000), FoxO4 (1:1000), and α-tubulin (1:2000). After three washes, the membrane was incubated with the horseradish peroxidase (HRP)-conjugated secondary antibody diluted (1:8000) in the blocking buffer for 1 h.
Finally, the blots were washed three times and detected for immunoreactivity by chemiluminescence detection methods. Blots were then used to expose radiographic film to visualize immunoreactive signals.
2.5. Statistical analysis
Values were expressed as the mean±standard error of the mean (SEM). The data were analyzed using a one-way analysis of variance (ANOVA) as appropriate. P<0.05 was considered statistically significant. All experiments were repeated at least three times, and representative data are shown.
3. Results
3.1. Immunohistochemical localizations of FoxO1, FoxO3a, and FoxO4 in the rat duodenum
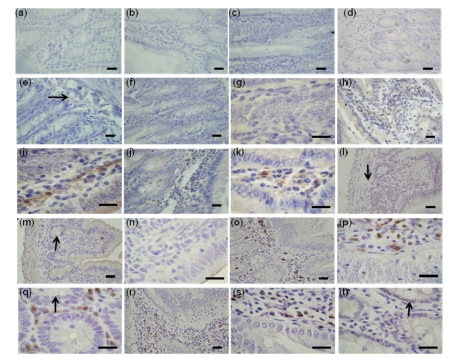
Immunohistochemical analyses of FoxO1, FoxO3a, and FoxO4 in the duodenum were performed by using rats at 21 d, 2 and 6 months old. The result indicated that FoxO3a existed mainly in the cell cytoplasm of the lamina propria but was negative in muscularis and any other parts of the duodenum at a low level at Months 2 (Figs. 1h and 1i) and 6 (Figs. 1j and 1k); in contrast, there was no detectable localization of FoxO3a at Day 21 (Figs. 1e, 1f, and 1g). Nevertheless, FoxO1 was not detectable in all parts and stages (Figs. 1a, 1b, 1c and 1d).
Fig. 1.
Immunohistochemical localizations of FoxO proteins in the duodenum of Sprague-Dawley rats
The immunohistochemical signals appear brown and the counterstaining background appears blue in color. Immunohistochemical localization of FoxO1: 21-d-old (b), 2-month-old (c), 6-month-old (d); Immunohistochemical localization of FoxO3a: 21-d-old (f, g), 2-month-old (h, i), 6-month-old (j, k); Immunohistochemical localization of FoxO4: 21-d-old (m, n), 2-month-old (o, p, q), 6-month-old (r, s, t). (q) and (t) are transverse sections. In control sections of FoxO1 (a), FoxO3a (e), and FoxO4 (l), normal albumin bovine V was used instead of primary antibody. →: muscularis mucosa; ↓: submucosa; ↑: intestinal gland. Bar=50 µm
While interestingly, the result indicated that no detectable localization of FoxO4 in the duodenum of 21-d-old rats (Figs. 1l, 1m and 1n) was found. FoxO4 staining was very distinct and primarily concentrated in the cell nuclei of the lamina propria around the intestinal gland of the duodenum at 2-month-old rats (Fig. 1q). The localization patterns were based on the mucosa, close to the muscularis mucosa (Figs. 1o and 1p). Compared with rats at the 2-month mark, FoxO4 staining could not be detected in the cell nuclei of the lamina propria around the intestinal gland and decreasing staining in the duodenum of rats at 6 months (Figs. 1r, 1s, and 1t).
3.2. Immunoblot analyses of FoxO3a and FoxO4 proteins in the rat duodenum
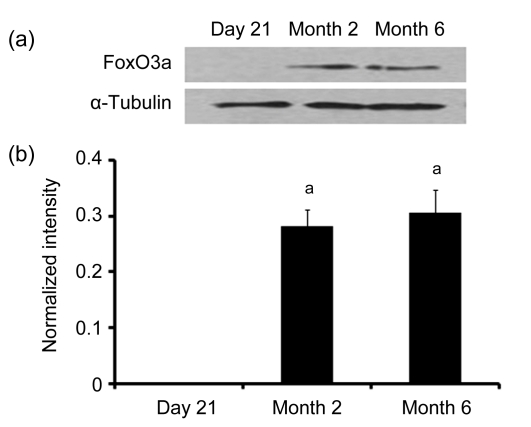
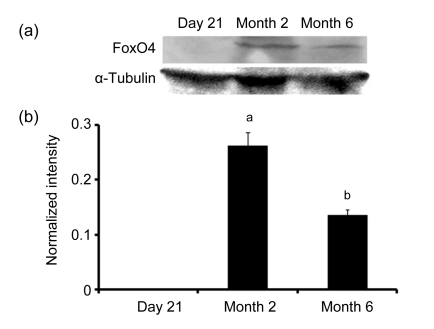
Immunoblot analyses of FoxO3a and FoxO4 levels in the duodenum were also performed in rats at different ages. The results showed that FoxO3a and FoxO4 levels were changed at different ages in the rat duodenum. No FoxO3a or FoxO4 expression was detected in the duodenum of 21-d-old rats, while, significant expressions of FoxO3a and FoxO4 in the duodenum were found in the rats at 2 and 6 months (Figs. 2 and 3). In addition, the FoxO4 levels in the duodenum of rats at 6 months decreased compared with 2 months (Figs. 3a and 3b). However, there were no significant differences of FoxO3a levels in the duodenum of rats between Months 2 and 6 (Figs. 2a and 2b).
Fig. 2.
Western blotting (a) and digital image analysis (b) of FoxO3a in the duodenum of rats at 21 d, 2 and 6 months old
After homogenization, the extracted duodenal mucosas (40 µg/lane) were fractionated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose, then followed by immunoblot analysis of FoxO3a. For the control, immunoblot analysis was performed with antibodies specific to α-tubulin. Then, following immunoblot analysis, the intensities of FoxO3a immunoreactive bands were determined by digital image analysis. Data (ratio to α-tubulin) represent means±SEM. Different letters above the bars indicate a statistically significant difference (P<0.05, n=6) among groups
Fig. 3.
Western blotting (a) and digital image analysis (b) of FoxO4 in the duodenum of rats at 21 d, 2 and 6 months old
After homogenization, the extracted duodenal mucosas (40 µg/lane) were fractionated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose, then followed by immunoblot analysis of FoxO4. For the control, immunoblot analysis was performed with antibodies specific to α-tubulin. Then, following immunoblot analysis, the intensities of FoxO4 immunoreactive bands were determined by digital image analysis. Data (ratio to α-tubulin) represent means±SEM. Different letters above the bars indicate a statistically significant difference (P<0.05, n=6) among groups
4. Discussion and conclusions
Our present study demonstrated clearly that the FoxO4 and FoxO3a expressions in the duodenum of rats showed age-dependent and cell-specific manners. FoxO4 is a main FoxO protein expressed in the rat duodenum, which is consistent with our previous works in the pig. FoxO3a existed in the cell cytoplasm of the lamina propria at a low level at Months 2 and 6, and there was no detectable localization at Day 21, which is not consistent with our previous works in the pig. Besides, FoxO1 was not detectable in all parts and stages.
The duodenum is the most critical nutrient absorbing organ which completes development in four weeks after birth in rats (Gao et al., 1995). In our current investigation, we selected three time points: weanling date (21 d), adolescence (2 months), and adulthood (6 months). The significant differences in FoxO4 expression among these three ages in the rat duodenum play some roles.
Recent discoveries suggested IGF-1/PI3K/PKB/FoxO might affect lifespan in higher eukaryotes (Burgering and Kops, 2002). These age-dependent changes may be caused, in part, by the change of hormonal circumstance. In particular, GH and IGF-1 were well known to decline with age (Furuyama et al., 2002). Since transcriptional activity of FoxOs increased as a result of the reduction of insulin or IGF-1 level (Brunet et al., 1999; Tomita et al., 2001), the peak of IGF-1 secretion after birth may result in no FoxO3a and FoxO4 expressions in the 21-d-old rats. Meanwhile, the expression of FoxO4 increased in the 2-month-old rats through a low plasma level of IGF-1. However, the reduced expression of FoxO4 in 6-month-old rats might lead to accelerated aging. Emerging evidence suggests the similar conclusions in the skeletal muscles (Furuyama et al., 2002). In other words, this suggests that FoxO4 may be involved in the development and growth performance of the rat duodenum.
Our present study demonstrated that FoxO4 mainly concentrated in the cell nuclei of the lamina propria around the intestinal gland in the duodenum of 2-month-old rats but not the 6-month-old rats. This may occur because the 2-month-old rats were in a higher rate of metabolism. Further research is required to identify the mechanisms of FoxO4 shuttling into cell nuclei to regulate apoptosis after synthesized in mucosa of duodenum in 2-month-old rats. Though many researches regarding shuttling mechanisms of FoxO proteins were mostly concentrated on FoxO proteins shuttling into cytoplasm phosphorylated by PKB proteins, there was only a hypothesis on entrance mechanisms of FoxO proteins into cell nuclei, which suggested that FoxO proteins followed common shuttling into nuclear model for the nuclear localization signal (van der Heide et al., 2004).
It was reported that FoxO3a was a downstream target of PKB (also known as Akt, a serine/threonine protein kinase) in endothelial cells that could promote apoptosis via down-regulation of Fas-associated death domain-like interleukin 1β-converting enzyme inhibiting protein (FLIP) and activation of the extrinsic apoptotic pathway (Skurk et al., 2004). Besides, in cerebellar neurons, fibroblasts and Jurkat cells, the transient overexpression of nonphosphorylatable FoxO3a induced apoptotic cell death by the up-regulation of Fas ligand expression and activation of the death receptor pathway (Brunet et al., 1999). Our previous work suggested that FoxO3a was detectable in lamina propria of jejunum and large intestine, but no localization was evident in the duodenum of pigs (Zhou et al., 2007). In contrast, from the present study, we found FoxO3a existed in the cell cytoplasm of the lamina propria at a low level at Months 2 and 6, and there was no detectable localization at Day 21 in the duodenum of rats. Our findings might suggest that the role of FoxO3a in mammalian gastrointestinal tracts has species specific manners.
The present study did not find any significant localization of FoxO1 in the duodenum of rats and this is inconsistent with our previous work in the pig (Zhou et al., 2007). However, Liu et al. (2008) showed that FoxO1 mRNA was expressed at a lesser level in the duodenum of pigs. This implied that FoxO1 may have a post-transcriptional regulatory mechanism in the duodenum.
In conclusion, our findings suggest that the cell-specific and age-dependent expressional patterns of FoxO4 and FoxO3a proteins in the duodenum play some roles in the development and growth performance of the rat duodenum.
Acknowledgments
We thank Mr. Wei ZHANG, a doctoral student of Nanjing Agricultural University, China for his technical assistance.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30771553) and the Basic Research Foundation for Science and Technology of Nanjing Agricultural University, China
References
- 1.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5(4):329–339. doi: 10.1016/S1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 2.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 3.Burgering BM, Kops GJ. Cell cycle and death control: long live forkheads. Trends Biochem Sci. 2002;27(7):352–360. doi: 10.1016/S0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 4.Ding W, Wang W, Zhou B, Zhang W, Huang P, Shi F, Taya K. Formation of primordial follicles and immunolocalization of PTEN, PKB and FOXO3A proteins in the ovaries of fetal and neonatal pigs. J Reprod Dev. 2010;56(1):162–168. doi: 10.1262/jrd.09-094H. [DOI] [PubMed] [Google Scholar]
- 5.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of FoxO1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech. 2002;59(4):331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Wang S, Xing S. Histological and histochemical observation on the development and differentiation of the mouse duodenum. Acta Anat Sin. 1995;26(5):426–431. [Google Scholar]
- 7.Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns. 2006;6(2):134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Wang Y, Shan T. The tissue-specific and developmental expression patterns of the forkhead transcription factor FoxO1 gene in pigs. J Anim Feed Sci. 2008;17(2):182–190. [Google Scholar]
- 9.Meng C, Shi F, Zhou Z, Huang R, Liu G, Watanabe G, Taya K. Cellular localization of inhibin α-subunit, PKB/Akt and FoxO3a proteins in the ovaries of minipigs. J Reprod Dev. 2007;53(2):229–236. doi: 10.1262/jrd.18078. [DOI] [PubMed] [Google Scholar]
- 10.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55(12):M716–M724. doi: 10.1093/gerona/55.12.M716. [DOI] [PubMed] [Google Scholar]
- 11.Saito Y, Gopalan B, Mhashilkar AM, Roth JA, Chada S, Zumstein L, Ramesh R. Adenovirus-mediated PTEN treatment combined with caffeine produces a synergistic therapeutic effect in colorectal cancer cells. Cancer Gene Ther. 2003;10(11):803–813. doi: 10.1038/sj.cgt.7700644. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284(41):28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi F, Stewart RLJr, Perez E, Chen JY, LaPolt PS. Cell-specific expression and regulation of soluble guanylyl cyclase α1 and β1 subunits in the rat ovary. Biol Reprod. 2004;70(6):1552–1561. doi: 10.1095/biolreprod.103.025510. [DOI] [PubMed] [Google Scholar]
- 14.Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, Walsh K. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279(2):1513–1525. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- 15.Tomita M, Shimokawa I, Higami Y, Yanagihara-Outa K, Kawahara T, Tanaka K, Ikeda T, Shindo H. Modulation by dietary restriction in gene expression related to insulin-like growth factor-1 in rat muscle. Aging. 2001;13(4):261–262. doi: 10.1007/BF03353423. [DOI] [PubMed] [Google Scholar]
- 16.van der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380(2):297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Wei Q, Wang Z, Ding W, Wang W, Shi F. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and the related nitric oxide/cyclic GMP signaling pathway in the ovaries of neonatal and immature rats. J Zhejiang Univ-Sci B. 2011;12(1):55–64. doi: 10.1631/jzus.B1000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Wang T, Pan L, Huang R, Shi F. FoxO4 is the main forkhead transcriptional factor localized in the gastrointestinal tracts of pigs. J Zhejiang Univ-Sci B. 2007;8(1):39–44. doi: 10.1631/jzus.2007.B0039. [DOI] [PMC free article] [PubMed] [Google Scholar]