Abstract
The effects of different fatty acid (FA) contents in diet on serum parameters, FA compositions of eggs and meat, and liver morphological changes were studied in Shaoxing laying ducks. A total of 264 ducks at 17 weeks were fed a control diet or a diet containing 30 g/kg fish oil (FO), 25 g/kg sunflower oil (SO), or 30 g/kg palm oil with 20 g/kg beef tallow (PBO). Malondialdehyde (MDA) content in the liver and the serum of ducks fed the PBO diet was significantly (P<0.05) higher than that of ducks fed the other diets. Triglyceride (TG) and total cholesterol (TC) levels were significantly lower (P<0.05) in ducks fed the FO diet. Serum TC also was lower in ducks fed the SO diet. Superoxide dismutase (SOD) activity was also affected by diets. The contents of polyunsaturated FAs (PUFAs) in eggs and meat were significantly higher (P<0.001) in ducks fed the FO and SO diets than in ducks fed the control diet. The level of C22:6 (n-3) FA in ducks fed the FO diet was significantly higher than that in ducks fed the other diets. However, the conversion efficiency of the longer-chain C20:5 (n-3) FA was higher than that of C22:6 (n-3). Ducks fed the PBO diet exhibited lipid droplet accumulation in the liver. These results demonstrate that a diet enriched with different FAs has strong effects on serum lipid levels and the deposition of PUFAs into tissue lipids.
Keywords: Duck, Liver, Egg, Meat, Fatty acid, Lipid oxidation
1. Introduction
The health benefits of polyunsaturated fatty acids (PUFAs) are being increasingly recognized. Thus, the consumption of PUFAs is now widely recommended to be increased in the human diet, along with PUFA n-6/n-3 and lineolic acid (LA)/α-linolenic acid (ALA) ratios being lowered to between 1 and 4 (ANC, 2001). However, the amounts of PUFAs, especially n-3 PUFAs, ingested by most subjects are lower than the recommended levels. The increasing awareness of the need for diets containing higher levels of n-3 PUFAs has focused on the importance of meats and eggs as natural suppliers of these fatty acids (FAs) (Gillingham et al., 2005; Bovet et al., 2007).
LA and ALA are members of two well-known classes of PUFAs, namely the n-6 (ω-6) and n-3 (ω-3) series, respectively. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which belong to the n-3 series of FA, are abundant in fish and shellfish. The composition of FAs stored in the muscle and egg largely reflects that of ingested lipids (Garcia-Rebollar et al., 2008; Guillevic et al., 2009a; Woods and Fearon, 2009). Thus, increasing the human intake of n-3 FAs can be achieved by enriching the traditionally consumed products such as meat with long chain PUFAs.
Studies have suggested that PUFAs not only reduce the levels of serum lipids but also alter membrane FA composition in humans and animals (Camara et al., 1996; Demirel et al., 2004; de Smet et al., 2004; Mach et al., 2006; Haak et al., 2008). However, a high concentration of PUFA makes membranes sensitive to peroxidative degradation. PUFAs become preferential targets for the action of free radicals that induce an oxidative stress. This type of stress is facilitated if an imbalance occurs between the respective amounts of PUFA and antioxidant systems. Recent studies have established that dietary lipids and nutrients play important roles in determining the strength of cellular antioxidative defense mechanisms (Reddy and Lokesh, 1994; Scislowski et al., 2005).
In avian species, lipogenesis takes place primarily in the liver, which accounts for 95% of de novo FA synthesis. In geese, overfeeding with a carbohydrate-rich diet results in a dramatic increase in hepatic lipogenesis and induces liver steatosis (Hermier et al., 1991; Mourot et al., 2006). Hepatic steatosis has a strong relationship with metabolic syndromes (Ueno et al., 1997; Deng et al., 2005; Tendler et al., 2007; Petta et al., 2009). Some studies have also demonstrated the effects of diets on liver steatosis in ducks (Hermier, 1997; Saez et al., 2010).
The aim of the present work was to study the effect of various dietary FAs on body and egg lipid compositions, serum parameters, and liver histological changes. Consequently, we expect to improve the physiological state of laying ducks, and the ability to produce eggs and meat that meet human nutritional demands.
2. Materials and methods
2.1. Animals and diets
A total of 264 Shaoxing laying ducks, at 17 weeks of age, were divided into four groups; each group was further divided into three subgroups with each having 22 ducks. The experimental ducks were fed different diets for 70 d. The control group was fed a basal diet (Table 1), the FO group was fed a diet with the addition of 30 g/kg fish oil, the SO group fed a diet with the addition of 25 g/kg sunflower oil, and the PBO group fed a diet with the addition of 30 g/kg palm oil and 20 g/kg beef tallow. All ducks had feed and water available ad libitum. At the end of the experiment, six eggs from each group were collected for lipid extraction and further measurement. Subsequently, all experimental ducks were fasted for 12 h with the exception of water being available ad libitum before sampling. The next morning, three ducks were randomly selected from each subgroup (i.e., 36 ducks in total), weighed, and slaughtered. Individual blood samples were taken and separated by centrifugation at 3 000×g for 15 min. Serum samples were frozen at −20 °C until assayed. The thigh muscles, pectoral muscles, and liver were quickly dissected out, placed into a plastic container, and stored at −20 °C until further analysis. All procedures followed established protocols approved by the Institutional Animal Care and Use Committees, Zhejiang Academy of Agricultural Sciences, China.
Table 1.
Composition and main characteristics of the basal diet
| Ingredient | Content (g/kg) | Nutrient | Content (g/kg) |
| Maize grain | 400 | Metabolizable energy | 11.20b |
| Wheat | 290 | Crude protein | 16.50 |
| Soybean meal | 120 | Total phosphorus | 0.70 |
| Wheat bran | 90 | Total calcium | 3.35 |
| Calcium hydrophosphate | 12 | Total lysine | 0.79 |
| Stone powder | 80 | Total methionine | 0.40 |
| Salt | 3 | Ether extract | 29.00 |
| Premixa | 5 |
Supplied per kg of diet: vitamin A 1 500 U, cholecalciferol 200 U, vitamin E (DL-α-tocopheryl acetate) 10 U, riboflavin 3.5 mg, pantothenic acid 10 mg, niacin 30 mg, cobalamin 10 μg, choline chloride 1 000 mg, biotin 0.15 mg, folic acid 0.5 mg, thiamine 1.5 mg, pyridoxine 3.0 mg, Fe 80 mg, Zn 40 mg, Mn 60 mg, I 0.18 mg, Cu 8 mg, Se 0.3 mg
Unit: MJ/kg
2.2. Measurement of serum and liver parameters
Total cholesterol (TC) and triglyceride (TG) were determined by cholesterol oxidase p-aminophenol (COD-PAP) and glycerol-3-phosphate oxidase p-aminophenol (GPO-PAP) methods, respectively. Serum high-density lipoprotein cholesterol (HDL-C) was determined by a precipitation method; the levels of superoxide dismutase (SOD) and malondialdehyde (MDA) were measured by the xanthine oxidase method and thiobarbituric acid (TBA) coloration, respectively. Commercial kits were purchased from Nanjing Jiancheng Bioengineering Institute, China.
2.3. Fatty acid analysis
The lipids were extracted from samples of the liver, muscle, egg, and oil using the chloroform/methanol procedure of Folch et al. (1957). FA composition was measured after methylation of samples (Table 2). Fatty acid methyl esters (FAMEs) were prepared with boron trifluoride methanol according to Morrissson and Smith (1964), and analyzed by using a gas chromatography (6890; Agilent, Santa Clara, CA, USA) on an HP-5MS column (30 m×0.25 mm×0.2 µm; Agilent). Helium was used as carrier gas, and the temperature was increased from 80 to 280 °C at 20–35 °C/min. The injector and detector temperatures were maintained at 220 and 280 °C, respectively. Retention time and peak area were determined using chromatography software (Agilent ChemStation B.02.01). Methyl heptadecanoate (No. 51633, Fluka, USA) was dissolved into n-hexane as an internal standard to quantify individual and total FAs.
Table 2.
Fatty acid profile of experimental diets
| Fatty acid | Content (g/100 g total fatty acids) |
|||
| Control | FO | SO | PBO | |
| SFA | 21.52 | 27.81 | 18.71 | 38.55 |
| MUFA | 29.08 | 29.88 | 22.15 | 35.17 |
| PUFA | 49.40 | 42.30 | 59.14 | 26.28 |
| C18:3 (n-3) | 6.12 | 4.90 | 3.93 | 2.58 |
| C20:5 (n-3) | 0.98 | 5.91 | 0.59 | 0.82 |
| C22:5 (n-3) | 0.65 | 0.97 | 0.39 | 0.64 |
| C22:6 (n-3) | 0.23 | 7.76 | 0.14 | 0.60 |
| ∑n-3 | 7.98 | 19.53 | 5.05 | 4.63 |
| C18:2 (n-6) | 40.90 | 21.13 | 53.20 | 20.84 |
| C20:2 (n-6) | 0.00 | 0.34 | 0.27 | 0.12 |
| C20:4 (n-6) | 0.45 | 0.78 | 0.26 | 0.41 |
| C22:3 (n-6) | 0.07 | 0.52 | 0.36 | 0.27 |
| ∑n-6 | 41.42 | 22.77 | 54.09 | 21.65 |
| PUFA/SFA | 2.30 | 1.52 | 3.16 | 0.68 |
| ∑n-6/∑n-3 | 5.19 | 1.17 | 10.72 | 4.68 |
FO: fish oil diet; SO: sunflower oil diet; PBO: palm oil and beef tallow diet; SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; ∑n-3: sum of (n-3) fatty acids; ∑n-6: sum of (n-6) fatty acids
2.4. Histological studies
At the end of the experiment, nine ducks were randomly selected from each group and the liver was removed and fixed in a 0.1 g/ml paraformaldehyde buffered solution for 24 h, then embedded in paraffin and sectioned at 6–8 μm. Sections were stained with hematoxylin-eosin (HE) and evaluated under a microscope (Olympus, Tokyo, Japan).
2.5. Statistical analysis
Data are shown as mean±standard error (SE) for each treatment. All data were tested with one-way analysis of variance (ANOVA) and Tukey’s post-hoc test using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to be statistically significant.
3. Results
3.1. Serum parameters and lipid peroxidation
In the current experiment, the TG and TC levels were significantly lower (P<0.05) in ducks fed the FO and SO diets than in ducks fed the control and PBO diets. The serum HDL-C concentration was higher in ducks fed the FO and SO diets than in ducks fed the other diets (Table 3).
Table 3.
Effect of dietary fatty acids on biochemical indicators in serum from 27 weeks old female ducks
| Diet | TG (mmol/L) | TC (mmol/L) | HDL (mmol/L) | SOD (U/ml) | MDA (nmol/ml) |
| Control | 22.13±1.12b | 6.50±0.81b | 1.22±0.45b | 75.30±5.83b | 14.74±1.13b |
| FO | 18.95±1.53c | 5.75±0.92c | 1.60±0.22a | 91.27±4.68a | 17.43±1.43b |
| SO | 19.76±1.26c | 6.14±1.23bc | 1.55±0.30ab | 94.56±7.97a | 16.97±1.78b |
| PBO | 27.50±2.34a | 7.60±0.84a | 1.24±0.67b | 100.20±8.55a | 22.80±3.23a |
FO: fish oil diet; SO: sunflower oil diet; PBO: palm oil and beef tallow diet; TG: triglyceride; TC: total cholesterol; HDL: high density lipoprotein; SOD: superoxide dismutase; MDA: malondialdehyde. Values are expressed as mean±SE (n=9). Values in a column not sharing the same superscripts are significantly different (P<0.05)
The results for lipid peroxidation (measured as MDA values) are shown in Table 3. MDA was significantly lower (P<0.05) in blood of ducks fed the control diet than in that of ducks fed the PBO diet, but no significant difference was observed between the FO and SO diets (P>0.05). The SOD activity for the control diet was lower (P<0.05) than that for the other diets.
3.2. Fatty acid composition and proportion
No significant effect of diet was seen on the proportions of saturated fatty acid (SFA), except that the SFA content was significantly different in eggs (P<0.001). The content of PUFA in muscles and eggs was significantly different in the four groups (P<0.001). SFA was more abundant in the leg muscles, while monounsaturated fatty acid (MUFA) was more abundant in the chest muscles and PUFA was more abundant in eggs. With the FO and SO diets, the leg muscle EPA and DHA contents were significantly (P<0.05) higher than with the control diet. However, with the PBO diet, the EPA and DHA levels were significantly lower than with the control diet (P<0.05). Likewise, findings in the chest and leg muscles were similar for the ∑n-6/∑n-3 ratio; however, in all the diets the ratio was below 4 (Tables 4 and 5).
Table 4.
Effect of dietary fatty acids on the fatty acid composition of leg muscles from 27 weeks old female ducks
| Fatty acid | Content (g/100 g total fatty acids) |
SE | P | |||
| Con. | FO | SO | PBO | |||
| EE | 2.34c | 2.41b | 2.39b | 2.43a | 0.04 | ** |
| SFA | 52.60 | 51.30 | 50.66 | 54.21 | 0.47 | NS |
| MUFA | 24.23b | 25.45a | 25.89a | 25.10a | 0.32 | * |
| PUFA | 22.57b | 23.25a | 23.45a | 20.69c | 0.33 | *** |
| C18:3 (n-3) | 0.71 | 0.60 | 0.63 | 0.65 | 0.05 | NS |
| C20:5 (n-3) | 2.89c | 3.17a | 3.02b | 2.41d | 0.03 | *** |
| C22:5 (n-3) | 0.87b | 1.05a | 0.95ab | 0.65c | 0.05 | * |
| C22:6 (n-3) | 0.87b | 1.01a | 0.91bc | 0.56d | 0.03 | ** |
| ∑n-3 | 5.34b | 5.83a | 5.51ab | 4.27c | 0.12 | ** |
| C18:2 (n-6) | 15.96ab | 16.26a | 16.50a | 15.54b | 0.22 | * |
| C20:2 (n-6) | 0.22 | 0.18 | 0.19 | 0.21 | 0.01 | NS |
| C20:4 (n-6) | 0.71b | 0.64c | 0.84a | 0.42d | 0.01 | ** |
| C22:3 (n-6) | 0.19a | 0.12b | 0.23a | 0.13b | 0.02 | * |
| t10c12CLA | 0.15ab | 0.22a | 0.18a | 0.12b | 0.01 | * |
| ∑n-6 | 17.23b | 17.42ab | 17.94a | 16.42c | 0.18 | ** |
| PUFA/SFA | 0.43ab | 0.45a | 0.46a | 0.39b | 0.02 | ** |
| ∑n-6/∑n-3 | 3.24c | 2.99b | 3.25c | 3.84a | 0.08 | ** |
EE: ether extract; SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; ∑n-3: sum of (n-3) fatty acids; ∑n-6: sum of (n-6) fatty acids; FO: fish oil diet; SO: sunflower oil diet; PBO: palm oil and beef tallow diet; SE: standard error
P<0.05
P<0.01
P<0.001
NS: no significant difference (P>0.05). Values (mean, n=9) in a row not sharing the same superscripts are significantly different (P<0.05)
Table 5.
Effect of dietary fatty acids on the fatty acid composition of chest muscles from 27 weeks old female ducks
| Fatty acid | Content (g/100 g total fatty acids) |
SE | P | |||
| Con. | PO | SO | PBO | |||
| EE | 2.14d | 2.32b | 2.21c | 2.37a | 0.06 | *** |
| SFA | 48.72 | 47.44 | 48.14 | 50.61 | 0.31 | NS |
| MUFA | 28.45 | 28.17 | 28.49 | 28.18 | 0.16 | NS |
| PUFA | 22.78b | 23.39b | 23.37a | 21.12c | 0.29 | *** |
| C18:3 (n-3) | 0.74a | 0.61b | 0.62b | 0.63b | 0.03 | ** |
| C20:5 (n-3) | 2.92b | 3.06a | 2.99ab | 2.31c | 0.03 | *** |
| C22:5 (n-3) | 0.91b | 1.03a | 0.94b | 0.66c | 0.02 | *** |
| C22:6 (n-3) | 0.79c | 1.01a | 0.88b | 0.65d | 0.02 | *** |
| ∑n-3 | 5.36b | 5.71a | 5.43b | 4.25c | 0.16 | *** |
| C18:2 (n-6) | 15.58b | 16.52a | 16.50a | 15.64b | 0.21 | * |
| C20:2 (n-6) | 0.31a | 0.21b | 0.18b | 0.19b | 0.01 | *** |
| C20:4 (n-6) | 0.73b | 0.64c | 0.81a | 0.52d | 0.02 | *** |
| C22:3 (n-6) | 0.20a | 0.13b | 0.22a | 0.12b | 0.01 | ** |
| t10c12CLA | 0.16bc | 0.18b | 0.23a | 0.12c | 0.09 | *** |
| ∑n-6 | 16.98b | 17.68a | 17.94a | 16.59b | 0.22 | ** |
| PUFA/SFA | 0.47a | 0.49a | 0.48a | 0.42b | 0.02 | ** |
| ∑n-6/∑n-3 | 3.17c | 3.10c | 3.30b | 3.90a | 0.12 | *** |
EE: ether extract; SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; ∑n-3: sum of (n-3) fatty acids; ∑n-6: sum of (n-6) fatty acids; FO: fish oil diet; SO: sunflower oil diet; PBO: palm oil and beef tallow diet; SE: standard error.
P<0.05
P<0.01
P<0.001
NS: no significant difference (P>0.05). Values (mean, n=9) in a row not sharing the same superscripts are significantly different (P<0.05)
Unsaturated FAs were more abundant in eggs than in muscles, and the PUFA/SFA ratio was greater than 1.5 in eggs, which was much higher than that in muscles. The ∑n-6/∑n-3 ratios of the FO and SO groups decreased and that of the PBO group increased, but only the FO group had a ratio lower than 4. In eggs, the EPA and DHA contents and proportions were higher (P<0.05) in ducks fed the FO and SO diets than in ducks fed the control diet. However, the EPA and DHA contents and proportions were not affected by the PBO diet (Table 6).
Table 6.
Effect of dietary fatty acids on the fatty acid composition of eggs from 27 weeks old female ducks
| Fatty acid | Content (g/100 g total fatty acids) |
SE | P | |||
| Con. | FO | SO | PBO | |||
| SFA | 22.87a | 20.44b | 20.36b | 22.75a | 0.32 | *** |
| MUFA | 41.67 | 41.79 | 42.76 | 41.97 | 0.46 | NS |
| PUFA | 35.39b | 37.68a | 36.88a | 35.28b | 0.38 | *** |
| C18:3 (n-3) | 2.35a | 1.91b | 1.80b | 1.86b | 0.07 | ** |
| C20:5 (n-3) | 1.03b | 1.81a | 1.72a | 1.09b | 0.13 | *** |
| C22:5 (n-3) | 0.11b | 0.16a | 0.14b | 0.05c | 0.01 | *** |
| C22:6 (n-3) | 2.89c | 4.23a | 3.61bc | 3.11c | 0.18 | *** |
| ∑n-3 | 6.38a | 8.11b | 7.27c | 6.11 a | 0.16 | *** |
| C18:2 (n-6) | 27.72 | 28.11 | 28.03 | 28.16 | 0.49 | NS |
| C20:4 (n-6) | 1.21c | 1.43b | 1.55a | 1.01d | 0.02 | *** |
| C22:3 (n-6) | 0.03a | 0.03a | 0.03a | 0.02b | 0.005 | ** |
| ∑n-6 | 28.96 | 29.57 | 29.61 | 29.19 | 0.25 | NS |
| PUFA/SFA | 1.55b | 1.84a | 1.81a | 1.55b | 0.19 | ** |
| ∑n-6/∑n-3 | 4.53ab | 3.64b | 4.07b | 4.79a | 0.12 | ** |
SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; ∑n-3: sum of (n-3) fatty acids; ∑n-6: sum of (n-6) fatty acids; FO: fish oil diet; SO: sunflower oil diet; PBO: palm oil and beef tallow diet; SE: standard error.* P<0.05
P<0.01
P<0.001
NS: no significant difference (P>0.05). Values (mean, n=6) in a row not sharing the same superscripts are significantly different (P<0.05)
3.3. Histological examination
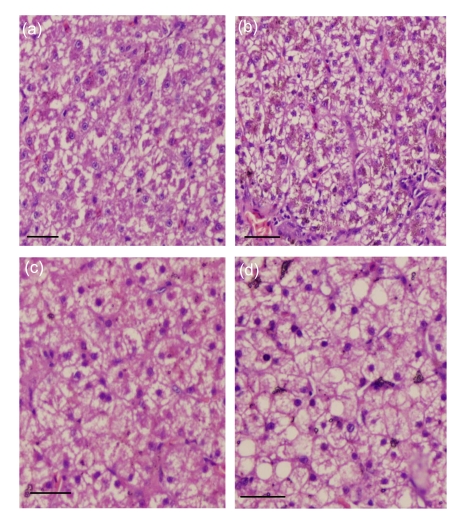
The livers from ducks fed the SO diet had the same histological characteristics as those fed the control diet. Hepatocytes showed large and spherical nuclei that were centrally located, with a prominent nucleolus and a moderate eosinophilic cytoplasm (Figs. 1a and 1c). Ducks fed the FO diet had nuclei that were homogenous in shape and chromatin density, but had migrated to the cell periphery (Fig. 1b). In contrast, ducks fed the PBO diet had larger lipid droplets and foci of swelling hepatocytes, with enlarged irregular nuclei located at the periphery of the cell (Fig. 1d).
Fig. 1.
Representative microphotographs of a 6 µm-thick section of duck liver stained with hematoxylin-eosin (HE)
Ducks were fed a control diet (a), a 3% (w/w) FO diet (b), a 2.5% (w/w) SO diet (c), or a 5% (w/w) PBO diet (d). In (d), note the swollen hepatocytes with enlarged irregular nuclei located at the periphery of the cell. Bar=60 mm
4. Discussion
Diets containing PUFAs have been reported to result in decreased TC, TG, and low-density lipoprotein cholesterol (LDL-C), but increase the beneficial HDL-C (Schaefer et al., 1981; Sunitha et al., 1997; Ortiz-Munoz et al., 2009). In the present study, the FO and SO diets enhanced the activity of the antioxidant enzyme SOD compared to the control diet, which is consistent with the previous reports (Prasad, 1997; Kratz et al., 2002). MDA levels in serum did not change significantly after dietary treatments in ducks, except for when they were fed the PBO diet, which indicated that PUFAs induced an increase in the activities of antioxidant enzymes and a decrease in lipid peroxidation (Yang et al., 2008). However, the MDA level increased significantly in ducks fed the PBO diet. It is possible that TG level in serum was higher in ducks fed the PBO diet than in ducks fed the other diets, which could then induce a higher production of MDA.
Recent studies have clearly established that dietary lipids and nutrients play important roles in determining the strength of cellular antioxidative defense mechanisms (Chow, 1979; Avula and Fernandes, 1999). Antioxidants constitute a major cell defense against acute oxygen toxicity and protect membrane components against damage caused by free radicals.
In the PBO group, the high serum lipid was concluded to be the major cause of liver enriched with lipids, and will cause liver injury (Abdeen et al., 2006; Liu et al., 2006). In addition, the MDA level in the serum was higher due to the high serum lipid concentration. Thus, a liver enriched with lipids is believed to be closely related to blood lipid metabolism and lipoperoxidation.
In our experiments, the dietary treatment changed the concentrations of n-3 and n-6 PUFAs in duck meat and eggs, confirming previous results reported by Abraham et al. (1983), Raes et al. (2004), Zhao et al. (2008), and Woods and Fearon (2009). The increased n-6 PUFA concentration in meat was obtained by using an SO or FO diet, the increased n-3 PUFA concentration was achieved by using an FO diet, and the SFA concentration was increased with a PBO diet. The current findings are consistent with previous reports (Cachaldora et al., 2008; Garcia-Rebollar et al., 2008; Guillevic et al., 2009b).
In eggs, the FO diet resulted in significantly higher EPA and DHA concentrations, which is consistent with Cachaldora et al. (2008)’s results. They fed laying hens diets containing 0, 15, or 30 g FO/kg, resulting in significantly higher EPA and DHA concentrations in egg yolk (increased by 0.11%, 1.03% and 2.44%, and 8.96%, 29.7% and 41.3%, respectively).
Conversion of ALA to n-3 PUFA such as EPA and DHA requires elongase and desaturase, which also use n-6 PUFA as a substrate, in both animals and humans (Woods and Fearon, 2009). Other possible reasons may explain the limited accumulation of long-chain FAs observed. For example, longer-chain FAs can compete with, or be displaced by, increased C18:3 (n-3) in the phospholipid fraction, which could prevent them from accumulating. No information is available regarding the differences among meat-producing animals in the activities of the Δ5 and Δ6 desaturases that are responsible for desaturation during the synthesis of longer-chain PUFAs (C20–C24) from dietary LA and ALA (de Smet et al., 2004).
The retention of FAs appears to be tissue-specific. The retention efficiency of FAs in the muscles is closely correlated with lipogenic enzyme activity (Kouba et al., 2003). Unexpectedly, C18:3 (n-3) in the FO and SO groups was lower than that in the control group, which differs from previous results (Bovet et al., 2007; Guillevic et al., 2009b). One possible reason for the decrease is that a great quantity of C18:3 (n-3) transformed into a longer-chain PUFA. Another explanation could be β-oxidation. A larger sample size would allow a more rigorous investigation of the reason for this effect.
The liver plays a crucial role in controlling FA and TG metabolism by synthesizing, storing, secreting, and oxidizing free fatty acids (FFAs). The influx of plasma FFAs from adipose tissue to the liver accounts for about 60%–80% of intrahepatic fat (Donnelly et al., 2005). In all animal species, hepatic steatosis results from an imbalance between synthesis and the secretion and storage of TG by the liver (Alpers et al., 1993). Ducks fed the PBO diet had increased deposition of dietary fat in hepatocytes; the excessive dietary intake of lipids saturated the physiological capability of the liver, leading to lipid droplet accumulation in the liver. Similar hepatic steatosis due to excessive caloric intake has been reported in several studies (Spisni et al., 1998; Caballero et al., 1999; Cazeils et al., 1999; Hesham A-Kader et al., 2009).
Nuclear displacement to the hepatocyte periphery in the livers from ducks fed the FO diet, and the larger lipid droplets seen in this study (Fig. 1b), may be a reflection of liver adaptation to increased dietary lipid content. It can be considered an indicator of a well-fed status rather than a nutritional disorder.
5. Conclusions
In the present study, the FO and SO diets modified the TG and TC concentrations in sera. The level of lipid peroxidation (MDA) remarkably increased in the PBO group. Ducks fed a 3% FO diet had a lower ∑n-6/∑n-3 ratio, laid eggs richer in n-3 PUFAs, and produced meat more suitable for human health needs. In the 5% PBO group, the excessive dietary intake of lipids led to lipid droplet accumulation. Therefore, in the PBO group, the lipid content in diet should be then reduced in a further study.
Acknowledgments
The authors thank Dr. Bo-jing LIU, Institute of Feed Science, Zhejiang University, China for her technical assistance.
Footnotes
Project supported by the Key Technology Project of Zhejiang Province (No. 2007C14023) and the Earmarked Fund for Modern Agro-industry Technology Research System of China (No. nycytx-45-02)
References
- 1.Abdeen MB, Chowdhury NA, Hayden MR, Ibdah JA. Nonalcoholic steatohepatitis and the cardiometabolic syndrome. J Cardiometab Syndr. 2006;1(1):36–40. doi: 10.1111/j.0197-3118.2006.05523.x. [DOI] [PubMed] [Google Scholar]
- 2.Abraham S, Hillyard LA, Lin CY, Schwartz RS. Effect of specific dietary fatty acids on lipogenesis in the livers and mammary glands of lactating mice. Lipids. 1983;18(11):820–829. doi: 10.1007/BF02534642. [DOI] [PubMed] [Google Scholar]
- 3.Alpers DH, Sabesin SM, White HM. Fatty Liver: Biochemical and Clinical Aspects. In: Schiff L, Schiff ER, editors. Diseases of Liver. II. Philadelphia: J.B. Lippincott; 1993. pp. 825–855. [Google Scholar]
- 4.ANC. Apports Nutritionnels Conseillés Pour la Population Française. Paris: CNRS/CNERNA/AFSSA, Tec et Doc Lavoisier; 2001. p. 605. (in French) [Google Scholar]
- 5.Avula CP, Fernandes G. Modulation of antioxidant enzymes and apoptosis in mice by dietary lipids and treadmill exercise. J Clin Immunol. 1999;19(1):35–44. doi: 10.1023/A:1020562518071. [DOI] [PubMed] [Google Scholar]
- 6.Bovet P, Faehb D, Madeleine G, Viswanathan B, Paccaud F. Decrease in blood triglycerides associated with the consumption of eggs of hens fed with food supplemented with fish oil. Nutr Metat Cardiovas. 2007;17(4):280–287. doi: 10.1016/j.numecd.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Caballero MJ, Lopez-Calero G, Socorro J, Roo FJ, Izquierdo MS, Fernandez AJ. Combined effect of lipid level and fish meal quality on liver histology of gilthead seabream. Aquaculture. 1999;179(1-4):277–290. doi: 10.1016/S0044-8486(99)00165-9. [DOI] [Google Scholar]
- 8.Cachaldora P, Garcia-Rebollar P, Alvarez C, de Blas JC, Mendez J. Effect of type and level of basal fat and level of fish oil supplementation on yolk fat composition and n-3 fatty acids deposition efficiency in laying hens. Anim Feed Sci Tech. 2008;141(1-2):104–114. doi: 10.1016/j.anifeedsci.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Camara M, Mourot J, Février C. Influence of two dairy fats on lipid synthesis in the pig: comparative study of liver, muscle and the two backfat layers. Ann Nutr Metab. 1996;40(5):287–295. doi: 10.1159/000177970. [DOI] [PubMed] [Google Scholar]
- 10.Cazeils JL, Bouillier-Oudot M, Auvergne A, Candau M, Babile R. Lipid composition of hepatocyte plasma membranes from geese overfed with corn. Lipids. 1999;34(9):937–942. doi: 10.1007/s11745-999-0443-z. [DOI] [PubMed] [Google Scholar]
- 11.Chow CK. Nutritional influence on cellular antioxidant defense systems. Am J Clin Nutr. 1979;32(5):1066–1081. doi: 10.1093/ajcn/32.5.1066. [DOI] [PubMed] [Google Scholar]
- 12.Demirel G, Wachira AM, Sinclair LA, Wilkinson RG, Wood JD, Eraser M. Effects of dietary n-3 polyunsaturated fatty acids, breed and dietary vitamin E on the fatty acids of lamb muscle, liver and adipose tissue. Brit J Nutr. 2004;91(4):551–565. doi: 10.1079/BJN20031079. [DOI] [PubMed] [Google Scholar]
- 13.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42(4):905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 14.de Smet S, Raes K, Demeyer D. Meat fatty acid composition as affected by fatness and genetic factors: a review. Anim Res. 2004;53(2):81–89. doi: 10.1051/animres:2004003. [DOI] [Google Scholar]
- 15.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folch J, Lees M, Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 17.Garcia-Rebollar P, Cachaldora P, Alvarez C, de Blas C, Mendez J. Effect of the combined supplementation of diets with increasing levels of fish and linseed oils on yolk fat composition and sensorial quality of eggs in laying hens. Anim Feed Sci Tech. 2008;140(3):337–348. doi: 10.1016/j.anifeedsci.2007.03.006. [DOI] [Google Scholar]
- 18.Gillingham LG, Caston L, Leeson S, Hourtovenko K, Holub BJ. The effects of consuming docosahexaenoic acid (DHA)-enriched eggs on serum lipids and fatty acid compositions in statin-treated hypercholesterolemic male patients. Food Res Int. 2005;38(10):1117–1123. doi: 10.1016/j.foodres.2005.03.006. [DOI] [Google Scholar]
- 19.Guillevic M, Kouba M, Mourot J. Effect of a linseed diet on lipid composition, lipid peroxidation and consumer evaluation of French fresh and cooked pork meats. Meat Sci. 2009;81(4):612–618. doi: 10.1016/j.meatsci.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Guillevic M, Kouba M, Mourot J. Effect of a linseed diet or a sunflower diet on performances, fatty acid composition, lipogenic enzyme activities and stearoyl-CoA-desaturase activity in the pig. Livest Sci. 2009;124(1-3):288–294. doi: 10.1016/j.livsci.2009.02.009. [DOI] [Google Scholar]
- 21.Haak L, de Smeet A, Fremaut D, van Walleghem K, Raes K. Fatty acid profile and oxidative stability of pork influence by duration and time of dietary linseed or fish oil supplementation. J Anim Sci. 2008;86(6):1418–1425. doi: 10.2527/jas.2007-0032. [DOI] [PubMed] [Google Scholar]
- 22.Hermier D. Lipoprotein metabolism and fattening in poultry. J Nutr. 1997;127(5):805–808. doi: 10.1093/jn/127.5.805S. [DOI] [PubMed] [Google Scholar]
- 23.Hermier D, Saadoun A, Salichon M, Sellier N, Rousselot-Paillet D, Chapman MJ. Plasma lipoproteins and liver lipids in two breeds of geese with different susceptibility to hepatic steatosis: changes induced by development and force-feeding. Lipids. 1991;26(5):331–339. doi: 10.1007/BF02537194. [DOI] [PubMed] [Google Scholar]
- 24.Hesham A-Kader H. Nonalcoholic fatty liver disease in children living in the obeseogenic society. Word J Pediatr. 2009;5(4):245–254. doi: 10.1007/s12519-009-0048-8. [DOI] [PubMed] [Google Scholar]
- 25.Kouba M, Enser M, Whittington FM, Nute GR, Wood JD. Effect of a high-linolenic acid diet on lipogenic enzyme activities, fatty acid composition, and meat quality in the growing pig. J Anim Sci. 2003;81(8):1967–1979. doi: 10.2527/2003.8181967x. [DOI] [PubMed] [Google Scholar]
- 26.Kratz M, Cullen P, Kannenberg F, Kassner A, Fobker M, Abuja PM, Assmann G, Wahrburg U. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur J Clin Nutr. 2002;56(1):72–81. doi: 10.1038/sj.ejcn.1601288. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Lu XC, Ge J, Wang YW. The dynamic research of establishing fatty liver rats induced by high-fat diet. Chin Pharmacol Bull. 2006;22(11):1399–1403. (in Chinese) [Google Scholar]
- 28.Mach N, Devant M, Díaz I, Font-Furnols M, Oliver MA, García JA, Bach A. Increasing the amount of n-3 fatty acids in meat from young Holstein bulls through nutrition. J Anim Sci. 2006;84(11):3039–3048. doi: 10.2527/jas.2005-632. [DOI] [PubMed] [Google Scholar]
- 29.Morrissson WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 30.Mourot J, Guy G, Peiniau P, Hermier D. Effects of overfeeding on lipid synthesis, transport and storage in two breeds of geese differing in their capacity for fatty liver production. Anim Res. 2006;55(5):427–442. doi: 10.1051/animres:2006027. [DOI] [Google Scholar]
- 31.Ortiz-Munoz G, Houard X, Martin-Ventura JL, Ishida BY, Loyau S, Rossignol P, Moreno JA, Kane JP, Chalkley RJ, Burlingame AL, et al. HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J. 2009;23(9):3129–3139. doi: 10.1096/fj.08-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petta S, Muratore C, Craxì A. Non-alcoholic fatty liver disease pathogenesis: the present and the future. Digest Liver Dis. 2009;41(9):615–625. doi: 10.1016/j.dld.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Prasad K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flaxseed. Mol Cell Biochem. 1997;168(1-2):117–123. doi: 10.1023/A:1006847310741. [DOI] [PubMed] [Google Scholar]
- 34.Raes K, de Smet S, Demeyer D. Effect of dietary fatty acids on incorporation of long chain polyunsaturated fatty acids and conjugated linoleic acid in lamb, beef and pork meat: a review. Anim Feed Sci Tech. 2004;113(1-4):199–221. doi: 10.1016/j.anifeedsci.2003.09.001. [DOI] [Google Scholar]
- 35.Reddy CP, Lokesh BR. Dietary unsaturated fatty acids vitamin E, curcumin and eugenol alter serum, and liver lipid peroxidation in rats. Nutr Res. 1994;14(9):1423–1437. doi: 10.1016/S0271-5317(05)80301-X. [DOI] [Google Scholar]
- 36.Saez G, Baéza E, Bernadet MD, Davail S. Is there a relationship between the kinetics of lipoprotein lipase activity after a meal and the susceptibility to hepatic steatosis development in ducks? Poult Sci. 2010;89(11):2453–2460. doi: 10.3382/ps.2010-00683. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer EJ, Levy RI, Ernst ND, van Sant FD, Brewer HB. The effect of low cholesterol, high polyunsaturated fat and low fat diets on plasma lipid and lipoprotein cholesterol levels in normal and hypocholesterolemic subjects. Am J Clin Nutr. 1981;34(9):1758–1763. doi: 10.1093/ajcn/34.9.1758. [DOI] [PubMed] [Google Scholar]
- 38.Scislowski V, Bauchart D, Gruffat D, Laplaud PM, Durand D. Effect of dietary n-6 and n-3 polyunsaturated fatty acids on peroxidizability of lipoproteins in steers. Lipids. 2005;40(12):1245–1256. doi: 10.1007/s11745-005-1492-z. [DOI] [PubMed] [Google Scholar]
- 39.Spisni E, Tugnoli M, Ponticelli A, Mordenti T, Tomasi V. Hepatic steatosis in artificially fed marine teleosts. J Fish Dis. 1998;21(3):177–184. doi: 10.1111/j.1365-2761.1998.00089.x. [DOI] [PubMed] [Google Scholar]
- 40.Sunitha T, Manoram A, Rukmini C. Lipid profile of rats fed blends of rice bran oil in combination with sunflower and safflower oil. Plant Foods Hum Nutr. 1997;51(3):219–230. doi: 10.1023/A1007993016796. [DOI] [PubMed] [Google Scholar]
- 41.Tendler D, Lin S, Yancy JS, Mavropoulos J, Sylvestre P, Rockey DC. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52(2):589–593. doi: 10.1007/s10620-006-9433-5. [DOI] [PubMed] [Google Scholar]
- 42.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27(1):103–107. doi: 10.1016/S0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 43.Woods VB, Fearon AM. Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: a review. Livest Sci. 2009;126(1-3):1–20. doi: 10.1016/j.livsci.2009.07.002. [DOI] [Google Scholar]
- 44.Yang XJ, He X, He LX, Liu YX, Yang Y, Guo YM. The effect of PUFA on antioxidation parameter of broiler chickens. Chin J Anim Nutr. 2008;20(3):299–304. (in Chinese) [Google Scholar]
- 45.Zhao ZY, Wu TX, Tang HG, Zhang JZ. Influence of dietary conjugated linoleic acid on growth, fatty acid composition and hepatic lipogenesis in large yellow croaker (Pseudosciaena crocea R.) J Zhejiang Univ-Sci B. 2008;9(9):691–700. doi: 10.1631/jzus.B0820181. [DOI] [PMC free article] [PubMed] [Google Scholar]