Abstract
To address the role of β1 integrins in pancreatic cancer progression, we stably knocked down β1 integrin subunit expression in human FG-RFP pancreatic cancer cells using lentiviral-based RNA interference. We then examined the effects of β1 integrin subunit knockdown on pancreatic cancer cell adhesion, migration, and proliferation on tumor microenvironment-specific ECM proteins in vitro, and on tumor progression in vivo using a clinically-relevant fluorescent orthotopic mouse model of pancreatic cancer. Knockdown of the β1 integrin subunit inhibited cell adhesion, migration, and proliferation on types I and IV collagen, fibronectin, and laminin in vitro. In vivo, knockdown of the β1 integrin subunit reduced primary tumor growth by 50% and completely inhibited spontaneously occurring metastasis. These observations indicate a critical role for the β1 integrin subunit in pancreatic cancer progression, and metastasis in particular. Our results suggest the β1 integrin subunit as a therapeutic target for the treatment of pancreatic cancer, especially in the adjuvant setting to prevent metastasis of this highly aggressive cancer.
Keywords: integrins, extracellular matrix, pancreatic cancer, orthotopic mouse models, RNA interference
INTRODUCTION
Pancreatic cancer is characterized by a desmoplastic response 1 that includes transforming growth factor β1-mediated up-regulation of the extracellular matrix (ECM) by surrounding stellate cells 1–3. Integrins, the family of heterodimeric transmembrane receptors that mediate cellular interactions with the ECM, regulate a variety of tumor cell functions, including adhesion, migration, invasion, proliferation, and survival 2, 4–10. The integrin family consists of 18 α subunits and 8 β subunits that assemble as non-covalently linked heterodimers, forming 24 different integrins 6. The β1 integrin subunit, in particular, forms heterodimers with at least 6 different α subunits in human pancreatic cancer and pancreatic cancer cell lines, including α1, α2, α3, α5, α6, and αv2.
Our previously published studies, along with other independent reports, provide substantial in vitro evidence that β1 integrin-mediated tumor cell interactions with tumor microenvironment-specific ECM proteins, including types I and IV collagen, fibronectin, and laminin, regulate the malignant phenotype in pancreatic cancer 2, 11–19. Additionally, in an experimental metastasis model, pre-incubation of human PaTu 8988s pancreatic cancer cells with function-blocking monoclonal antibodies directed against the β1 integrin subunit decreased lung colony formation four weeks after tail vein injection into nude mice 20.
In the present report, we further investigated the role of β1 integrins in the regulation of the malignant phenotype in pancreatic cancer utilizing shRNA-mediated stable suppression of the β1 integrin subunit in human FG-RFP pancreatic cancer cells. Functional effects of β1 integrin subunit knockdown were examined in cell adhesion, migration, and proliferation assays on tumor microenvironment-specific ECM proteins in vitro. Tumor growth and metastasis were assessed in vivo using a physiologically relevant orthotopic mouse model. These data identify the β1 integrin subunit as essential for tumor progression, and metastasis in particular, in pancreatic cancer.
MATERIALS AND METHODS
Cell Culture
FG cells are a fast-growing (FG), metastatic variant of the Colo-357 cell line 21, kindly provided by Dr. Steve Silletti (Moores Cancer Center, University of California, San Diego), and engineered to express red fluorescent protein (RFP) by methods previously described 22, 23. Cells were cultured in DMEM supplemented with 10% FBS in a humidified atmosphere containing 95% air and 5% CO2 at 37°C. Stable knockdowns were cultured in media supplemented with 0.2 μg/ml puromycin (Sigma-Aldrich, St. Louis, MO).
Suppression of α2, α3 and β1 integrin subunit expression using RNA interference
For long-term silencing of α2, α3 and β1 integrin subunit gene expression in FG-RFP cells, FG-RFP cells were infected with shRNA lentiviral particles targeted to specific integrin subunit mRNAs (five different shRNA insert sequences were investigated for each integrin subunit) according to manufacturer’s instructions (Sigma-Aldrich). The specific shRNA insert sequences for each integrin subunit resulting in the greatest knockdown at the mRNA level, and used for subsequent clonal selection in functional studies in vitro and in vivo, were as follows: For the non-target control – CCG GCA ACA AGA TGA AGA GCA CCA ACT CGA GTT GGT GCT CTT CAT CTT GTT GTT TTT; for the human α2 integrin subunit – CCG GCC GGC CAG ATA GTG CTA TAT ACT CGA GTA TAT AGC ACT ATC TGG CCG GTT TTT; for the human α3 integrin subunit – CCG GCG GAT GAA CAT CAC AGT GAA ACT CGA GTT TCA CTG TGA TGT TCA TCC GTT TTT; for the human β1 integrin subunit – CCG GGC CTT GCA TTA CTG CTG ATA TCT CGA GAT ATC AGC AGT AAT GCA AGG CTT TTT.
Reverse Transcriptase-quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was prepared using an RNeasy kit and then treated with RNase-Free DNase to remove any genomic DNA contamination according to manufacturer’s instructions (Qiagen, Valencia, CA). The RT-qPCR reactions were carried out using Power SYBR® Green RT-PCR Mix (Applied Biosystems, Foster City, CA) in 2-step reactions. Briefly, cDNAs were generated from 100 ng total RNA using MultiScribe Reverse Transcriptase. PCR amplification was performed using a Stratagene Mx3000P Real-time PCR System and 100 nM specific target primer sets (Real Time Primers, LLC, Elkins Park, PA). Primers used were as follows: For the human α2 integrin subunit – Forward 5′-ATG CAG ATG GAC CAC ACT TT -3′, Reverse 5′-AAG CAT CAC TGC TGA ACT CC -3′; For the human α3 integrin subunit – Forward 5′-ATG GTA AAT CAC CGG CTA CA -3′, Reverse 5′-GTG ATC TCC GTG GGA TAC AG -3′; For the human β1 integrin subunit – Forward 5′-GGA TTC TCC AGA AGG TGG TT -3′, Reverse 5′-GGT AAA ACA ATG CCA CCA AG -3′; For GAPDH – Forward 5′-GAG TCA ACG GAT TTG GTC GT -3′, Reverse 5′-TTG ATT TTG GAG GGA TCT CG -3′.
ECM Proteins
Human fibronectin and vitronectin, bovine types I and IV collagen, and mouse laminin were purchased from Millipore Inc. (Temecula, CA).
Antibodies
The monoclonal antibodies for the particular integrin subunits/integrins used in these studies, including FB12 (α1), P1E6 (α2), P1B5 (α3), P1H4 (α4), GoH3 (α6), P5D2 (β1), LM609 (αvβ3), P1F6 (αvβ5), 10D5 (αvβ6), R6G9 (β6), and ASC-3 (β4) (Millipore Inc., Temecula, CA), have been described 24–32. Rabbit polyclonal antisera directed against the α5,αv, β3, and β5 integrin subunits (Millipore), have also been previously described 33–35.
Immunoprecipitation
Immunoprecipitations (IP) were conducted as previously described in detail 18, 19 using 4 μg of the indicated antibodies adsorbed onto 25 μl packed anti-mouse IgG-agarose, anti-rat IgG-agarose or protein A-sepharose (Sigma-Aldrich), and 400 μg protein from cell surface-biotinylated cell lysates.
Densitometry
IP results were quantitatively analyzed with NIH Image J software (National Institutes of Health, Bethesda, MD). Bands intensities were assigned integrated density values, which represent the sum of all pixels in the box. Bands were quantified in equal area boxes and compared with β-actin immunoblotting protein loading controls to generate a ratio. Relative expression values from each integrin subunit knockdown were generated by comparing these ratios to those obtained for the non-target control knockdown, which was arbitrarily assigned a value of 100%.
Adhesion assays
Cell adhesion assays conducted under serum-free conditions were performed as previously described in detail 19 using 5 × 104 cells per well, non-tissue culture treated 96 well microtiter plates (Becton Dickinson, Franklin Lakes, NJ) previously coated with 5 μg/ml of types I or IV collagen, 25 μg/ml of fibronectin or laminin, and 10 μg/ml of vitronectin.
Migration Assays
Haptokinetic migration assays were conducted under serum-free conditions using the modified Boyden chamber as previously described in detail 19, 21, 36, 37 using 5 × 104 cells per well, 8 μM pore polycarbonate membrane filters (Neuro Probe, Inc., Gaithersburg, MD) that were previously coated on both sides with either type I or IV collagen, fibronectin, laminin or vitronectin, each at 5 μg/ml.
Proliferation assays
Proliferation assays were conducted as previously described in detail 19 using the ECM proteins and coating concentrations described above for cell adhesion assays. Cells (5 × 103 per well) were cultured under serum-free conditions on these ECM proteins over a four-day timecourse.
Animal care
Athymic nu/nu nude mice were maintained in a barrier facility on high efficiency particulate air (HEPA)-filtered racks. The animals were fed autoclaved laboratory rodent diet (Teckland LM-485; Western Research Products, Orange, CA). All animal studies were approved by the VA and UCSD Institutional Animal Care and Use Committees (IACUC) and conducted in accordance with principles and procedures outlined in the NIH Guide for the Care and Use of Animals.
Fluorescent orthotopic mouse model of pancreatic cancer
We used the RFP orthotopic mouse model of pancreatic cancer as previously described with modifications 23. Six-week old female nude mice were randomized into four groups of ten mice each. The first group received orthotopic implantation of non-target control cells (C); the second group received α2 integrin subunit knockdown cells (α2 KD); the third group received α3 integrin subunit knockdown cells (α3 KD); and the fourth group received β1 integrin subunit knockdown cells (β1 KD). Cells were prepared by trypsinization, washed with serum-containing DMEM, re-suspended in 1 mL of serum-free DMEM, and placed on ice prior to orthotopic implantation. Animals were anesthetized by intramuscular injection with 0.02 mL of a solution containing 100 mg/kg ketamine and 10 mg/kg xylazine. Orthotopic implantation was then performed in the following manner; a 6 to 10 mm transverse incision was made on the left flank of the mouse through the skin and peritoneum, and the tail of the pancreas was exposed. FG-RFP integrin subunit and non-target control knockdown cells (106 cells in 20 μL total volume/mouse) were injected into the pancreatic tail, which was subsequently returned into the abdomen. The incision was closed in two layers using 6.0 Ethibond non-absorbable sutures (Ethicon Inc., Somerville, NJ).
Fluorescence imaging
Mice were imaged weekly starting on post-implantation week 2 using the Olympus OV100 Small Animal Imaging System (Olympus Corp, Tokyo, Japan) equipped with an MT-20 light source (Olympus Biosystems, Planegg, Germany) and a DP71 CCD camera (Olympus Corp, Tokyo, Japan). For whole body fluorescence imaging, the mice were kept anesthetized by inhalation of isoflurane with 1–3% oxygen throughout the session. At six weeks post-implantation, the animals were euthanized by inhalation of 100% CO2 followed by cervical dislocation. Upon completion of necropsy, including removal of the primary tumor, the animals underwent intravital fluorescence imaging for identification of metastases with their thoracic cavity and abdomen fully exposed. Whole-body images were overlaid using Image J software (National Institutes of Health). All images were processed for contrast and brightness using Photoshop Elements 4 (Adobe Systems, Inc., San Jose, CA).
Statistics
Statistical significance of IP densitometry, cell adhesion, migration, and tumor weight and volume data was determined using Dunnett’s two-tailed t test post hoc comparison to the non-target control after ANOVA. For cell proliferation and real time externally visible fluorescent area analyses, statistical significance was determined using linear trend tests after ANOVA. For metastasis and ascites formation, statistical significance was determined using Fisher exact test.
RESULTS
RNA interference effectively suppressed the β1,α2, and α3 integrin subunits in human FG-RFP pancreatic cancer cells
Lentiviral-based shRNA was used to inhibit expression of the β1 as well as the α2 and α3 integrin subunits in human FG-RFP pancreatic cancer cells. After clonal selection using puromycin, five clones from each integrin subunit knockdown were examined for integrin mRNA expression by quantitative RT-PCR (RT-qPCR), and for integrin protein expression by immunoprecipitation (IP) of cell surface biotinylated cell extracts (Figure S1A and B). The two clones for each integrin subunit knockdown exhibiting the most effective suppression at the mRNA and protein levels (Figure S1C) were then analyzed for inhibition of function in cell adhesion assays on type I collagen, fibronectin, and laminin (Figure S1D). Based on these results, the single best clone from each integrin subunit knockdown (β1, α2, and α3) was quantitatively analyzed for mRNA and protein expression, and for functional effects in vitro and in vivo.
IP and subsequent densitometric analyses indicate inhibition of cell surface protein expression by more than 90% for each targeted integrin subunit relative to the non-target control (Figure 1A and B). RT-qPCR data show that expression of mRNA for each targeted integrin subunit was inhibited by more than 95% compared to the non-target control (Figure 1D). Other integrin subunits were generally not affected by knockdown of the α3 integrin subunit with the exception of the β1 integrin subunit (Figure 1C), its natural partner 2. For the β1 integrin subunit knockdown, in addition to reduced α2 and α3 integrin subunit expression, cell surface expression of the α1, α5, and αv integrin subunits was also reduced. All of these integrin α subunits have been previously shown form functional heterodimers with the β1 integrin subunit in pancreatic cancer cell lines, including FG cells 2. Surprisingly, in addition to reduced β1 integrin subunit expression, αv and β5 integrin subunit expression was also reduced in the α2 integrin subunit knockdown.
Figure 1. Stable suppression of β1,α2, and α3 integrin subunit mRNA and protein expression using shRNA.
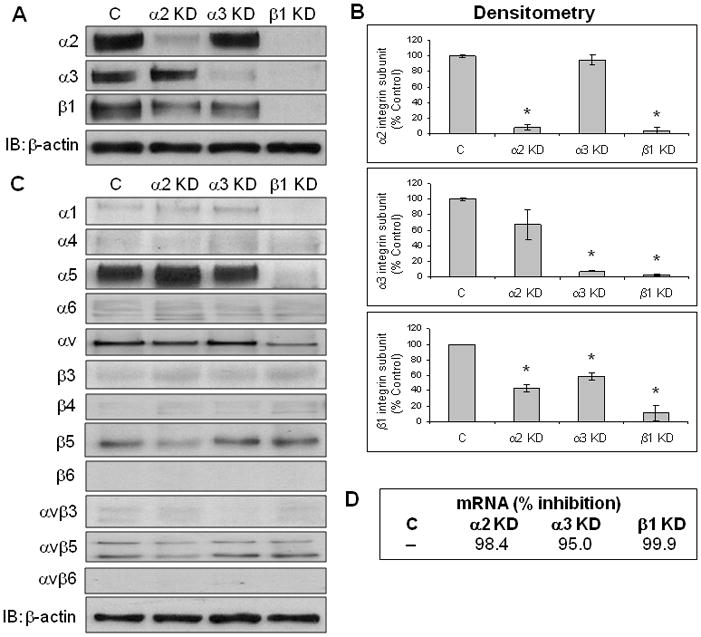
(A) IPs were conducted as described in Materials and Methods. Representative results for the α2, α3, and β1 integrin subunits are shown for each cell line, as is an independent immunoblot for β-actin (5 μg protein/lane). (C = non-target control; α2 KD – α2 integrin subunit knockdown; α3 KD – α3 integrin subunit knockdown; β1 KD – β1 integrin subunit knockdown). (B) Densitometry results from IP data for α2, α3, and β1 integrin subunit expression are shown for each cell line. Data represent the mean ± SEM from at least three independent experiments. *p ≤ 0.004. (C) IP data were also collected for non-target integrins and integrin subunits as indicated. Representative results from up to four independent experiments for each integrin or integrin subunit are shown for each knockdown as is an independent immunoblot for β-actin. (D) RT-qPCR data indicating relative mRNA (expressed as % inhibition) for each integrin subunit knockdown compared to the non-target control are shown.
Cell adhesion, proliferation, and migration on tumor microenvironment-specific ECM proteins are inhibited by integrin subunit knockdown
We next compared the best stable knockdown clones for each integrin subunit (Figures S1 and 1) in cell adhesion assays on types I and IV collagen, fibronectin, laminin, and vitronectin. Suppression of α2 integrin subunit expression reduced cell adhesion on types I and IV collagen, laminin, and vitronectin, compared to the non-target and α3 integrin subunit knockdowns (Figure 2). The α3 integrin subunit knockdown exhibited no inhibition of adhesion on any tested ECM protein. The β1 integrin subunit knockdown showed inhibition of adhesion on all tested ECM proteins, except vitronectin.
Figure 2. Inhibition of pancreatic cancer cell adhesion on specific ECM proteins by integrin subunit-specific shRNA.
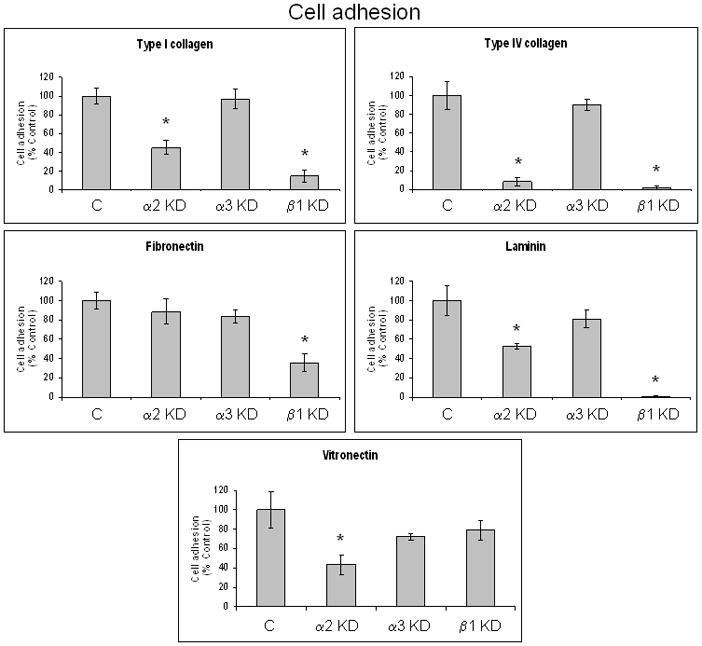
Cell adhesion assays were conducted with FG-RFP integrin subunit knockdown cell lines on the indicated ECM proteins under serum-free conditions as described in Materials and Methods. Results are expressed as % control and represent the mean ± SEM from at least three independent experiments conducted in triplicate. 100% = maximal absorbance at 595 nm for the non-target control cell line with non-specific adhesion to bovine serum albumin (BSA) subtracted from each absorbance value. *p ≤ 0.021.
Inhibition of α2 integrin subunit expression resulted in reduced cell proliferation on types I and IV collagen, as well as vitronectin, compared to the non-target and α3 integrin subunit knockdowns (Figure 3). In contrast to cell adhesion, however, no inhibition of cell proliferation was observed on laminin with the α2 integrin subunit knockdown. The α3 integrin subunit knockdown exhibited no inhibition of cell proliferation on any tested ECM protein, while the β1 integrin subunit knockdown showed inhibition of cell proliferation on all examined ECM proteins, including vitronectin. On tissue culture plastic using serum-containing media, no significant differences in proliferation were observed between any of the cell lines (Figure 3).
Figure 3. Inhibition of pancreatic cancer cell proliferation on specific ECM proteins by integrin subunit-specific shRNA.
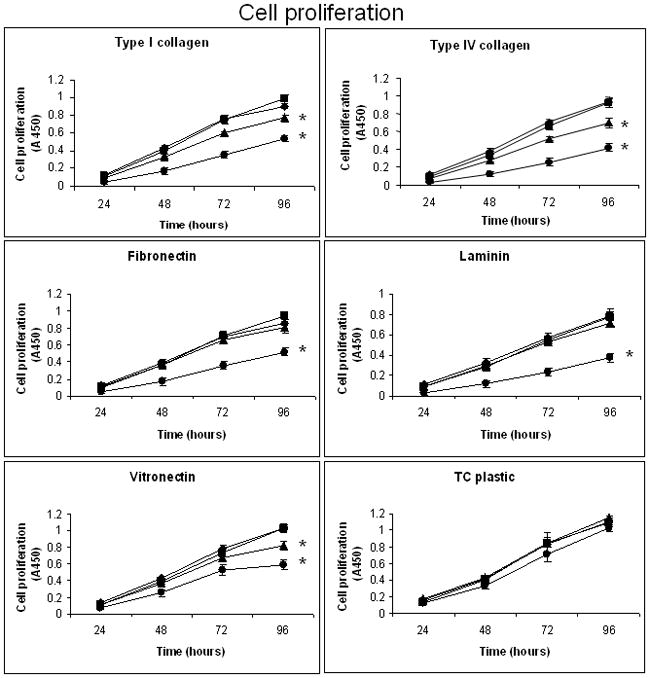
Cell proliferation assays were conducted under serum-free conditions over a four-day timecourse as previously described in detail 19 using type I collagen, type IV collagen, fibronectin, laminin or vitronectin, and FG-RFP integrin subunit knockdown cell lines (5 × 103/well). At the indicated timepoints, triplicate determinations were quantified for each cell line on each substrate by measuring the absorbance at 450 nm and subtracting the value obtained at initial seeding (T=0) using CellTiter 96 Aqueous One Solution Cell Proliferation Assay ™ reagent according to manufacturer’s instructions (Promega, Madison, WI). Proliferation assays were also conducted on uncoated tissue culture plastic with serum-containing media. Data presented represent the mean absorbance ± SEM from three to four independent experiments conducted in triplicate. *p ≤ 0.014 compared to the non-target control. ■ – C; ▲– α2 KD; ◆ - α3 KD; ● – β1 KD.
The α2 integrin subunit knockdown exhibited inhibition of haptokinetic cell migration on types I and IV collagen as well as laminin, compared to the non-target and α3 integrin subunit knockdowns (Figure 4). Migration of the α2 knock down was also reduced on vitronectin, though not significantly. The β1 integrin subunit knockdown exhibited inhibition of cell migration on all ECM proteins tested, except vitronectin. The α3 integrin subunit knockdown was not significantly different from the non-target control in migration assays on any of the substrates tested. Migration assays were conducted over 24 hours, consistent with our previous migration studies using pancreatic cancer cell lines 19, 21, 36, 37. Increasing concentrations of mitomycin C, up to 10 μg/ml, had no effect on non-target and α3 integrin subunit knockdown cell migration on any of the tested ECM proteins after 24 hours (data not shown). Differences in cell migration are thus not attributable to differences in cell proliferation.
Figure 4. Inhibition of pancreatic cancer cell migration on specific ECM proteins by integrin subunit-specific shRNA.
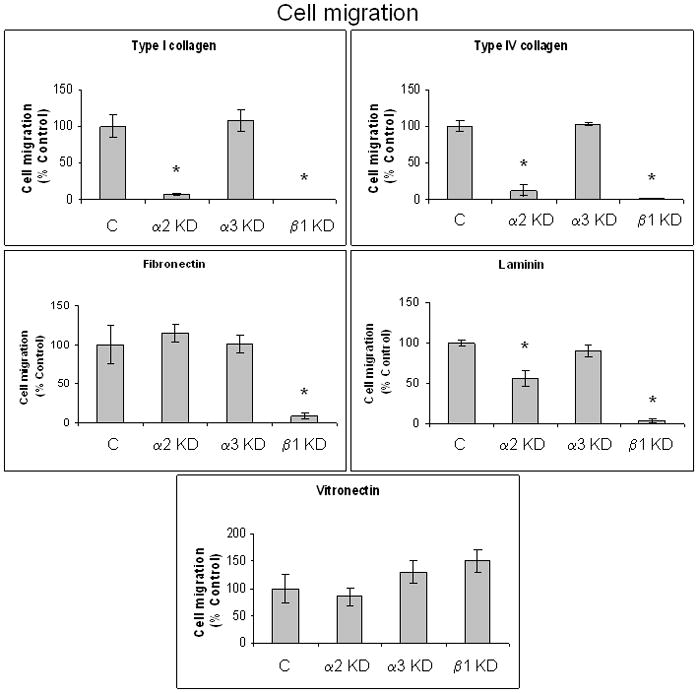
Haptokinetic cell migration was determined as previously describe in detail 19, 21, 36, 37 using 8 μm pore polycarbonate membrane filters coated with either type I collagen, type IV collagen, fibronectin, laminin, or vitronectin, 5 × 104 FG-RFP non-target control and integrin subunit knockdown cell lines that were serum-starved 24h prior to assay, and a 24 hour incubation period at 37°C. Migratory cells were quantified by counting four high-powered fields (100x magnification) per well using an inverted light microscope (Olympus BH 2). Results are expressed as % control, and represent the mean ± SEM from at least three independent experiments conducted in triplicate. *p ≤ 0.006 compared to the non-target control.
Knockdown of the β1 integrin subunit inhibits primary tumor growth in vivo
We next examined the effects of integrin subunit knockdown on primary tumor growth in vivo using a fluorescent orthotopic mouse model of pancreatic cancer. Equal numbers of integrin subunit knockdown FG-RFP cells were injected into the pancreatic tail of athymic nu/nu mice, 10 mice/group. One mouse in the β1 knockdown group died soon after implantation, reducing the number of mice in that group to nine. All 39 remaining mice successfully grew tumors in the pancreas that were visible by real-time, whole-body fluorescence imaging 22, 23, 38–40 as early as the first imaging session (post-implantation week 2). Tumor growth was monitored weekly through post-implantation week 6. As shown in Figure 5A and B, real time imaging over the six week time course indicates that primary tumors from the β1 integrin subunit knockdown were significantly smaller than tumors from the α2 andα3 integrin subunit knockdowns, as well as tumors from the non-target control.
Figure 5. Real-time fluorescence imaging demonstrates that knockdown of the β1 integrin subunit inhibits tumor progression in vivo.
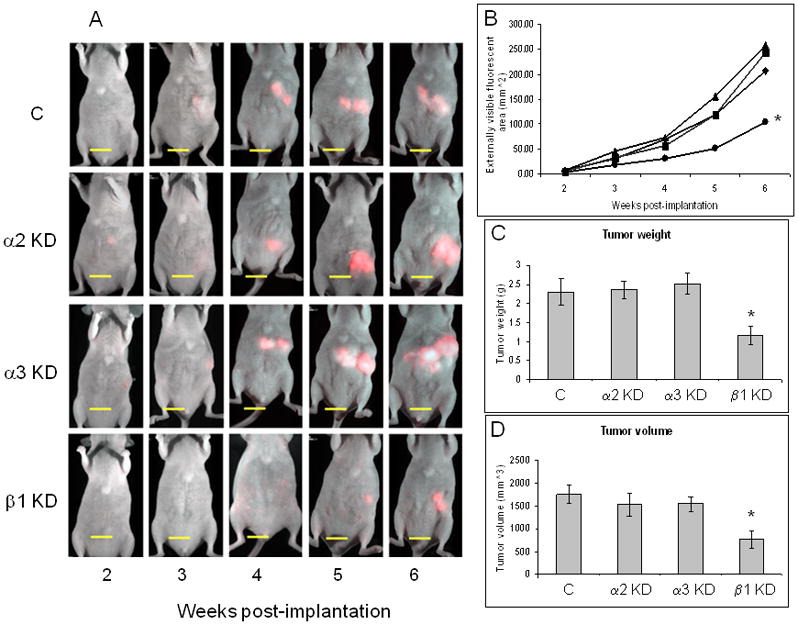
(A) Representative mice from each integrin subunit knockdown group throughout weeks 2–6 post-implantation are shown. Mice were imaged weekly starting on post-implantation week 2 using the Olympus OV100 Small Animal Imaging System equipped with an MT-20 light source and a monochrome camera to achieve maximum signal sensitivity. Bar, 10 mm. (B) Real-time imaging data from each knockdown group are shown throughout the 6 week post-implantation time course. Data are expressed as the mean visible fluorescence area (mm2) from 10 mice per group, except for the β1 KD group, where n=9. *p = 0.007. ■ – C; ▲ – α2 KD; ◆ -α3 KD; ● – β1 KD. Weight (C) and volume (D) of primary tumors are presented as the mean ± SEM from 10 animals per group, except for the β1 KD group, where n=9. Primary tumors were collected, weighed, and volumes measured on post-implantation week 6. *p = 0.033 and *p = 0.018 for tumor weight and volume, respectively.
Upon termination of the experiment (week 6), mice were sacrificed, tumors were excised, and their weights and volumes were determined. Consistent with the real-time fluorescent imaging results, tumor weights and volumes from the β1 knockdown group were significantly less than those recovered from mice in the non-target control knockdown group (1.16 g vs. 2.31 g, and 758.40 mm3 vs. 1748.40 mm3, respectively), representing about a 50% reduction in tumor weight and volume (Figure 5C and D). Strong and statistically significant direct correlations between real time fluorescence imaging and ex vivo tumor weights were observed for each integrin subunit knockdown group as well as the non-target control group (Figure S2). Tumors from the α2 and α3 integrin subunit knockdown groups were not significantly different from those of the non-target control group.
Knockdown of the β1 integrin subunit completely inhibits pancreatic cancer metastasis in vivo
At necropsy, the presence of ascites and metastases was also evaluated. Ascites were observed in mice from the non-target control, α2 and α3 knockdowns, but not in animals from the β1 knockdown group (Figure 6). Upon removal of the primary tumor, intravital fluorescence imaging of the abdominal and thoracic cavities was performed for all animals to identify metastases. Consistent with human disease, metastases were present in the mice of non-target control, α2 and α3 knockdowns (Figure 6), with the most common sites being the mesentery of the gastrointestinal tract, spleen, liver, the surface of the peritoneum, and the reproductive system 23, 38, 40, 41. Remarkably, none of the mice from the β1 integrin subunit knockdown group developed detectable metastases.
Figure 6. Knockdown of the β1 integrin subunit completely inhibits metastasis in vivo.
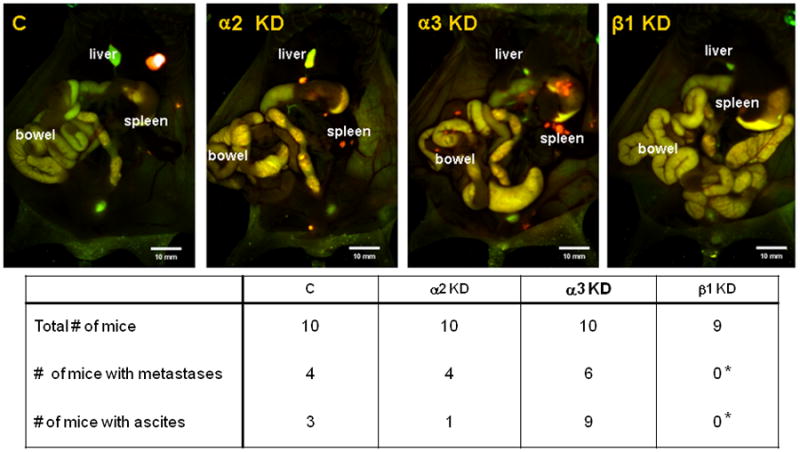
At the time of euthanasia, ascites formation was observed in all but the β1 KD group. Intravital fluorescent imaging after removal of the primary tumors indicates metastases in all but the β1 KD group. *p = 0.011 and *p ≤ 0.001 for metastasis and ascites formation, respectively, compared to the α3 KD.
DISCUSSION
In vitro studies show that knockdown of the β1 integrin subunit significantly inhibits pancreatic cancer cell adhesion, proliferation, and migration on types I and IV collagen, fibronectin, and laminin (Figures 2–4). These observations are consistent with previously published studies using multiple pancreatic cancer cell lines, where a function-blocking monoclonal antibody directed against the β1 integrin subunit inhibited interactions with these ECM proteins 18, 19. We also show that knockdown of the α2 integrin subunit significantly inhibits pancreatic cancer cell adhesion, proliferation, and migration on types I and IV collagen. These data are also consistent with the studies cited above, where a function-blocking monoclonal antibody directed against the α2 integrin subunit inhibited pancreatic cancer cell interactions with these ECM proteins.
It is perhaps surprising that knockdown of the α2 integrin subunit appears to reduceαvβ5 integrin expression (Figure 1), and also significantly inhibit cell adhesion and proliferation on vitronectin (Figures 2 and 3). Cell migration was also reduced (Figure 4), though not significantly. Previous adhesion studies using function-blocking monoclonal antibodies have shown with multiple pancreatic cancer cell lines that while the β5 integrin subunit (as the αvβ5 integrin) is mainly responsible for vitronectin adhesion, the β1 integrin subunit is also involved 37. While the particular α subunits involved in β1 integrin-mediated interactions with vitronectin were not determined, these data collectively suggest a potential regulatory relationship between the α2β1 andαvβ5 integrins in pancreatic cancer cells. It has been recently shown that suppression of αvβ5/ αvβ6 integrin expression, either by RNA interference or by lysosomal targeting strategies, increasesα2β1 integrin-mediated colon cancer cell migration on type I collagen, suggesting the existence of crosstalk between these particular integrins 42. It is possible, then, that inhibition ofα2 integrin subunit expression on pancreatic cancer cells may exert a negative regulatory effect on αvβ5 integrin expression and its interactions with vitronectin.
An inhibitory effect of α2 integrin subunit knockdown was also observed in adhesion and migration assays on laminin, but not in proliferation assays. Though previously unappreciated in pancreatic cancer cell lines, it has been shown that the α2β1 integrin can interact with different ligands, including types I or IV collagen, and laminin, depending on the cell type on which it is expressed 43. Our data suggest that the α2β1 integrin may be both a collagen and laminin receptor in FG-RFP pancreatic cancer cells. It remains to be determined whether this is consistently observed in all pancreatic cancer cell lines.
The in vitro shRNA knockdown data presented here, along with the previously published in vitro results described above, and considering that type I collagen, in particular, is highly up-regulated in pancreatic cancer 2, suggest that knockdown of the α2 and β1 integrin subunits would both inhibit pancreatic cancer progression in vivo. As shown in Figures 5–6, however, only knockdown of the β1 integrin subunit significantly inhibited tumor progression.
It is possible that knockdown of the α2 integrin subunit by greater than 90% at the protein level (Figure 1) is not enough to significantly inhibit high-affinity 44, 45α2β1 integrin-mediated tumor cell interactions with type I collagen in vivo. The statistically significant cell adhesion, proliferation, and migration data shown in Figures 2–4 indicate a greater loss of function in the β1 integrin subunit knockdown than the α2 integrin subunit knockdown.
It is also possible that tumor cell interactions with type I collagen other than the α2β1 integrin are in effect in pancreatic cancer. While the α2β1 integrin is the only known type I collagen-binding integrin expressed on pancreatic cancer cells 2, it has been recently shown that the receptor tyrosine kinase, discoidin domain receptor (DDR1), also binds to and is activated by type I collagen 46. In addition, cross-talk between both the α2β1 integrin and DDR1 receptor tyrosine kinase signaling elements are required to produce the characteristic N-cadherin-mediated epithelial-mesenchymal-like transition on type I collagen in pancreatic cancer cells.
And while targeting the α2 integrin subunit may lead to a significant reduction in tumor cell function on types I and IV collagen as well as laminin under serum-free conditions in vitro, compensatory interactions of other integrins with other ECM proteins may override any inhibitory effects aimed at one particular α subunit in vivo. That may be why targeting the β1 integrin subunit, which forms non-covalently linked heterodimers with at least 6 known α subunits in pancreatic cancer 2, completely inhibits the occurrence of detectable metastases in vivo.
As stated earlier, in a previous study by Vogelmann et al., pre-incubation of human PaTu 8988s pancreatic cancer cells with function-blocking monoclonal antibodies directed against the α6 and β1 integrin subunits both significantly decreased lung colony formation four weeks after tail vein injection 20. This experimental metastasis model is quite different from the orthotopic model used in our study, where metastases spontaneously arise from established primary tumors 23. However, their data regarding the β1 integrin subunit are in support of our current findings.
The β1 integrin subunit has been previously shown to be a potential therapeutic target in pre-clinical models of other cancers. For example, a function-blocking monoclonal antibody directed against the β1 integrin subunit significantly inhibited the growth of human breast cancer tumor cells in nude mice 47. Also, ablation of β1 integrin subunit expression in β tumor cells of the pancreas in Rip1Tag2 mice reduced primary tumor growth and inhibited metastasis 48. Additionally, osteosarcoma tumor growth in vivo was inhibited in SCID mice with antibodies directed against β1 integrins 49. These data are in agreement with our current results in pancreatic cancer, and suggest the β1 integrin subunit as a therapeutic target in other cancers as well.
Current studies are focused on elucidating the mechanism by which knockdown of the β1 integrin subunit reduces primary tumor growth and inhibits metastasis in pancreatic cancer. Besides inhibition of cell adhesion, migration, and proliferation on known β1 integrin ECM proteins (Figures 2–4), preliminary studies indicate a rounded morphology in the β1 knockdown cell line compared to the other integrin knockdown cell lines and the non-target control, where cell protrusions and extensions are apparent (Figure S3). Additionally, primary tumors derived from the β1 knockdown, besides being smaller than tumors derived from the other groups, show a great deal of central necrosis compared to primary tumors derived from the other integrin or non-target control knockdowns (Figure S4). These data collectively suggest that anoikis (apoptosis caused by lack of cell attachment) 50, necrosis, or cellular senescence are responsible for inhibition of metastasis in the β1 integrin subunit knockdown. It has been previously shown in breast cancer models that, besides inhibition of metastasis, loss of β1 integrin correlates with loss of tumorigenic morphology, reduced phosphorylation of focal adhesion kinase (FAK), an increased expression of the cell cycle inhibitory protein p21Cip1, and the arrest of cells in the G1 phase of the cell cycle 48. Similar results have been reported when β1 integrin subunit expression was inhibited in β tumor cells of the pancreas in Rip1Tag2 mice as described in the preceding paragraph 48.
In conclusion, our data suggest that the desmoplastic response characteristic of pancreatic cancer, including highly up-regulated ECM expression, is not merely a host defense mechanism against the tumor, but through interactions with β1 integrins on the tumor cell surface, plays an active and critical role in tumor progression. Our results also suggest the exciting possibility of targeting the β1 integrin subunit for the adjuvant treatment of pancreatic cancer to prevent metastasis of this highly aggressive disease.
Supplementary Material
Acknowledgments
This work was funded by VA Merit Review grants (MB and LJD), NCI grant R21CA135435 (MB and RMH), NIH and Tobacco Related Disease Research Program grants (LJD), and T32 training grant CA121938 (HSTC).
References
- 1.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 2.Grzesiak JJ, Ho JC, Moossa AR, Bouvet M. The Integrin-Extracellular Matrix Axis in Pancreatic Cancer. Pancreas. 2007;35:293–301. doi: 10.1097/mpa.0b013e31811f4526. [DOI] [PubMed] [Google Scholar]
- 3.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117(1):50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 5.Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 6.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 7.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 8.Ruoslahti E. Integrins. J Clin Invest. 1991;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruoslahti E, Noble NA, Kagami S, Border WA. Integrins. Kidney Int Suppl. 1994;44:S17–22. [PubMed] [Google Scholar]
- 10.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 11.Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66(9):4662–71. doi: 10.1158/0008-5472.CAN-05-2804. [DOI] [PubMed] [Google Scholar]
- 12.Menke A, Philippi C, Vogelmann R, Seidel B, Lutz MP, Adler G, Wedlich D. Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res. 2001;61(8):3508–17. [PubMed] [Google Scholar]
- 13.Imamichi Y, Konig A, Gress T, Menke A. Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer. Oncogene. 2006 doi: 10.1038/sj.onc.1210012. [DOI] [PubMed] [Google Scholar]
- 14.Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66(24):11745–53. doi: 10.1158/0008-5472.CAN-06-2322. [DOI] [PubMed] [Google Scholar]
- 15.Grzesiak JJ, Clopton P, Chalberg C, Smith K, Burton DW, Silletti S, Moossa AR, Deftos LJ, Bouvet M. The extracellular matrix differentially regulates the expression of PTHrP and the PTH/PTHrP receptor in FG pancreatic cancer cells. Pancreas. 2004;29(2):85–92. doi: 10.1097/00006676-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC, Iredale JP. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10(21):7427–37. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 17.Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128(4):907–21. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Grzesiak JJ, Bouvet M. Determination of the ligand binding specificities of the a2b1 and a1b1 integrins in a novel three-dimensional in vitro model of pancreatic cancer. Pancreas. 2007;34:220–28. doi: 10.1097/01.mpa.0000250129.64650.f6. [DOI] [PubMed] [Google Scholar]
- 19.Grzesiak JJ, Bouvet M. The a2b1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br J Cancer. 2006;94(9):1311–9. doi: 10.1038/sj.bjc.6603088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogelmann R, Kreuser ED, Adler G, Lutz MP. Integrin alpha6beta1 role in metastatic behavior of human pancreatic carcinoma cells. Int J Cancer. 1999;80(5):791–5. doi: 10.1002/(sici)1097-0215(19990301)80:5<791::aid-ijc25>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Grzesiak JJ, Smith KC, Burton DW, Deftos LJ, Bouvet M. GSK3 and PKB/Akt are associated with integrin-mediated regulation of PTHrP, IL-6 and IL-8 expression in FG pancreatic cancer cells. Int J Cancer. 2005;114:522–30. doi: 10.1002/ijc.20748. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5(10):796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 23.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. An imageable highly metastatic orthotopic red fluorescent protein model of pancreatic cancer. Clin Exp Metastasis. 2004;21(1):7–12. doi: 10.1023/b:clin.0000017160.93812.3b. [DOI] [PubMed] [Google Scholar]
- 24.Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987;105(4):1873–84. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wayner EA, Carter WG, Piotrowicz RS, Kunicki TJ. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988;107(5):1881–91. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wayner EA, Gil SG, Murphy GF, Wilke MS, Carter WG. Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for alpha 3 beta 1 positive T lymphocytes. J Cell Biol. 1993;121(5):1141–52. doi: 10.1083/jcb.121.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbri M, Castellani P, Gotwals PJ, Kotelianski V, Zardi L, Zocchi MR. A functional monoclonal antibody recognizing the human alpha 1-integrin I-domain. Tissue Antigens. 1996;48(1):47–51. doi: 10.1111/j.1399-0039.1996.tb02604.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262(36):17703–11. [PubMed] [Google Scholar]
- 29.Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336(6198):487–9. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- 30.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 31.Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269(9):6940–8. [PubMed] [Google Scholar]
- 32.Pinco KA, He W, Yang JT. alpha4beta1 integrin regulates lamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Mol Biol Cell. 2002;13(9):3203–17. doi: 10.1091/mbc.02-05-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238(4826):491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 34.Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- 35.Aplin JD, Spanswick C, Behzad F, Kimber SJ, Vicovac L. Integrins beta 5, beta 3 and alpha v are apically distributed in endometrial epithelium. Mol Hum Reprod. 1996;2(7):527–34. doi: 10.1093/molehr/2.7.527. [DOI] [PubMed] [Google Scholar]
- 36.Grzesiak JJ, Bouvet M. Activation of the alpha(2)beta(1) integrin-mediated malignant phenotype on type I collagen in pancreatic cancer cells by shifts in the concentrations of extracellular Mg(2+) and Ca(2+) Int J Cancer. 2008;122(10):2199–209. doi: 10.1002/ijc.23368. [DOI] [PubMed] [Google Scholar]
- 37.Grzesiak JJ, Bouvet M. Divalent cations modulate the integrin-mediated malignant phenotype in pancreatic cancer cells. Cancer Science. 2008;99:1553–63. doi: 10.1111/j.1349-7006.2008.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouvet M, Wang J, Nardin SR, Nassirpour R, Yang M, Baranov E, Jiang P, Moossa AR, Hoffman RM. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002;62(5):1534–40. [PubMed] [Google Scholar]
- 39.Yang M, Baranov E, Jiang P, Sun FX, Li XM, Li L, Hasegawa S, Bouvet M, Al-Tuwaijri M, Chishima T, Shimada H, Moossa AR, et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci U S A. 2000;97(3):1206–11. doi: 10.1073/pnas.97.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res. 2003;113(1):151–60. doi: 10.1016/s0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 41.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89(12):5645–9. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Defilles C, Lissitzky JC, Montero MP, Andre F, Prevot C, Delamarre E, Marrakchi N, Luis J, Rigot V. alphavbeta5/beta6 integrin suppression leads to a stimulation of alpha2beta1 dependent cell migration resistant to PI3K/Akt inhibition. Exp Cell Res. 2009;315(11):1840–9. doi: 10.1016/j.yexcr.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Kirchhofer D, Languino LR, Ruoslahti E, Pierschbacher MD. Alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem. 1990;265(2):615–8. [PubMed] [Google Scholar]
- 44.Popova SN, Lundgren-Akerlund E, Wiig H, Gullberg D. Physiology and pathology of collagen receptors. Acta Physiol (Oxf) 2007;190(3):179–87. doi: 10.1111/j.1748-1716.2007.01718.x. [DOI] [PubMed] [Google Scholar]
- 45.White DJ, Puranen S, Johnson MS, Heino J. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol. 2004;36(8):1405–10. doi: 10.1016/j.biocel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180(6):1277–89. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66(3):1526–35. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kren A, Baeriswyl V, Lehembre F, Wunderlin C, Strittmatter K, Antoniadis H, Fassler R, Cavallaro U, Christofori G. Increased tumor cell dissemination and cellular senescence in the absence of beta1-integrin function. Embo J. 2007;26(12):2832–42. doi: 10.1038/sj.emboj.7601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miura K, Uniyal S, Leabu M, Oravecz T, Chakrabarti S, Morris VL, Chan BM. Chemokine receptor CXCR4-beta1 integrin axis mediates tumorigenesis of osteosarcoma HOS cells. Biochem Cell Biol. 2005;83(1):36–48. doi: 10.1139/o04-106. [DOI] [PubMed] [Google Scholar]
- 50.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9(5):701–6. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


