Abstract
In the past two decades, over 1000 clinical trials have failed to demonstrate a benefit in treating stroke, with the exception of thrombolytics. Although many targets have been pursued, including antioxidants, calcium channel blockers, glutamate receptor blockers, and neurotrophic factors, often the focus has been on neuronal mechanisms of injury. Broader attention to loss and dysfunction of non-neuronal cell types is now required to increase the chance of success. Of the several glial cell types, this review will focus on astrocytes. Astrocytes are the most abundant cell type in the higher mammalian nervous system, and they play key roles in normal CNS physiology and in central nervous system injury and pathology. In the setting of ischemia astrocytes perform multiple functions, some beneficial and some potentially detrimental, making them excellent candidates as therapeutic targets to improve outcome following stroke and in other central nervous system injuries. The older neurocentric view of the central nervous system has changed radically with the growing understanding of the many essential functions of astrocytes. These include K+ buffering, glutamate clearance, brain antioxidant defense, close metabolic coupling with neurons, and modulation of neuronal excitability. In this review, we will focus on those functions of astrocytes that can both protect and endanger neurons, and discuss how manipulating these functions provides a novel and important strategy to enhance neuronal survival and improve outcome following cerebral ischemia.
Keywords: Brain ischemia, astrocytes, neurons, inflammation, neuroprotection, clinical trials
1. INTRODUCTION
Stroke is the third leading cause of death in the United States and results in substantial health-care expenditures; the mean lifetime cost resulting from an ischemic stroke is estimated at $140,048 per patient, and this estimation is higher for people over 45 years. Nationwide in 2010, the estimated direct and indirect costs of stroke totaled $73.7 billion [1]. Although many clinical trials have been completed in stroke patients, none of these have demonstrated protective efficacy except for thrombolysis [2, 3]. In the case of cardiac arrest and resuscitation only hypothermia has been shown to have clinical utility [4]. In some sense the two therapies that have been effective thus far clinically have broad targets, and do not only affect a single injury mechanism. In contrast, of the failed trials, many targeted neuron-specific injury mechanisms [5]. This may reflect too narrow a view of what is needed for brain preservation. A large body of work has shown that astrocytes play key roles both in normal and pathological central nervous system functioning [6]. Astrocytes are the most abundant brain cell type, and in addition to their multiple important homeostatic roles, they organize the structural architecture of the brain, help organize communication pathways, and modulate neuronal plasticity (for recent review see [7, 8]). Thus, astrocytes are now thought to be important potential targets for manipulation.
Ischemic stroke is caused by an interruption of cerebral blood flow that leads to stress, cell death, and inflammation. Neurons are more susceptible to injury than astrocytes when studied under some in vitro conditions [9, 10]. Neurons have less endogenous antioxidants and are susceptible to excito-toxicity [10]. Both normally and after ischemia, astrocytes support neurons by providing antioxidant protection [11, 12], substrates for neuronal metabolism [13], and glutamate clearance REF. Although astrocytes are sometimes more resilient than neurons, injury can result in impaired astrocyte function even when astrocytes do not die. Impaired astrocyte function can amplify neuronal death [14]. Therefore, many recent efforts have focused on the astrocyte-neuron interaction and how astrocyte function can be improved after stroke to enhance neuronal support and survival [10, 15, 16]. A growing body of data demonstrates that astrocytes play a multifaceted and complex role in the response to ischemia, with potential to both enhance and impair neuronal survival and regeneration [17]. Many recent studies focus on the astrocyte-neuron interaction and several investigate ways in which astrocyte function can be improved after stroke to enhance neuronal survival.
This review provides a brief overview of the pathophysiological events underlying ischemic brain damage, and considers how these events affect astrocyte-mediated support of neurons. In addition, we discuss some experimental approaches to enhance the neuronal supportive role of astrocytes as a novel strategy against stroke. Finally, we explore how these approaches may eventually be applied in the clinical setting to improve stroke outcome for patients.
2. ASTROCYTE VIABILITY AFTER ISCHEMIA
2.1. In Vitro Studies
In vitro studies have provided substantial insight into the mechanisms governing the survival of astrocytes following simulated ischemia. These investigations have shown that astrocytes are generally more resistant than neurons to oxygen-glucose deprivation (OGD) performed in media at physiologically normal pH, an in vitro model of ischemia [10, 18]. Most neurons in astrocyte-neuronal co-cultures will die after 60–90 min of OGD, while astrocyte cultures only suffer a similar extent of injury after 4–6 hours [9, 18, 19]. Different astrocyte populations exist and astrocytes isolated from different brain regions such as cortex, striatum, and hippocampus differ in their sensitivity to OGD [15, 20, 21]. Furthermore, Lukaszevicz and colleagues [22] reported that protoplasmic astrocytes lose their integrity faster than fibrous astrocytes, which may explain the regional differences in susceptibility to ischemia between white matter astrocytes which are fibrous and grey matter astrocytes that are protoplasmic. Although less susceptible to OGD-induced damaged in vitro studies have highlighted certain elements that are highly toxic to astrocytes. For example, acidosis has been found to be very effective in killing astrocytes [23–26], in contrast to neurons, which are protected in acidic conditions [24, 26].
2.2. Focal Cerebral Ischemia
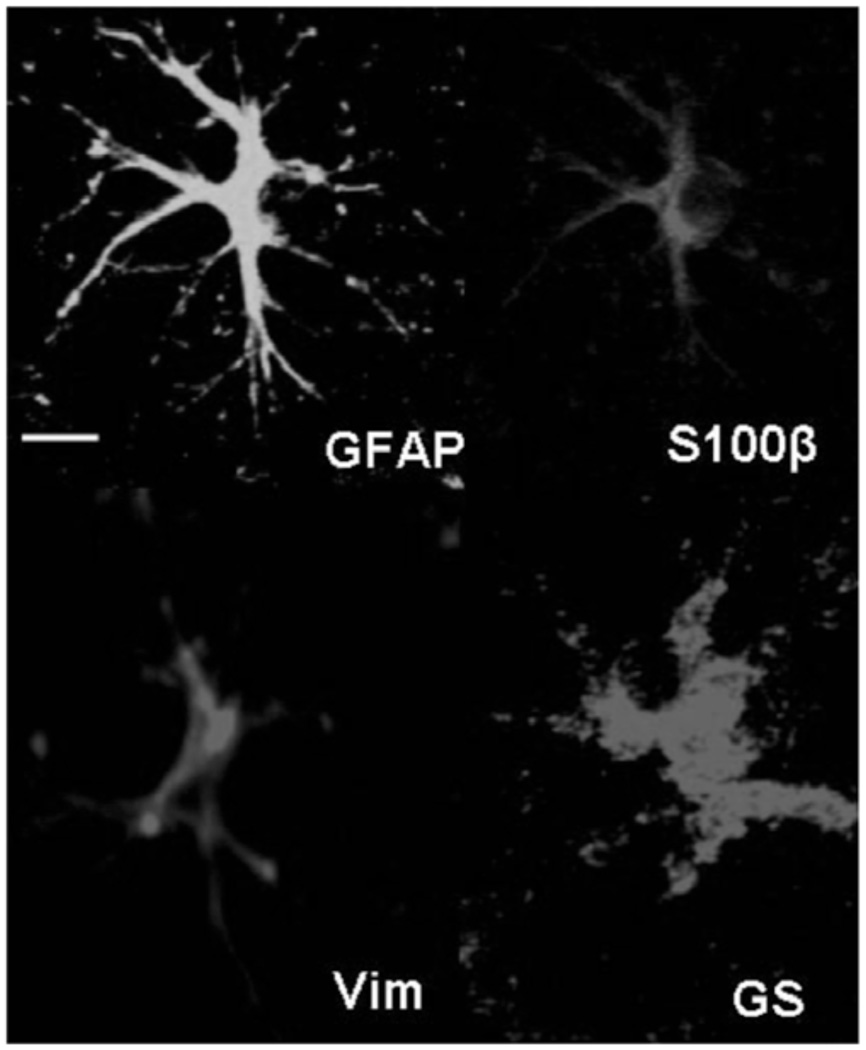
Much of the information about the recovery of astrocytes in vivo has been provided by studies using immunohistological markers for astrocyte specific proteins, such as glial fibrillary acidic protein (GFAP) and glutamine synthetase GS; Fig. 1. Using these markers as tools, several investigations suggest that astrocytes are better preserved than neurons in animal models of stroke outside the core where all cells die [27–29]. Though neuronal markers are decreased as soon as 1 hour after MCAO, GFAP expression is preserved over the first 3 hours of reperfusion after 2 hour MCAO [29] and GS is increased 3 hours following a 3 hour MCAO [28]. At later reperfusion periods, GFAP increases in the peri-infarct area that later develops into the glial scar [29–32]. In contrast, Liu and colleagues [33] reported the deterioration of some astrocyte markers prior to that of neuronal markers. Discrepancy in findings may be due to differences in detection (i.e., protein vs. mRNA) and injury paradigms.
Fig. (1).
Expression of different astrocytic proteins following stroke. Increased expression of GFAP is a hallmark of astrocytes activation, as is induction/re-expression of vimentin. Astrocytes normally express glutamine synthetase (GS) and S100β, genes that are widely expressed in both reactive and non-reactive astrocytes. The GFAP and S100β labelling are for the same cell, while the Vim and GS staining labels are for of other cells. Scale bar, 50µm.
2.3. Forebrain Ischemia
Excitotoxic neuronal injury is a common mechanism in both acute and chronic neurodegenerative diseases. It has long been appreciated that inhibition of astrocyte glutamate uptake [34, 35], and more recently inhibition of astrocyte mitochondrial function [36], impairs neuronal survival from excitotoxic injury. Brief forebrain ischemia is a model of the delayed hippocampal neuronal loss seen in patients following cardiac arrest and resuscitation, and in part involves excitotoxicity. Increased generation of reactive oxygen species (ROS) and mitochondrial dysfunction in CA1 astrocytes contributes to ischemia-induced loss of GLT-1 and ultimately to delayed death of CA1 neurons [15]. Our studies and those of other laboratories have demonstrated that selective dysfunction of hippocampal CA1 subregion astrocytes, with loss of glutamate transport activity and immunoreactivity for glutamate transporter 1 (GLT-1), occurs at early reperfusion times, hours to days before the death of CA1 neurons [15, 37, 38].
The heterogeneous degeneration of astrocytic processes and mitochondria was tightly associated with the appearance of disseminated selective neuronal necrosis and its maturation after temporary ischemia [39]. By electronmicroscopy the same investigators [40] found that focal infarction is exacerbated by temporary microvascular obstruction due to compression by swollen astrocytic end-feet. However, hypoxia has multiple effects on astrocytes and their ability to support neuronal viability [41]. For example, hypoxia induces astrocyte-dependent protection of neurons following hypoxic preconditioning. Yet, hypoxia induces processes in astrocytes that augment neuronal death in other situations, such as the coincidence of hypoxia with inflammatory signaling.
3. REACTIVE ASTROGLIA: GOOD OR BAD AFTER STROKE?
The astrocyte response to ischemia has traditionally been viewed as detrimental to recovery, as the astrocyte-rich glial scar has both physical and chemical inhibitory properties [42, 43]. As components of the glial scar, astrocytes exhibit hypertrophied, interdigitated processes that form a physical barrier. Astrocytes produce inhibitory molecules including chondroitin sulfate proteoglycans (CSPGs) that contribute to chemical inhibition [44, 45]. In the acute setting, astrocytic gap junctions may remain open following ischemia [46], allowing substances such as proapoptotic factors to spread through the syncytium, thereby expanding the size of the infarct [47]. As discussed below, astrocytes can also produce a variety of pro-inflammatory cytokines.
Many studies have shown that decreased astrogliosis often correlates with decreased infarct size. Nonspecific inhibition of cell proliferation following ischemia using a cyclin kinase inhibitor decreases astrocyte proliferation and results in improved functional recovery [48]. In addition, treatment with alpha-melanocyte stimulating hormone [49], cysteinyl leukotriene receptor antagonist [50], cliostazol [51], and caffeic acid [52] result in reduced infarct size accompanied by a decrease in astrogliosis. Treadmill exercise [28] and acupuncture [53] are similarly associated with improved outcome and reduced astrogliosis. Thus, results from several studies suggest that treatments that decrease infarct size are often accompanied by attenuated astrocyte response. Despite the frequent association of decreased astrogliosis with improved outcome, it is difficult to determine cause and effect, since the extent of astrogliosis likely reflects the severity of the injury, as well as influencing it.
In addition to their role in glial scar formation, astrocytes also respond to ischemia with functions important for neuroprotection and repair. These include protecting spared tissue from further damage [14], taking up excess glutamate as discussed above, rebuilding the blood brain barrier [54, 55], and producing neurotrophic factors [10]. GFAP knockout mice exhibit larger lesions than their wild-type littermates following focal ischemia [56], and mice lacking both GFAP and vimentin have impaired astrocyte activation, decreased glutamate uptake abilities, and attenuated PAI-1 expression after ischemia [57]. Application of astrocyte-conditioned media after transient MCAO results in decreased infarct volume and regained blood-brain barrier function [58], suggesting that factors released by astrocytes following ischemia are important for neuroprotection.
Although few studies other than the use of animals lacking vimentin and GFAP have specifically targeted astrocyte activation after ischemia, there is correlational evidence suggesting that astrogliosis may be beneficial. Environmental enrichment, which results in reduced infarct size and improved recovery following ischemia, also leads to increased astrocyte proliferation [59, 60]. After focal ischemia, aged rats exhibit increased tissue damage and increased astrocyte hypertrophy, but have decreased astrocyte proliferation compared to young rats [61]. Systemic infusion of bone marrow stromal cells following MCAO increases gliogenesis and decreases lesion size [62, 63]. In addition, administration of transforming growth factor α (TGFα), a known mitogen for astrocytes [64], following MCAO leads to reduced infarct size and improved functional recovery [65]. Furthermore, ischemic preconditioning that produces a neuroprotective state leads to prolonged astrocyte expression of Hsp27 [66]. Finally, mice lacking connexin 43, the gap junction connecting astrocyte networks that is needed for proper neurotransmitter and potassium regulation, have increased infarcts following MCAO [67]. Thus, astrocytes have the potential to be both detrimental and beneficial following ischemic insult, making them promising targets for manipulation to improve outcome.
4. ASTROCYTE-MEDIATED INFLAMMATION AFTER STROKE: A DOUBLE-EDGED SWORD
Inflammation plays both detrimental and beneficial roles in brain ischemia, depending upon the timing and severity of the inflammation. Within minutes after injury, injured neurons in the core and penumbra of the lesion and glial cells in the core produce pro-inflammatory mediators, cytokines, and reactive oxygen species, which activate both astrocytes and microglia [68]. Activated astrocytes can produce the proinflammatory cytokines IL-6, TNFα, IL-1α and β, interferon γ, and others [68–70]. High levels of these cytokines can be detrimental to ischemic recovery [71–75] by directly inducing apoptosis of neuronal cells and/or increasing toxic nitric oxide levels [76] and inhibiting neurogenesis [77]. Indeed, inactivation of astrocyte NfκB signaling, shown to induce astrocyte production of pro-inflammatory cytokines [78], decreases cytokine production and protects neurons after ischemic injury [79]. Hsp72 overexpression is associated with lower NfκB activation and lower TNFα [80]. In addition to cytokines, reactive astrocytes also produce chemokines following ischemia [81]. Chemokines upregulate adhesion molecules in vascular endothelial cells, resulting in attraction of immune cells, which may worsen ischemia-induced damage [82]. Overall, some aspects of the local inflammatory response contribute to secondary injury to potentially viable tissue and lead to apoptotic and necrotic neuronal cell death hours to days after injury [83], while other aspects are beneficial.
Although the potential benefits of inflammation after stroke have received relatively little attention so far, indirect evidence suggests that some specific inflammatory reactions are neuroprotective and neuroregenerative [84–91]. In addition to providing defense against the invasion of pathogens, inflammation is also involved in clearing damaged tissue, and in angiogenesis, tissue remodeling, and regeneration [89]. This is probably best studied in wound healing, which is severely compromised if inflammation is inhibited [89, 91]. There is also evidence suggesting that specific inflammatory factors can be protective in some circumstances. IL-6, produced by astrocytes acutely after MCAO [69], is likely neuroprotective early after ischemia [84]. Interestingly, ischemic preconditioning resulting in protection appears to be dependent on TLR-4 signaling, and is accompanied by increased TNFα, NFκB, and COX-2 expression [90]. Indeed, in vitro work has shown that administration of TNFα in combination with Hsp70 results in decreased expression of pro-apoptotic proteins following hypoxia [88]. Thus, it is important to consider these factors, along with timing, when trying to determine the best strategy to reduce damage and improve recovery and regeneration.
5. ASTROCYTE SUPPORT OF NEURONS AFTER STROKE
5.1. Antioxidant Production
One hallmark of the cellular response to ischemia is a rapid, dramatic increase in damaging free radicals, including nitric oxide (NO), superoxide, and peroxynitrite [92]. Nitric oxide synthetase levels increase as soon as 10 minutes after induction of MCAO [93], followed by NO production that persists for at least one week after MCAO [94]. Nitric oxide can cause cell death by inducing the release of cytochrome-c from mitochondria, leading to apoptosis [95]. Nitric oxide can also induce necrotic death [96]. Furthermore, the production of nitric oxide and other free radicals can modify oxidative metabolism and impair ATP production [13, 19]. Changes in mitochondrial properties can further limit oxidative metabolism [97]. Not surprisingly, several studies have shown that antioxidant treatment enhances neuroprotection and recovery after stroke [98–101].
The release of glutathione and SOD by astrocytes has been reported and is suggested to play an important role in maintaining and enhancing neuronal survival, yet they are able to reduce ascorbate for further neuronal antioxidant defense Fig. (2) [10, 102–106]. Interestingly, neurons cocultured with astrocytes exhibit higher levels of glutathione compared with neurons cultured alone [107]. Although astrocytes upregulate SOD after cerebral ischemia [108], they do not appear to increase levels of glutathione in ischemic conditions [109]. It is unknown whether ischemia alters astrocytic ascorbate levels, but osmotic swelling from ischemia results in increased astrocyte release of ascorbate in vitro [110], suggesting that similar mechanisms may occur in vivo.
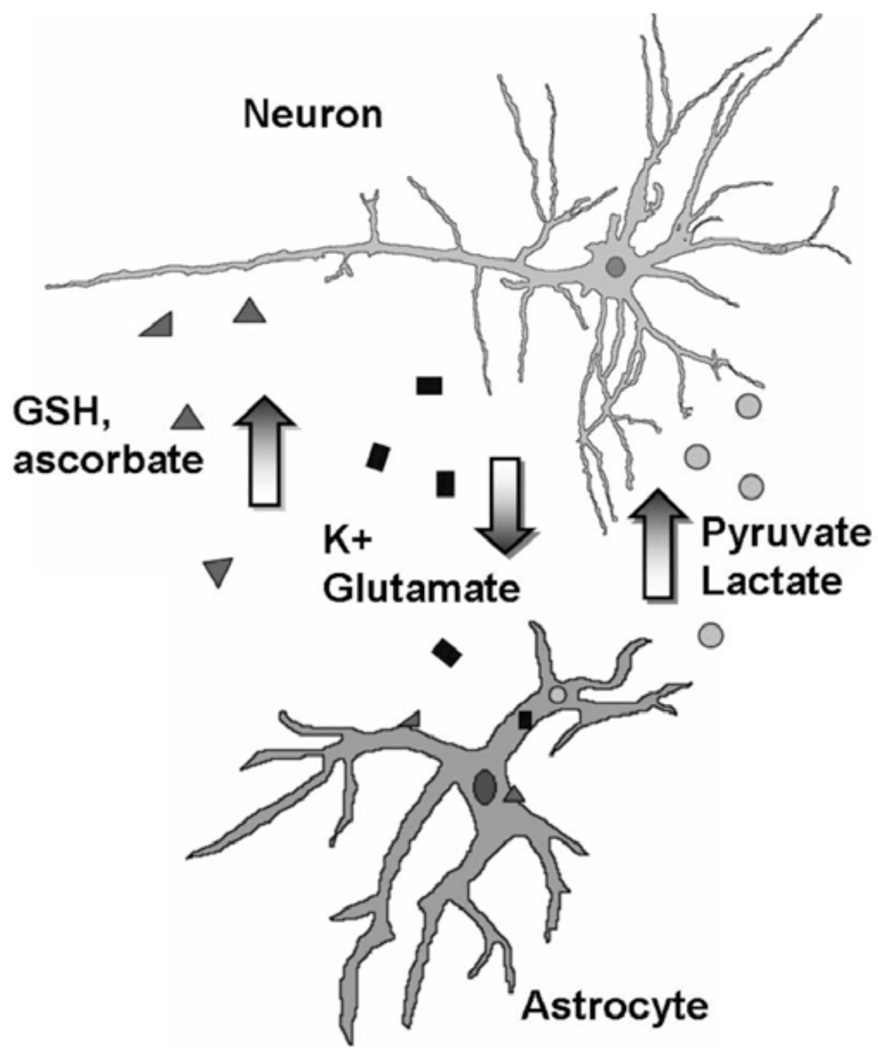
Fig. (2).
Mechanisms of astrocyte support of neurons important in stroke. Antioxidant defense includes release of glutathione and ascorbate. Regulation of extracellular levels of ions and neuro-transmitters, especially K+ and glutamate, strongly influences neuronal excitability. Elevated extracellular K+ triggers astrocyte glycolysis and enhances lactate and pyruvate release which support neuronal metabolism. Sodium dependent glutamate uptake by astrocytes activates the Na+/K+ ATPase, stimulating glycolytic activity and production of lactate. Astrocytes and neurons are also coupled by the glutamate-glutamine cycle. Astrocytes take up glutamate, convert it to glutamine, and release glutamine to the extracellular space where it is taken up by neurons and used to synthesize glutamate to replenish the neurotransmitter pool.
Several treatments that attenuate ischemic injury result in increased glutathione levels [111, 112]. SOD converts superoxide into oxygen and hydrogen peroxide. Similar to glutathione, many treatments that ameliorate stroke damage are accompanied by an increase in SOD [113, 114]. Furthermore, rodents overexpressing SOD1 have significantly smaller injuries after both focal and global ischemia [115, 116], while mice with decreased SOD1 have larger infarcts [117]. Finally, ascorbate can also reduce oxidative stress [118]. Treatment with dehydroascorbic acid, a blood-brain-barrier-permeable precursor to ascorbic acid, is protective after MCAO [119]. Dehydroascorbic acid is taken up by astrocytes and released as ascorbic acid [12], a process increased by propofol [120], a treatment that can be protective after stroke [121]. In summary, astrocytes are important producers of antioxidants in the normal CNS, and astrocyte production of these molecules after stroke may enhance neuronal survival and protect astrocyte function.
5.2. Glutamate Regulation
Astrocytes are key players in the regulation of neuro-transmitters in the CNS. Astrocytes make glutamine, the precursor for the neurotransmitters glutamate and GABA [122] Fig. (2). Astrocyte production of neurotransmitter precursors is impaired after MCAO, and alterations in neuro-transmitter levels occur throughout the brain following stroke, possibly contributing to neuronal death [123, 124].
Astrocytes are primarily responsible for glutamate uptake in the normal brain using the astrocyte specific glutamate transporters GLAST and GLT-1 (Fig. 2) [125–127], as excess glutamate leads to cell death via excitotoxicity [128]. Glutamate transporter levels in astrocytes decrease acutely following global ischemia [38, 129] and neonatal hypoxia-ischemia [130], most likely exacerbating neuronal death as a result of glutamate-induced excitoxicity. Despite the therapeutic potential of increasing astrocyte glutamate transport after stroke, few groups have explored this possibility. Carnosine, shown to be protective after focal ischemia, may partially be effective because it prevents loss of GLT-1 on astrocytes, resulting in attenuated excitotoxicity [131]. In a more direct assessment of how post-ischemic astrocyte glutamate transporters contribute to neuronal survival, our laboratory has shown that upregulation of GLT-1 on astrocytes using ceftriaxone protects CA1 neurons after global ischemia [129], similar to its effects in focal cerebral ischemia [132].
5.3. Potassium Uptake and Energy Metabolism
Astrocytes also regulate neuronal activation by extracellular potassium uptake [133] Fig. (2). Neurons release potassium after activation, and increased extracellular potassium leads to neuronal hyperexcitability [133], a phenomenon that occurs in ischemic conditions [134]. In addition to regulating neuronal activation, proper maintenance of ion gradients, such as potassium, is important in regulating cell volume in both normal and ischemic conditions [135, 136]. Astrocytes increase potassium transporter activity in response to transient in vitro ischemia [137]. Due to its effects on both neuronal activity and cell volume, increasing astrocytic potassium uptake may be a possible therapeutic target for stroke.
Astrocytes are also integral to normal maintenance of neuronal metabolism. When astrocytes take up extracellular glutamate as a result of neuronal activity, the Na+/ K+-ATPase, along with AMPA signaling, triggers astrocyte uptake of glucose from the blood, as astrocytic endfeet contact capillaries [138, 139]. This glucose is then made into lactate, a substrate for neuronal energy, to further “fuel” active neurons [140] Fig. (2). As mentioned above, astrocytes produce glutathione. In addition to its antioxidant properties, glutathione is needed for the conversion of methylglyoxal, a toxic by-product of metabolism, into D-Lactate by glyoxalase 1 [141]. Although the role of astrocyte metabolism is relatively well-established in normal tissue, the post-ischemic role of astrocyte metabolism maintenance is less clear [142]. After ischemia, astrocytes upregulate glucose transporters in order to provide energy to stressed/dying neuronal cells [143, 144]. Ethyl pyruvate, a derivative of the energy substrate pyruvate, is neuroprotective after stroke only when astrocytes are viable, suggesting that astrocytes are necessary for improvement in post-ischemic energy metabolism [122].
6. NOVEL STRATEGIES TO IMPROVE THE NEURONAL SUPPORTIVE ROLE OF ASTROCYTES
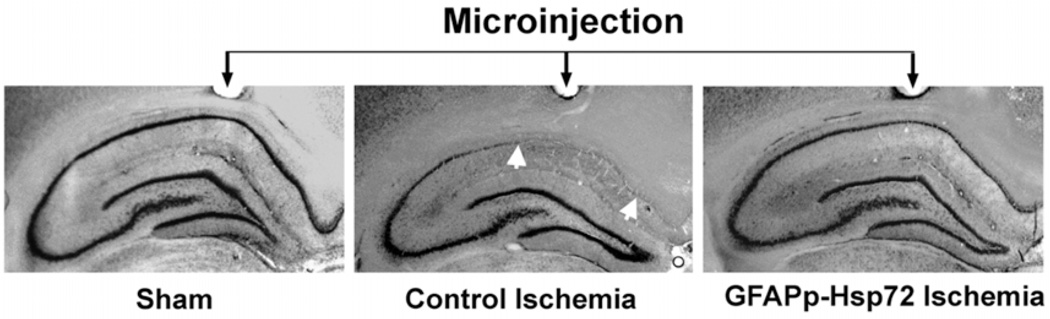
Although few studies have specifically targeted astrocytes for repair after stroke, there is some evidence that this can be a successful strategy. Recent results indicate that induction of BDNF in astrocytes by galectin-1 reduces neuronal apoptosis in ischemic boundary zone and improves functional recovery [145]. In addition, protection by pyruvate against glutamate neurotoxicity is mediated by astrocytes through a glutathione-dependent mechanism [146]. Our recent study demonstrated that enhancing astrocyte resistance to ischemic stress by overexpressing protective proteins or antioxidant enzyme results in improved survival of CA1 neurons following forebrain ischemia Fig. (3) [16]. Two well-studied protective proteins, heat shock protein 72 (Hsp72) and mitochondrial SOD, were genetically targeted for expression in astrocytes using the astrocyte-specific human GFAP promoter. In both cases protection was accompanied by preservation of the astrocytic glutamate transporter GLT-1, and reduced evidence of oxidative stress in the CA1 region [16]. Similarly, selective overexpression of excitatory amino acid transporter 2 (EAAT2) in astrocytes enhances neuroprotection from moderate hypoxia-ischemia [147].
Fig. (3).
Targeted over-expression of Hsp72 in astrocytes reduces the vulnerability of CA1 neurons to forebrain ischemia. Selective overexpression of Hsp72 in astrocytes by expressing it from the astrocyte specific GFAP promoter was achieved by unilateral stereotaxic injection of the expression plasmid just above the CA1 region of the hippocampus (black arrows for microinjection tracks) 2 days before rats were subjected to 15 min forebrain ischemia. Selective loss of CA1 hippocampal neurons (between white arrows in middle panel) was observed at 7 days reperfusion by cresyl violet staining. The loss of CA1 hippocampal neurons was significantly reduced with astrocytic Hsp72 overex-pression (right panel) compared to the neuronal loss seen with injection of control DNA (middle panel). Modified from [16].
7. TRANSLATING INSIGHTS INTO PROTECTION INTO CLINICAL APPLICATIONS
Many factors have been identified that likely contribute to the failure in translation seen so far with stroke therapies. Currently, the only approved stroke therapy is thrombolysis induced by intravenous administration of recombinant tissue plasminogen activator [148]; however, because of a short therapeutic time window, only a small fraction of patients benefit from this treatment. Hypothermia is the only accepted acute treatment to reduce brain injury following cardiac arrest and resuscitation [4]. Thus far many clinical trials have focused on treatments that would likely be beneficial to neurons, with fewer studies focused on mechanisms that might benefit all cell types or specifically targeting other cell types, such as astrocytes. Often the consequence of these treatments on the astrocyte response is not considered. Several examples of past and ongoing clinical trials are discussed below, with specific attention to how these treatments may alter astrocyte response or viability.
Several clinical trials have targeted manipulation of the inflammatory response to ischemia, as stroke patients with systemic inflammation exhibit poorer outcomes [149]. Although anti-inflammatory therapy decreases infarct size and improves neurological sequelae in experimental animal models of stroke [150], human trials with anti-neutrophil therapy have not shown a clear benefit [151, 152]. In addition, recent clinical trials in which anti-CD11/18 antibodies were administered to human subjects were unsuccessful [153]. Likewise, a double-blinded, placebo-controlled clinical trial in which anti–ICAM-1 antibody was administered within 6 hours of stroke symptoms showed disappointing results [151]. In understanding these results it is important to recall that while experimental stroke is relatively homogeneous concerning size, territory, and etiology, with more consistent inflammatory response, human stroke is extremely heterogeneous [154], with different vascular territories and extents of injury. In addition, these mediators are known to affect many organ systems beyond the central nervous system. Systemic administration of anti-inflammatory agents may have exacerbated the relative state of immunocompromise seen in stroke patients, thereby confounding the outcome. Furthermore, inflammation and astrocyte response are likely closely connected. Although there is little evidence for a direct relationship between neutrophils and astrocytes, it has been shown that mice with a blunted inflammatory response exhibit increased loss of GFAP-positive astrocytes after cortical stab injury [155]. Because astrocytic glial scar formation is important in protection of spared tissue from further damage [156], it is possible that treatments that drastically attenuate inflammation lead to a stunted astrocyte response that is deleterious to recovery.
Another drug that has advanced to clinical study is DP-b99, currently in phase III studies for acute stroke. DP-b99 is a membrane active chelator derivative of the known calcium chelator, BAPTA spell out [157]. A lipophilic chelator of calcium, zinc and copper ions, DP-b99 sequesters metal ions only within and in to cell membranes. This clinical trial is especially attractive because sequestration of calcium, zinc, and copper are potentially beneficial not only to neurons, but also to astrocytes. It has been shown in Alzheimer’s disease that beta amyloid increases astrocyte calcium influx, which causes decreased glutathione levels [158]. Zinc chloride is toxic to astrocytes as well as neurons in vitro [159]. Similarly, astrocytes exposed to neocuprine exhibit increased copper influx and undergo apoptotic cell death [160]. Approaches that benefit multiple cell types, including astrocytes, are more likely to be successful.
Other current ongoing clinical trials focus on neuroprotective agents for the purpose of aiding neurological recovery after stroke. Minocycline (Phase I), edavarone (Phase IV), propanolol (a β-blocker; phase II and III), and more recently arundic acid have been previously shown to be protective and enhance neuronal survival in stroke [161–165], though by targeting different mechanisms. Some additional completed and ongoing trials are summarized in Table 1. Preclinical research needs to consider these clinical results, and assess effects on astrocytes as well as neurons.
Table 1.
Overview of Some Completed and Ongoing Clinical Trials for Stroke
| Mechanism and Compound | |
|---|---|
| Anticoagulants | |
| Ancrod® (Viprinex) | |
| Aspirin added to IV TPA | |
| Angiotensin II Receptor Antagonist | |
| Diovan® (valsartan) | |
| Losartan and amlodipine | |
| Calcium Channel Blockers | |
| Cilnidipine | |
| Amlodipine | |
| Inhibiton of Myeloperoxidases | |
| Dapsone | |
| Antiplatelet Therapy | |
| Eptifibatide and rt-PA | |
| Glutamate Antagonists | |
| YM872 | |
| Granulocyte Colony Stimulating Factor | |
| AX200 (G-CSF) | |
| Free Radical Scavenging | |
| NXY-059 | |
Although anti-inflammatory strategies to diminish ischemic brain injury have failed thus far, continued elucidation of the complex interactions involved in modulating the inflammatory response may still enable novel therapeutic approaches that translate successfully into clinical efficacy.
CONCLUSIONS
Traditionally, stroke research has focused on neurons and often ignored effects on glial cells. It is increasingly evident that glia are vital to both normal CNS functioning and also play important roles in neuropathological conditions. Although astrocytes form an inhibitory glial scar following ischemia, they also perform functions necessary for neuronal survival and well-being, such as maintaining low extracellular glutamate levels and providing antioxidant protection. Because they have a great many functions, astrocytes are attractive candidates as therapeutic targets. By striving to shift astrocytes towards a pro-reparative, neuronal-supportive phenotype following stroke, future clinical therapies may well be more successful in protecting neurons from ischemic damage and promoting repair.
ACKNOWLEDGEMENTS
This work was supported by NIH grants CM49831, N5053898, and NS014543 to RGG.
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Blakeley JO, Llinas RH. Thrombolytic therapy for acute ischemic stroke. J. Neurol. Sci. 2007;261:55–62. doi: 10.1016/j.jns.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 3.Marder VJ, Jahan R, Gruber T, Goyal A, Arora V. Thrombolysis with plasmin: implications for stroke treatment. Stroke. 2010;41:S45–S49. doi: 10.1161/STROKEAHA.110.595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.Howells DW, Porritt MJ, Rewell SS, O'Collins V, Sena ES, van der Worp HB, Traystman RJ, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kettenmann H, Ramson BR. Neuroglia. New York: Oxford University Press; 1995. [Google Scholar]
- 7.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J. Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr. Mol. Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 11.Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol. Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can. J. Physiol. Pharmacol. 1997;75:1149–1163. [PubMed] [Google Scholar]
- 13.Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedergaard M, Dirnagl U. Role of glial cells in cerebral ischemia. Glia. 2005;50:281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J. Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: networking for survival? Neurochem. Res. 2003;28:293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- 18.Giffard RG, Swanson RA. Ischemia-induced programmed cell death in astrocytes. Glia. 2005;50:299–306. doi: 10.1002/glia.20167. [DOI] [PubMed] [Google Scholar]
- 19.Almeida A, Delgado-Esteban M, Bolanos JP, Medina JM. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J. Neurochem. 2002;81:207–217. doi: 10.1046/j.1471-4159.2002.00827.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Sapolsky RM, Giffard RG. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp. Neurol. 2001;169:416–424. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G, Flavin MP. Differential sensitivity of rat hippocampal and cortical astrocytes to oxygen-glucose deprivation injury. Neurosci. Lett. 2000;285:177–180. doi: 10.1016/s0304-3940(00)01056-9. [DOI] [PubMed] [Google Scholar]
- 22.Lukaszevicz AC, Sampaio N, Guegan C, Benchoua A, Couriaud C, Chevalier E, Sola B, Lacombe P, Onteniente B. High sensitivity of protoplasmic cortical astroglia to focal ischemia. J. Cereb. Blood Flow Metab. 2002;22:289–298. doi: 10.1097/00004647-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Bondarenko A, Chesler M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001;34:134–142. doi: 10.1002/glia.1048. [DOI] [PubMed] [Google Scholar]
- 24.Giffard RG, Monyer H, Choi DW. Selective vulnerability of cultured cortical glia to injury by extracellular acidosis. Brain Res. 1990;530:138–141. doi: 10.1016/0006-8993(90)90670-7. [DOI] [PubMed] [Google Scholar]
- 25.Swanson RA, Farrell K, Stein BA. Astrocyte energetics, function, and death under conditions of incomplete ischemia: a mechanism of glial death in the penumbra. Glia. 1997;21:142–153. doi: 10.1002/(sici)1098-1136(199709)21:1<142::aid-glia16>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Tombaugh GC, Sapolsky RM. Mechanistic distinctions between excitotoxic and acidotic hippocampal damage in an in vitro model of ischemia. J. Cereb. Blood Flow Metab. 1990;10:527–535. doi: 10.1038/jcbfm.1990.94. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Swanson RA. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 28.Lee MH, Kim H, Kim SS, Lee TH, Lim BV, Chang HK, Jang MH, Shin MC, Shin MS, Kim CJ. Treadmill exercise suppresses ischemia-induced increment in apoptosis and cell proliferation in hippocampal dentate gyrus of gerbils. Life Sci. 2003;73:2455–2465. doi: 10.1016/s0024-3205(03)00655-6. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Chopp M, Zhang ZG, Zhang RL. Expression of glial fibrillary acidic protein in areas of focal cerebral ischemia accompanies neuronal expression of 72-kDa heat shock protein. J. Neurol. Sci. 1995;128:134–142. doi: 10.1016/0022-510x(94)00228-g. [DOI] [PubMed] [Google Scholar]
- 30.Schroeter M, Schiene K, Kraemer M, Hagemann G, Weigel H, Eysel UT, Witte OW, Stoll G. Astroglial responses in photochemically induced focal ischemia of the rat cortex. Exp Brain Res. 1995;106:1–6. doi: 10.1007/BF00241351. [DOI] [PubMed] [Google Scholar]
- 31.Van Beek J, Chan P, Bernaudin M, Petit E, MacKenzie ET, Fontaine M. Glial responses, clusterin, and complement in permanent focal cerebral ischemia in the mouse. Glia. 2000;31:39–50. doi: 10.1002/(sici)1098-1136(200007)31:1<39::aid-glia40>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita K, Vogel P, Fritze K, Back T, Hossmann KA, Wiessner C. Monitoring the temporal and spatial activation pattern of astrocytes in focal cerebral ischemia using in situ hybridization to GFAP mRNA: comparison with sgp-2 and hsp70 mRNA and the effect of glutamate receptor antagonists. Brain Res. 1996;735:285–297. doi: 10.1016/0006-8993(96)00578-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Smith CL, Barone FC, Ellison JA, Lysko PG, Li K, Simpson IA. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res. Mol. Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 34.Dugan LL, Bruno VM, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: glia exacerbate AMPA neurotoxicity. J. Neurosci. 1995;15:4545–4555. doi: 10.1523/JNEUROSCI.15-06-04545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci. Lett. 1989;103:162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- 36.Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J. Neurochem. 2007;102:1383–1394. doi: 10.1111/j.1471-4159.2007.4634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen JC, Hsu-Chou H, Lu JL, Chiang YC, Huang HM, Wang HL, Wu T, Liao JJ, Yeh TS. Down-regulation of the glial glutamate transporter GLT-1 in rat hippocampus and striatum and its modulation by a group III metabotropic glutamate receptor antagonist following transient global forebrain ischemia. Neuro-pharmacology. 2005;49:703–714. doi: 10.1016/j.neuropharm.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Yeh TH, Hwang HM, Chen JJ, Wu T, Li AH, Wang HL. Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol. Dis. 2005;18:476–483. doi: 10.1016/j.nbd.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Ito U, Hakamata Y, Kawakami E, Oyanagi K. Degeneration of astrocytic processes and their mitochondria in cerebral cortical regions peripheral to the cortical infarction: heterogeneity of their disintegration is closely associated with disseminated selective neuronal necrosis and maturation of injury. Stroke. 2009;40:2173–2181. doi: 10.1161/STROKEAHA.108.534990. [DOI] [PubMed] [Google Scholar]
- 40.Ito U, Hakamata Y, Kawakami E, Oyanagi K. Temporary focal cerebral ischemia results in swollen astrocytic end-feet that compress microvessels and lead to focal cortical infarction. J. Cereb. Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vangeison G, Rempe DA. The Janus-faced effects of hypoxia on astrocyte function. Neuroscientist. 2009;15:579–588. doi: 10.1177/1073858409332405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bidmon HJ, Jancsik V, Schleicher A, Hagemann G, Witte OW, Woodhams P, Zilles K. Structural alterations and changes in cytoskeletal proteins and proteoglycans after focal cortical ischemia. Neuroscience. 1998;82:397–420. doi: 10.1016/s0306-4522(97)00289-3. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda Y, Tateishi N, Shimoda T, Satoh S, Ogitani E, Fu-jita S. Relationship between S100beta and GFAP expression in astrocytes during infarction and glial scar formation after mild transient ischemia. Brain Res. 2004;1021:20–31. doi: 10.1016/j.brainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- 45.Gris P, Tighe A, Levin D, Sharma R, Brown A. Transcriptional regulation of scar gene expression in primary astrocytes. Glia. 2007;55:1145–1155. doi: 10.1002/glia.20537. [DOI] [PubMed] [Google Scholar]
- 46.Martinez AD, Saez JC. Regulation of astrocyte gap junctions by hypoxia-reoxygenation. Brain Res. Brain Res. Rev. 2000;32:250–258. doi: 10.1016/s0165-0173(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 47.Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, Hansen TW, Goldman S, Nedergaard M. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Redecker C, Yu ZY, Xie MJ, Tian DS, Zhang L, Bu BT, Witte OW. Rat focal cerebral ischemia induced astrocyte proliferation and delayed neuronal death are attenuated by cyclin-dependent kinase inhibition. J. Clin. Neurosci. 2008;15:278–285. doi: 10.1016/j.jocn.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Forslin Aronsson S, Spulber S, Popescu LM, Winblad B, Post C, Oprica M, Schultzberg M. alpha-Melanocyte-stimulating hormone is neuroprotective in rat global cerebral ischemia. Neuropeptides. 2006;40:65–75. doi: 10.1016/j.npep.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Fang SH, Wei EQ, Zhou Y, Wang ML, Zhang WP, Yu GL, Chu LS, Chen Z. Increased expression of cysteinyl leukotriene receptor-1 in the brain mediates neuronal damage and astrogliosis after focal cerebral ischemia in rats. Neuroscience. 2006;140:969–979. doi: 10.1016/j.neuroscience.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 51.Ye YL, Shi WZ, Zhang WP, Wang ML, Zhou Y, Fang SH, Liu LY, Zhang Q, Yu YP, Wei EQ. Cilostazol, a phosphodiesterase 3 inhibitor, protects mice against acute and late ischemic brain injuries. Eur. J. Pharmacol. 2007;557:23–31. doi: 10.1016/j.ejphar.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Y, Fang SH, Ye YL, Chu LS, Zhang WP, Wang ML, Wei EQ. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2006;27:1103–1110. doi: 10.1111/j.1745-7254.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 53.Chung JH, Lee EY, Jang MH, Kim CJ, Kim J, Ha E, Park HK, Choi S, Lee H, Park SH, Leem KH, Kim EH. Acupuncture decreases ischemia-induced apoptosis and cell proliferation in dentate gyrus of gerbils. Neurol. Res. 2007;29 Suppl 1:S23–S27. doi: 10.1179/016164107X172239. [DOI] [PubMed] [Google Scholar]
- 54.del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr. Neurovasc. Res. 2008;5:71–81. doi: 10.2174/156720208783565645. [DOI] [PubMed] [Google Scholar]
- 56.Nawashiro H, Brenner M, Fukui S, Shima K, Hallenbeck JM. High susceptibility to cerebral ischemia in GFAP-null mice. J. Cereb. Blood Flow Metab. 2000;20:1040–1044. doi: 10.1097/00004647-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Mara-gakis N, Nilsson M, Pekny M. Protective role of reactive astrocytes in brain ischemia. J. Cereb. Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 58.Kinoshita A, Yamada K, Kohmura E, Hayakawa T. Effect of astrocyte-derived factors on ischemic brain edema induced by rat MCA occlusion. APMIS. 1990;98:851–857. doi: 10.1111/j.1699-0463.1990.tb05006.x. [DOI] [PubMed] [Google Scholar]
- 59.Buchhold B, Mogoanta L, Suofu Y, Hamm A, Walker L, Kessler C, Popa-Wagner A. Environmental enrichment improves functional and neuropathological indices following stroke in young and aged rats. Restor. Neurol. Neurosci. 2007;25:467–484. [PubMed] [Google Scholar]
- 60.Keiner S, Wurm F, Kunze A, Witte OW, Redecker C. Rehabilitative therapies differentially alter proliferation and survival of glial cell populations in the perilesional zone of cortical infarcts. Glia. 2008;56:516–527. doi: 10.1002/glia.20632. [DOI] [PubMed] [Google Scholar]
- 61.Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113:277–293. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 63.Yang M, Wei X, Li J, Heinel LA, Rosenwasser R, Iacovitti L. Changes in Host Blood Factors and Brain Glia Accompanying the Functional Recovery after Systemic Administration of Bone Marrow Stem Cells in Ischemic Stroke Rats. Cell Transplant. 2010 doi: 10.3727/096368910X503415. [DOI] [PubMed] [Google Scholar]
- 64.Sharif A, Legendre P, Prevot V, Allet C, Romao L, Studler JM, Chneiweiss H, Junier MP. Transforming growth factor alpha promotes sequential conversion of mature astrocytes into neural progenitors and stem cells. Oncogene. 2007;26:2695–2706. doi: 10.1038/sj.onc.1210071. [DOI] [PubMed] [Google Scholar]
- 65.Justicia C, Perez-Asensio FJ, Burguete MC, Salom JB, Planas AM. Administration of transforming growth factor-alpha reduces infarct volume after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2001;21:1097–1104. doi: 10.1097/00004647-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Currie RW, Ellison JA, White RF, Feuerstein GZ, Wang X, Barone FC. Benign focal ischemic preconditioning induces neuronal Hsp70 and prolonged astrogliosis with expression of Hsp27. Brain Res. 2000;863:169–181. doi: 10.1016/s0006-8993(00)02133-8. [DOI] [PubMed] [Google Scholar]
- 67.Nakase T, Fushiki S, Naus CC. Astrocytic gap junctions composed of connexin 43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke. 2003;34:1987–1993. doi: 10.1161/01.STR.0000079814.72027.34. [DOI] [PubMed] [Google Scholar]
- 68.Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr. Pharm. Des. 2008;14:3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 69.Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J. Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- 70.Orzylowska O, Oderfeld-Nowak B, Zaremba M, Januszewski S, Mossakowski M. Prolonged and concomitant induction of astroglial immunoreactivity of interleukin-1beta and interleukin-6 in the rat hippocampus after transient global ischemia. Neurosci. Lett. 1999;263:72–76. doi: 10.1016/s0304-3940(99)00043-9. [DOI] [PubMed] [Google Scholar]
- 71.Yang GY, Gong C, Qin Z, Liu XH, Lorris Betz A. Tumor necrosis factor alpha expression produces increased blood-brain barrier permeability following temporary focal cerebral ischemia in mice. Brain Res. Mol. Brain Res. 1999;69:135–143. doi: 10.1016/s0169-328x(99)00007-8. [DOI] [PubMed] [Google Scholar]
- 72.Wang ZQ, Wu DC, Huang FP, Yang GY. Inhibition of MEK/ERK 1/2 pathway reduces pro-inflammatory cytokine inter-leukin-1 expression in focal cerebral ischemia. Brain Res. 2004;996:55–66. doi: 10.1016/j.brainres.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 73.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 74.Clark WM, Lutsep HL. Potential of anticytokine therapies in central nervous system ischaemia. Exp. Opin. Biol. Ther. 2001;1:227–237. doi: 10.1517/14712598.1.2.227. [DOI] [PubMed] [Google Scholar]
- 75.Venters HD, Dantzer R, Kelley KW. Tumor necrosis factor-alpha induces neuronal death by silencing survival signals generated by the type I insulin-like growth factor receptor. Ann. N. Y. Acad. Sci. 2000;917:210–220. doi: 10.1111/j.1749-6632.2000.tb05385.x. [DOI] [PubMed] [Google Scholar]
- 76.Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog. Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 77.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 78.Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem. Res. 2005;30:883–893. doi: 10.1007/s11064-005-6961-x. [DOI] [PubMed] [Google Scholar]
- 79.Dvoriantchikova G, Barakat D, Brambilla R, Agudelo C, Hernandez E, Bethea JR, Shestopalov VI, Ivanov D. Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury. Eur. J. Neurosci. 2009;30:175–185. doi: 10.1111/j.1460-9568.2009.06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoehn B, Ringer TM, Xu L, Giffard RG, Sapolsky RM, Steinberg GK, Yenari MA. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J. Cereb. Blood Flow Metab. 2001;21:1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Kim JS. Cytokines and adhesion molecules in stroke and related diseases. J. Neurol. Sci. 1996;137:69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- 82.Sofroniew MV. Astrocyte failure as a cause of CNS dysfunction. Mol. Psychiatry. 2000;5:230–232. doi: 10.1038/sj.mp.4000753. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J. Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali C, Nicole O, Docagne F, Lesne S, MacKenzie ET, Nouvelot A, Buisson A, Vivien D. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J. Cereb. Blood Flow Metab. 2000;20:956–966. doi: 10.1097/00004647-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 85.Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.del Zoppo GJ, Becker KJ, Hallenbeck JM. Inflammation after stroke: is it harmful? Arch Neurol. 2001;58:669–672. doi: 10.1001/archneur.58.4.669. [DOI] [PubMed] [Google Scholar]
- 87.Dirnagl U. Inflammation in stroke: the good, the bad, and the unknown. Ernst. Schering Res. Found. Workshop. 2004:87–99. doi: 10.1007/978-3-662-05426-0_5. [DOI] [PubMed] [Google Scholar]
- 88.Goel G, Guo M, Ding J, Dornbos D, 3rd, Ali A, Shenaq M, Guthikonda M, Ding Y. Combined effect of tumor necrosis factor (TNF)-alpha and heat shock protein (HSP)-70 in reducing apoptotic injury in hypoxia: a cell culture study. Neurosci. Lett. 2010;483:162–166. doi: 10.1016/j.neulet.2010.07.069. [DOI] [PubMed] [Google Scholar]
- 89.Hart J. Inflammation. 2: Its role in the healing of chronic wounds. J. Wound. Care. 2002;11:245–249. doi: 10.12968/jowc.2002.11.7.26416. [DOI] [PubMed] [Google Scholar]
- 90.Pradillo JM, Fernandez-Lopez D, Garcia-Yebenes I, Sobrado M, Hurtado O, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J. Neurochem. 2009;109:287–294. doi: 10.1111/j.1471-4159.2009.05972.x. [DOI] [PubMed] [Google Scholar]
- 91.Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos. Trans. R Soc. Lond. B Biol. Sci. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suzuki M, Tabuchi M, Ikeda M, Tomita T. Concurrent formation of peroxynitrite with the expression of inducible nitric oxide synthase in the brain during middle cerebral artery occlusion and reperfusion in rats. Brain Res. 2002;951:113–120. doi: 10.1016/s0006-8993(02)03145-1. [DOI] [PubMed] [Google Scholar]
- 93.Kader A, Frazzini VI, Solomon RA, Trifiletti RR. Nitric oxide production during focal cerebral ischemia in rats. Stroke. 1993;24:1709–1716. doi: 10.1161/01.str.24.11.1709. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q, Chen C, Lu J, Xie M, Pan D, Luo X, Yu Z, Dong Q, Wang W. Cell cycle inhibition attenuates microglial proliferation and production of IL-1beta, MIP-1alpha, and NO after focal cerebral ischemia in the rat. Glia. 2009;57:908–920. doi: 10.1002/glia.20816. [DOI] [PubMed] [Google Scholar]
- 95.Sugawara T, Chan PH. Reactive oxygen radicals and patho-genesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 96.Mitrovic B, Ignarro LJ, Vinters HV, Akers MA, Schmid I, Uittenbogaart C, Merrill JE. Nitric oxide induces necrotic but not apoptotic cell death in oligodendrocytes. Neuroscience. 1995;65:531–539. doi: 10.1016/0306-4522(94)00491-m. [DOI] [PubMed] [Google Scholar]
- 97.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem. Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 98.Clark WM, Rinker LG, Lessov NS, Lowery SL, Cipolla MJ. Efficacy of antioxidant therapies in transient focal ischemia in mice. Stroke. 2001;32:1000–1004. doi: 10.1161/01.str.32.4.1000. [DOI] [PubMed] [Google Scholar]
- 99.Cash D, Beech JS, Rayne RC, Bath PM, Meldrum BS, Williams SC. Neuroprotective effect of aminoguanidine on transient focal ischaemia in the rat brain. Brain Res. 2001;905:91–103. doi: 10.1016/s0006-8993(01)02508-2. [DOI] [PubMed] [Google Scholar]
- 100.Zhao Z, Cheng M, Maples KR, Ma JY, Buchan AM. NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat. Brain Res. 2001;909:46–50. doi: 10.1016/s0006-8993(01)02618-x. [DOI] [PubMed] [Google Scholar]
- 101.Thiyagarajan M, Kaul CL, Sharma SS. Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br. J. Pharmacol. 2004;142:899–911. doi: 10.1038/sj.bjp.0705811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem. Res. 2004;29:601–608. doi: 10.1023/b:nere.0000014830.06376.e6. [DOI] [PubMed] [Google Scholar]
- 103.Dringen R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 104.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 105.Lindenau J, Noack H, Possel H, Asayama K, Wolf G. Cellular distribution of superoxide dismutases in the rat CNS. Glia. 2000;29:25–34. doi: 10.1002/(sici)1098-1136(20000101)29:1<25::aid-glia3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 106.Sims NR, Nilsson M, Muyderman H. Mitochondrial glutathione: a modulator of brain cell death. J. Bioenerg. Biomembr. 2004;36:329–333. doi: 10.1023/B:JOBB.0000041763.63958.e7. [DOI] [PubMed] [Google Scholar]
- 107.Giordano G, Kavanagh TJ, Costa LG. Mouse cerebellar astrocytes protect cerebellar granule neurons against toxicity of the polybrominated diphenyl ether (PBDE) mixture DE-71. Neurotoxicology. 2009;30:326–329. doi: 10.1016/j.neuro.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu XH, Kato H, Nakata N, Kogure K, Kato K. An immunohistochemical study of copper/zinc superoxide dismutase and manganese superoxide dismutase in rat hippocampus after transient cerebral ischemia. Brain Res. 1993;625:29–37. doi: 10.1016/0006-8993(93)90134-9. [DOI] [PubMed] [Google Scholar]
- 109.Bragin DE, Zhou B, Ramamoorthy P, Muller WS, Connor JA, Shi H. Differential changes of glutathione levels in astrocytes and neurons in ischemic brains by two-photon imaging. J. Cereb. Blood Flow Metab. 2010;30:734–738. doi: 10.1038/jcbfm.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siushansian R, Dixon SJ, Wilson JX. Osmotic swelling stimulates ascorbate efflux from cerebral astrocytes. J. Neurochem. 1996;66:1227–1233. doi: 10.1046/j.1471-4159.1996.66031227.x. [DOI] [PubMed] [Google Scholar]
- 111.Hoda MN, Singh I, Singh AK, Khan M. Reduction of lipoxidative load by secretory phospholipase A2 inhibition protects against neurovascular injury following experimental stroke in rat. J. Neuroinflammation. 2009;6:21. doi: 10.1186/1742-2094-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rong ZT, Gong XJ, Sun HB, Li YM, Ji H. Protective effects of oleanolic acid on cerebral ischemic damage in vivo and H(2)O(2)-induced injury in vitro. Pharm. Biol. 2010 doi: 10.3109/13880209.2010.499130. [DOI] [PubMed] [Google Scholar]
- 113.Shukla PK, Khanna VK, Ali MM, Khan MY, Srimal RC. Anti-ischemic effect of curcumin in rat brain. Neurochem. Res. 2008;33:1036–1043. doi: 10.1007/s11064-007-9547-y. [DOI] [PubMed] [Google Scholar]
- 114.Dhote V, Balaraman R. Anti-oxidant activity mediated neuroprotective potential of trimetazidine on focal cerebral ischaemia-reperfusion injury in rats. Clin. Exp. Pharmacol. Physiol. 2008;35:630–637. doi: 10.1111/j.1440-1681.2008.04845.x. [DOI] [PubMed] [Google Scholar]
- 115.Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, Epstein CJ. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J. Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang G, Chan PH, Chen J, Carlson E, Chen SF, Wein-stein P, Epstein CJ, Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- 117.Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J. Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rose RC, Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J. 1993;7:1135–1142. [PubMed] [Google Scholar]
- 119.Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, Fox WD, Israel RJ, Boyd TA, Golde DW, Connolly ES., Jr Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc. Natl. Acad. Sci. USA. 2001;98:11720–11724. doi: 10.1073/pnas.171325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Daskalopoulos R, Korcok J, Tao L, Wilson JX. Accumulation of intracellular ascorbate from dehydroascorbic acid by astrocytes is decreased after oxidative stress and restored by propofol. Glia. 2002;39:124–132. doi: 10.1002/glia.10099. [DOI] [PubMed] [Google Scholar]
- 121.Wang HY, Wang GL, Yu YH, Wang Y. The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res. 2009;1297:177–184. doi: 10.1016/j.brainres.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 122.Tokumaru O, Kuroki C, Yoshimura N, Sakamoto T, Takei H, Ogata K, Kitano T, Nisimaru N, Yokoi I. Neuroprotective effects of ethyl pyruvate on brain energy metabolism after ischemia-reperfusion injury: a 31P-nuclear magnetic resonance study. Neurochem. Res. 2009;34:775–785. doi: 10.1007/s11064-008-9871-x. [DOI] [PubMed] [Google Scholar]
- 123.Haberg A, Qu H, Saether O, Unsgard G, Haraldseth O, Sonnewald U. Differences in neurotransmitter synthesis and intermediary metabolism between glutamatergic and GABAergic neurons during 4 hours of middle cerebral artery occlusion in the rat: the role of astrocytes in neuronal survival. J. Cereb. Blood Flow Metab. 2001;21:1451–1463. doi: 10.1097/00004647-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 124.Haberg AK, Qu H, Sonnewald U. Acute changes in intermediary metabolism in cerebellum and contralateral hemisphere following middle cerebral artery occlusion in rat. J. Neurochem. 2009;109 Suppl 1:174–181. doi: 10.1111/j.1471-4159.2009.05940.x. [DOI] [PubMed] [Google Scholar]
- 125.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 126.Romera C, Hurtado O, Botella SH, Lizasoain I, Cardenas A, Fernandez-Tome P, Leza JC, Lorenzo P, Moro MA. In vitro ischemic tolerance involves upregulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway. J. Neurosci. 2004;24:1350–1357. doi: 10.1523/JNEUROSCI.1596-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schousboe A, Waagepetersen HS. Glial modulation of GABAergic and glutamat ergic neurotransmission. Curr. Top. Med. Chem. 2006;6:929–934. doi: 10.2174/156802606777323719. [DOI] [PubMed] [Google Scholar]
- 128.Westbrook GL. Glutamate receptors and excitotoxicity. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1993;71:35–50. [PubMed] [Google Scholar]
- 129.Ouyang C, Guo L, Lu Q, Xu X, Wang H. Enhanced activity of GABA receptors inhibits glutamate release induced by focal cerebral ischemia in rat striatum. Neurosci. Lett. 2007;420:174–178. doi: 10.1016/j.neulet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 130.Fukamachi S, Furuta A, Ikeda T, Ikenoue T, Kaneoka T, Rothstein JD, Iwaki T. Altered expressions of glutamate transporter subtypes in rat model of neonatal cerebral hypoxia-ischemia. Brain Res. Dev. Brain Res. 2001;132:131–139. doi: 10.1016/s0165-3806(01)00303-0. [DOI] [PubMed] [Google Scholar]
- 131.Shen Y, He P, Fan YY, Zhang JX, Yan HJ, Hu WW, Ohtsu H, Chen Z. Carnosine protects against permanent cerebral ischemia in histidine decarboxylase knockout mice by reducing glutamate excitotoxicity. Free Radic. Biol. Med. 2010;48:727–735. doi: 10.1016/j.freeradbiomed.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 132.Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- 133.Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 134.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol. Rev. 1985;65:101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 135.Kahle KT, Simard JM, Staley KJ, Nahed BV, Jones PS, Sun D. Molecular mechanisms of ischemic cerebral edema: role of electroneutral ion transport. Physiology (Bethesda) 2009;24:257–265. doi: 10.1152/physiol.00015.2009. [DOI] [PubMed] [Google Scholar]
- 136.Leis JA, Bekar LK, Walz W. Potassium homeostasis in the ischemic brain. Glia. 2005;50:407–416. doi: 10.1002/glia.20145. [DOI] [PubMed] [Google Scholar]
- 137.Stanimirovic DB, Ball R, Durkin JP. Glutamate uptake and Na,K-ATPase activity in rat astrocyte cultures exposed to ischemia. Acta Neurochir. Suppl. 1997;70:1–3. doi: 10.1007/978-3-7091-6837-0_1. [DOI] [PubMed] [Google Scholar]
- 138.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 139.Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J. Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos. Trans. R Soc. Lond. B Biol. Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cliffe EE, Waley SG. The mechanism of the glyoxalase I reaction, and the effect of ophthalmic acid as an inhibitor. Biochem. J. 1961;79:475–482. doi: 10.1042/bj0790475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dienel GA, Hertz L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia. 2005;50:362–388. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- 143.Lee WH, Bondy CA. Ischemic injury induces brain glucose transporter gene expression. Endocrinology. 1993;133:2540–2544. doi: 10.1210/endo.133.6.8243275. [DOI] [PubMed] [Google Scholar]
- 144.Vannucci SJ, Reinhart R, Maher F, Bondy CA, Lee WH, Vannucci RC, Simpson IA. Alterations in GLUT1 and GLUT3 glucose transporter gene expression following unilateral hypoxia-ischemia in the immature rat brain. Brain Res. Dev. Brain Res. 1998;107:255–264. doi: 10.1016/s0165-3806(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 145.Qu WS, Wang YH, Wang JP, Tang YX, Zhang Q, Tian DS, Yu ZY, Xie MJ, Wang W. Galectin-1 Enhances Astrocytic BDNF Production and Improves Functional Outcome in Rats Following Ischemia. Neurochem. Res. 2010 doi: 10.1007/s11064-010-0234-z. [DOI] [PubMed] [Google Scholar]
- 146.Miao Y, Qiu Y, Lin Y, Miao Z, Zhang J, Lu X. Protection by pyruvate against glutamate neurotoxicity is mediated by astrocytes through a glutathione-dependent mechanism. Mol. Biol. Rep. 2010 doi: 10.1007/s11033-010-9998-0. [DOI] [PubMed] [Google Scholar]
- 147.Weller ML, Stone IM, Goss A, Rau T, Rova C, Poulsen DJ. Selective overexpression of excitatory amino acid transporter 2 (EAAT2) in astrocytes enhances neuroprotection from moderate but not severe hypoxia-ischemia. Neuroscience. 2008;155:1204–1211. doi: 10.1016/j.neuroscience.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wardlaw JM, Sandercock PA, Berge E. Thrombolytic therapy with recombinant tissue plasminogen activator for acute ischemic stroke: where do we go from here? A cumulative meta-analysis. Stroke. 2003;34:1437–1442. doi: 10.1161/01.STR.0000072513.72262.7E. [DOI] [PubMed] [Google Scholar]
- 149.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J. Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial Hsp70/Hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J. Cereb. Blood Flow Metab. 2009;29:365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Investigators E.A.S.T. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 152.Schneider D, Berrouschot J, Brandt T, Hacke W, Ferbert A, Norris SH, Polmar SH, Schafer E. Safety, pharmacokinetics and biological activity of enlimomab (anti-ICAM-1 antibody): an open-label, dose escalation study in patients hospitalized for acute stroke. Eur. Neurol. 1998;40:78–83. doi: 10.1159/000007962. [DOI] [PubMed] [Google Scholar]
- 153.Mocco J, Choudhri T, Huang J, Harfeldt E, Efros L, Klingbeil C, Vexler V, Hall W, Zhang Y, Mack W, Popilskis S, Pinsky DJ, Connolly ES., Jr HuEP5C7 as a humanized monoclonal anti-E/P-selectin neurovascular protective strategy in a blinded placebo-controlled trial of nonhuman primate stroke. Circ. Res. 2002;91:907–914. doi: 10.1161/01.res.0000042063.15901.20. [DOI] [PubMed] [Google Scholar]
- 154.Wu O, Christensen S, Hjort N, Dijkhuizen RM, Kucinski T, Fiehler J, Thomalla G, Rother J, Ostergaard L. Characterizing physiological heterogeneity of infarction risk in acute human ischaemic stroke using MRI. Brain. 2006;129:2384–2393. doi: 10.1093/brain/awl183. [DOI] [PubMed] [Google Scholar]
- 155.Hampton DW, Seitz A, Chen P, Heber-Katz E, Fawcett JW. Altered CNS response to injury in the MRL/MpJ mouse. Neuroscience. 2004;127:821–832. doi: 10.1016/j.neuroscience.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 156.Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 157.Rosenberg G, Angel I, Kozak A. Clinical pharmacology of DP-b99 in healthy volunteers: first administration to humans. Br. J. Clin. Pharmacol. 2005;60:7–16. doi: 10.1111/j.1365-2125.2005.02378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Swanson RA, Sharp FR. Zinc toxicity and induction of the 72 kD heat shock protein in primary astrocyte culture. Glia. 1992;6:198–205. doi: 10.1002/glia.440060307. [DOI] [PubMed] [Google Scholar]
- 160.Chen SH, Lin JK, Liu SH, Liang YC, Lin-Shiau SY. Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol. Sci. 2008;102:138–149. doi: 10.1093/toxsci/kfm292. [DOI] [PubMed] [Google Scholar]
- 161.Iwata M, Inoue S, Kawaguchi M, Nakamura M, Konishi N, Furuya H. Posttreatment but not pretreatment with selective beta-adrenoreceptor 1 antagonists provides neuroprotection in the hippocampus in rats subjected to transient forebrain ischemia. Anesth. Analg. 2010;110:1126–1132. doi: 10.1213/ANE.0b013e3181d278f7. [DOI] [PubMed] [Google Scholar]
- 162.Kikuchi K, Tancharoen S, Matsuda F, Biswas KK, Ito T, Morimoto Y, Oyama Y, Takenouchi K, Miura N, Arimura N, Nawa Y, Meng X, Shrestha B, Arimura S, Iwata M, Mera K, Sameshima H, Ohno Y, Maenosono R, Tajima Y, Uchikado H, Kuramoto T, Nakayama K, Shigemori M, Yoshida Y, Hashiguchi T, Maruyama I, Kawahara K. Edaravone attenuates cerebral ischemic injury by suppressing aquaporin-4. Biochem. Biophys. Res. Commun. 2009;390:1121–1125. doi: 10.1016/j.bbrc.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 163.Pettigrew LC, Kasner SE, Gorman M, Atkinson RP, Funakoshi Y, Ishibashi H. Effect of arundic acid on serum S-100beta in ischemic stroke. J. Neurol. Sci. 2006;251:57–61. doi: 10.1016/j.jns.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 164.Ueno Y, Zhang N, Miyamoto N, Tanaka R, Hattori N, Urabe T. Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience. 2009;162:317–327. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 165.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]