Abstract
The efficient induction of virus-specific mucosal antibodies is an important unmet objective in Human Immunodeficiency Virus Type-1 (HIV-1) vaccine research. One promising approach is sublingual (SL) immunization. We examined the effectiveness of SL delivery of two different viral vectors: (i) a recombinant adenovirus (rAd5), and (ii) a Herpes Simplex Virus Type-1 amplicon vector (HSV-1). Initial in vitro videomicroscopy experiments showed that rAd5 particles were trapped in saliva (i.e., that Ad5 was mucoadhesive) - unlike HSV-1 virions, which migrated freely in both saliva and water. In vivo imaging studies in mice revealed that only the rAd5 vector efficiently transduced the SL epithelium. Consistent with this, SL delivery of an rAd5 encoding HIV-1 envelope glycoprotein (Env) resulted in robust antigen-specific antibody responses in plasma and in vaginal washes, whereas SL delivery of a HSV-1 amplicon vector encoding HIV-1 Env failed to elicit Env-specific antibodies. In contrast, both vectors elicited equivalent humoral responses following intramuscular (IM) delivery. Finally, SL delivery of the rAd5:Env vector resulted in elevated levels of Env-specific serum IgA, and vaginal IgA and IgG, when compared to IM delivery of the same vector. These results findings shed light on vector properties (mucoadhesion, penetration of the sublingual barrier) which may be important for the induction of potent humoral immune responses following sublingual vector administration. Our data also show that SL delivery of an Env-encoding rAd5 vector can elicit a potent antigen-specific mucosal antibody response in the absence of adjuvant. Overall, these findings support the further exploration of the SL delivery route for HIV-1 vaccine delivery.
Keywords: sublingual, HIV-1, Env, adenovirus, HSV, herpes simplex virus, vaccine, saliva, antibody, IgA, mucosal
1. Introduction
Virus-specific mucosal antibodies have the potential to reduce or prevent the sexual transmission of the Human Immunodeficiency Virus Type-1 (HIV-1). To date, however, attempts to elicit robust HIV-1 specific mucosal antibodies through immunization have yielded suboptimal results. One approach that may have promise is sublingual (SL) vaccine delivery.
It has long been known that SL delivery of xenobiotics can result in efficient drug absorbtion into systemic circulation, as reflected by the early use of the SL route to administer nitroglycerin for treatment of angina pectoris [1, 2]. Sublingual immunotherapy has also been shown to be effective at modulating immune responses to inhaled allergens [3–5], and phase III trials have shown that SL grass pollen immunotherapy can reduce allergic symptoms in adults with allergic rhinitis [5–9].
The success of sublingual immunotherapy has led to the exploration of the SL administration route for vaccine delivery against other antigens. Several studies have shown that SL delivery can elicit strong, mucosal, antigen-specific immune responses against both soluble, non-replicating recombinant proteins [10–16] and virally encoded antigens [17]. In addition, sublingual immunization offers several important conceptual advantages over other vaccine delivery methods. First, it is needle-free. Second, it has a strong safety profile associated with minimal side-effects [5–9]. Third, it provides an attractive approach to mucosal immunization. Alternate sites of mucosal immunization, such as the intrarectal route, may prove to be less acceptable [18] or (in the case of intranasal delivery) have the potential to lead to vector penetration into the CNS [19].
Recently, it was shown that SL administration of a recombinant, replication-defective adenovirus serotype 5 (Ad5) vector encoding HIV-1 Gag induced strong antigen-specific cellular immune responses [20]. This response was not affected by preexisting Ad5 immunity. These studies point to the potential utility of SL delivery of viral vectors encoding HIV-1 antigens, and prompted us to examine whether SL administration of virus vectors encoding the HIV-1 envelope glycoprotein (Env) could elicit antigen-specific humoral immune responses.
Here, we show that Ad5 virus particles, but not Herpes Simplex Virus Type-1 (HSV-1) virions, were mucoadhesive (i.e., trapped by saliva) and recombinant rAd5 vectors, but not HSV-1 amplicon vectors, were able to efficiently induce expression of an encoded transgene following sublingual delivery. Consistent with this, SL delivery of a rAd5 vector encoding HIV-1 Env resulted in robust, antigen-specific serum and mucosal IgG and IgA antibody responses. IgA responses were markedly higher in both serum and vaginal washes following SL vector delivery, as compared to the more standard IM route of administration. In contrast, SL delivery of a HSV-1 amplicon vector encoding HIV-1 Env failed to elicit detectable antigen-specific antibody responses, even though IM delivery of the same vector elicited a potent serum IgG antibody response to Env. Collectively, these findings shed light on vector properties which may be important for the induction of potent humoral immune responses following sublingual vector administration.
2. Materials and Methods
2.1 Construction of recombinant virus vectors
Construction of an HSV-1 amplicon vector expressing HIV-1 gp120 (HSV-1:gp120) has been previously described [21, 22] as has the process of vector packaging and purification [22, 23]. The recombinant adenovirus serotype 5 (rAd5) vectors used in this study were constructed using the AdEasy™ vector system (QBiogene, Carlsbad, CA) [24]. Both a human codon optimized HIV-1 Env gp120 (strain MN) gene [22] as well as a human codon optimized HIV-1 Env gp140 (YU2 strain) gene [25] were PCR-amplified and cloned into the adenovirus shuttle vector pShuttle which contains a CMV promoter upstream of the desired gene insert [26]. Viral titers were determined by real-time DNA PCR using Taqman probes and primers directed to the adenovirus hexon gene as described [26].
2.2 Preparation of fluorescent virus particles
For studies requiring fluorescent HSV-1 virions, we used K26GFP virus kindly provided by Dr. Prashant Desai (Johns Hopkins University). This is a recombinant HSV-1 virus in which the enhanced green fluorescent protein (eGFP) has been cotranslationally fused with a virus capsid protein, VP26 [27]. As a result, the virus particles are fluorescent. HSV-1 particles were purified from VERO cell culture supernatants using a sucrose cushion, as described [28]. For studies requiring fluorescent Ad5 particles, we labeled purified adenovirus particles with YOYO-1 dye, as described [29], and then removed excess free dye by extensive dialysis. This method results in fluorescent adenovirus particles, containing YOYO-1 dye intercalated into virion DNA [29].
2.3 Collection of saliva
Whole unstimulated saliva was collected from healthy, unmedicated human subjects under an IRB-approved study protocol, by gentle expectoration into chilled 50-ml polypropylene tubes. Saliva was collected between the hours of 8:00–10:00AM and subjects were asked to forego food/beverage and oral hygiene prior to collection. After collection, large particulate matter was removed from the saliva by low speed centrifugation. For the experiment shown in Figure 3B, pilocarpine-stimulated whole saliva was collected manually using a micropipette from 3 female BALB/c mice, aged 6–8 weeks old, following I.P injection of 150 µg pilocarpine. The three samples were kept on ice, pooled and used immediately (within 1 hr of collection) in the experiment.
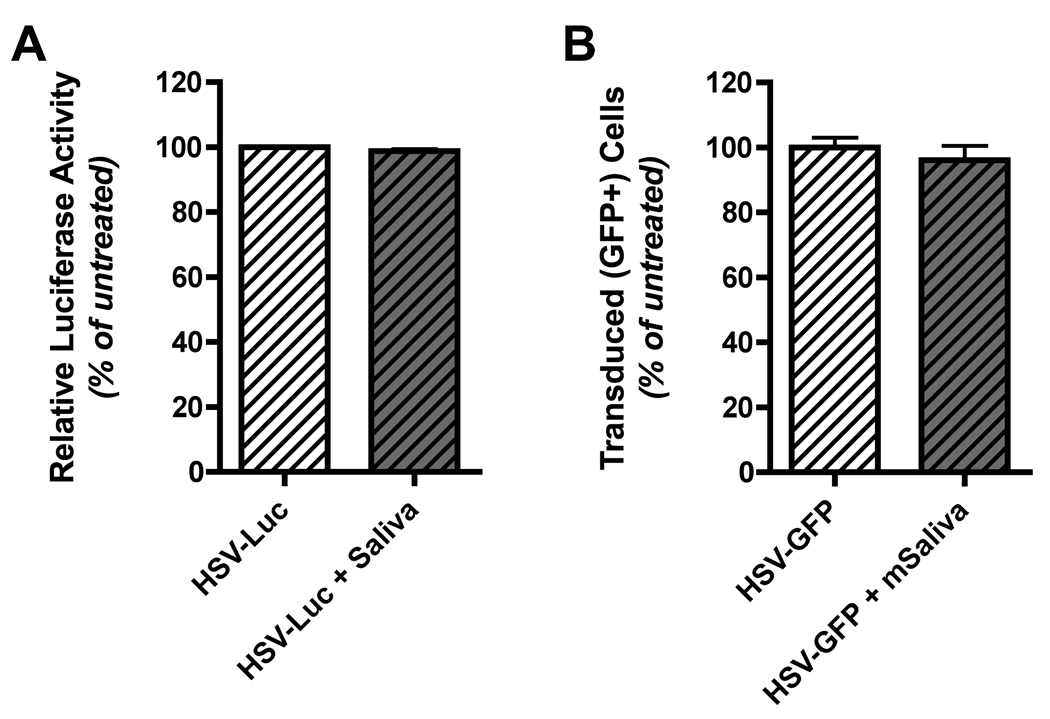
Figure 3. Saliva does not inactivate HSV-1 amplicon particles.
(A) A HSV-1 amplicon vector encoding luciferase was suspended in DMEM medium without FBS and incubated with an equal volume of whole unstimulated human saliva at 37°C for 30 minutes. Following incubation, complete DMEM (containing 10% FBS plus antibiotics) was added and the mixture was then added to HEK 293A cells. Twenty-four hours later the 293A cells were washed with PBS, lysed with passive lysis buffer (Promega), centrifuged at 13,000 RPM for 10 minutes to remove cell debris, and then the lysate was directly used to quantitate luciferase expression. Luciferase expression levels in cells exposed to control virus stocks (not incubated with saliva) was assigned a level of 100%, and luciferase levels in cells exposed to saliva-treated virus stocks was calculated as a percentage of this baseline. The results shown represent mean normalized luciferase expression data (% of baseline relative light units; RLU) from virus samples that were incubated with each of four different human saliva samples; error bars denote the standard error of the mean. (B) A HSV-1 amplicon vector encoding GFP was used to conduct an experiment analogous to that described for panel A. In this case, the vector was preincubated with pooled, pilocarpine-stimulated whole mouse saliva (collected from three female BALB/c mice). 24 hours following addition of control or saliva-treated vector to 293A cell cultures, the cells were collected and analyzed by flow cytometry. The percentage of GFP positive cells in cultures exposed to control virus stocks (not incubated with saliva) was assigned a level of 100%, and the transduction efficiency in cells exposed to saliva-treated virus stocks was calculated as a percentage of this baseline. The results shown represent the mean normalized transduction efficiency data calculated from four replicates; error bars denote the standard error of the mean.
2.4 Analysis of viral mobility in saliva
Fluorescent adenovirus or HSV virions were diluted 1:50 in water or fresh human saliva to a total volume of 250 µL. The virus-containing solutions were then loaded into custom-made microfluidic chambers of 5 micron height and 50 micron width using a syringe pump (NE300, New Era Pump Systems, Inc) at a flow rate of 10 µL/min. A total volume of 40 µL was injected to fully perfuse the system. Movies of the virus-containing solutions were captured with a Cooke SensiCam EM (The Cooke Corporation, Romulus, MI) at a rate of 13.44 frames per second (fps) for a duration of 15 seconds under 100X oil objective magnification (total magnification of 1,000X). The position of each particle was identified in each individual frame using custom MATLAB-based video analysis [30] and the closest particle from frame to frame was connected to define the trajectory of the virus. Linear regression was performed on the x- and y- coordinates of the trajectories to determine the x- and y-component of any background drift caused by residual convection, and these trends were subtracted from the original trajectories. Corrected trajectories were analyzed to determine the diffusion coefficients of Ad and HSV in water (Dw) and saliva (Ds). The diffusion coefficient of each virus particle was determined by taking the linear regression of the first third of the MSD of each trajectory as a function of time. Only those viral trajectories with a coefficient of determination greater than 0.99 were selected for further analysis. The mean of all the individual diffusion coefficients was determined to represent the diffusion coefficient of the entire viral population. Finally, the ratios of the virus diffusion coefficients in water and saliva were calculated (Ds/Dw) using the mean Ds and Dw values for the two viruses. The standard deviation of the ratio of the two means (Ds/Dw) was determined by the delta method [31].
2.5 In vitro analysis of saliva-mediated inhibition of virus infectivity
Human embryonic kidney (HEK 293A, ATCC CRL-1573) cells were plated in 24-well plates the day prior to infection at 2 × 105 cell per well. Experiments involving unstimulated whole human saliva were conducted as follows: Whole human saliva was collected from 4 volunteers and centrifuged at 2,000 RPM for 10 minutes to remove cells; the supernatant fraction was then collected and stored at −20° until use. For experimental analyses, 100 µl of saliva was then incubated with an equal volume of recombinant rAd5 vector encoding luciferase (rAd5:luc; multiplicity of infection [MOI] = 5), or recombinant HSV-1 amplicon vector encoding the same insert (HSV:luc; MOI = 0.1); in control experiments, virus preparations were mixed an equal volume of cell culture medium (DMEM, containing no fetal bovine serum). The virus/saliva mixture was incubated at 37°C for 30 minutes, after which complete DMEM medium (DMEM [Gibco-BRL] plus 10% fetal bovine serum [FBS] and 1% penicillin, streptomycin and glutamine [PSG]) was added and the mixture was transferred to cultures of HEK 293A cells. Cells were incubated for 24 hours at 37°C, and then washed with phosphate buffered saline (PBS [Gibco-BRL]), prior to lysis with passive lysis buffer (Promega, Madison, WI). Cell lysates were then used in a luciferase assay (Promega). Levels of luciferase activity (in relative light units, RLU) in 293A cell cultures exposed to saliva-treated virus preparations were then normalized to the level of luciferase activity in control cultures (i.e., 293A cells exposed to luciferase-encoding vector that was incubated in cell culture medium), which was set at 100%. For this study, whole saliva from 4 different human subjects was used in separate assays for each virus, and mean values of luciferase activity (% baseline RLU) were calculated.
For experiments involving mouse saliva, freshly collected, pilocarpine-stimulated whole mouse saliva was used. Briefly, 100 µl of saliva was incubated with an equal volume of recombinant rAd5 vector encoding green fluorescent protein (Ad5-GFP; MOI = 5), or recombinant HSV-1 amplicon vector encoding the same insert (HSV-GFP; MOI = 0.1); in control experiments, virus preparations were mixed an equal volume of cell culture medium (DMEM, without FBS). The virus/saliva mixture was incubated at 37°C for 30 minutes, after infection of HEK 293A cells was performed as described above. 24 hours later cells were trypsinized, washed, fixed with PBS + 4% formaldehyde and washed twice more in FACS buffer (3% FBS, 0.05% azide) prior to being subjected to flow cytometric analysis. The percentages of GFP positive cells in 293A cell cultures exposed to saliva-treated virus preparations were then normalized to the corresponding percentages of GFP positive cells in control cultures (i.e., 293A cells exposed to GFP-encoding vector that was incubated in cell culture medium), which was set at 100%. For this study, pilocarpine-stimulated whole saliva from 3 different mice was pooled; mean values of the percentage of GFP positive cells were calculated from quadruplicate wells. Note that GFP-encoding viruses were used in the experiment with mouse saliva because available stocks of the HSV-Luc amplicon vector had been exhausted.
2.6 Mice and immunization of mice
Female BALB/c female mice aged 4–6 weeks were purchased from Taconic Laboratories (Germantown, NY) and housed and handled according to institutional and NIH Guidelines. All injections (IM and IP) were done with 26-gauge needles (Becton Dickinson & Co., Franklin Lakes, NJ). Intramuscular injections (IM) were performed in volumes of 50 – 100 µl into the left hind quadriceps with virus inocula diluted in PBS. Animals receiving sublingual (SL) or gastric (IG) immunizations were anesthetized by intra-peritoneal (IP) delivery of ketamine and xylazine, diluted in sterile 0.9% NaCl. Gastric immunization was performed using a pre-filled 18-gauge feeding needle (Harvard Apparatus) with rAd5 vector diluted into a final volume of 50 µl PBS per mouse. SL immunizations were performed by slowly pipetting 10 µl of an appropriately diluted viral vector (rAd5 or HSV-1) under the tongue and placing the animal in the supine position to limit swallowing.
2.7 In vivo imaging system (IVIS)
Groups of 4 mice were administered either rAd5:Luc (1 × 108 viral particles [32]), HSV-1:Luc (5 × 105 VP), or PBS via either the IM or SL routes as described above. Twenty-four hours post administration each mouse was anesthetized with 240 mg/kg Avertin via IP injection. Mice were then injected IP with 250 mg/kg of D-Luciferin (Caliper Life Sciences) and imaged 10 minutes later. Firefly luciferase expression was detected using a Xenogen IVIS system (Hopkinton, MA), as described [33]. Expression per mouse was then determined using Living Image Software (Caliper Life Sciences, Hopkinton, MA), and expressed as total Flux (photons per second [p/s]).
2.8 Collection and preparation of sera and tissue samples
Whole blood was collected from mice by cardiac puncture using a 26-gauge needle (Becton Dickinson, Franklin Lakes, NJ). Sera were then collected by low speed centrifugation for 20 minutes and stored at −80 °C until use. Vaginal wash samples were collected by slowly washing the vaginal vault with 100 µl of PBS, and then stored at −80°C until use. For cell-based assays, spleens were collected and homogenized between the frosted ends of two sterile glass slides. Single cells suspensions were generated by passing the splenocytes through nylon mesh (0.45 µM), followed by suspension into cold complete RPMI media (RPMI-1640 [Gibco-BRL] plus 10% FBS and 1% PSG). Cells were counted by trypan blue exclusion assay and used immediately.
2.9 HIV-1 Env specific ELISA
ELISA plates (Dynex Technologies) were coated overnight with recombinant soluble HIV-1 MN gp160 (Protein Sciences) or purified oligomeric HIV-1 YU2 gp140 [25] at 500 ng/well in 0.1 M NaHCO3 (pH 9.6). Plates were washed 5X with PBS-T (PBS plus 0.1% Tween-20) buffer and then blocked for 2 hours at 37°C with PBS-T containing 3% bovine serum albumin (Sigma). Sera or vaginal wash samples were then added to the ELISA plates at pre-determined dilutions and incubated at 37°C for 1 hour. After extensive washing, peroxidase-conjugated anti-mouse immunoglobulin G (IgG) or peroxidase-conjugated anti-mouse immunoglobulin A (IgA) (Sigma) was added, as appropriate. After further washing, tetramethylbenzidine (TMB, Pierce) was added to the plates for 20 minutes and 4N sulfuric acid was used to stop the reaction. ELISA plates were then analyzed at 450 nm.
2.10 ELIspot analysis of HIV-1 Env specific antibody-secreting cells
Ninety-six-well ELIspot plates (Millipore) were coated with 500 ng per well of oligomeric HIV-1 YU2 gp140 [25] in 0.1 M NaHCO3 (pH 9.6) and incubated overnight at 4°C. Plates were then washed four times with PBS under sterile conditions and blocked with 200 µl per well of complete RPMI media for 1 hour at room temperature. Splenocytes (1 × 106) were then plated into the top row and serially diluted 2-fold down the plate. After incubation for 16 hours at 37°C, the plates were extensively washed with PBS-T and then incubated with alkaline phosphatase (AP) conjugated anti-mouse IgG or anti-mouse IgA antibodies (Southern Biotech; 1:1000) for 2 hours at room temperature. Wells were then extensively washed with PBS-T and spots visualized using Vector alkaline phosphatase substrate kit (Vector Laboratories, Berlingame, CA). Spots were counted using an automated ELIspot reader (Cellular Technology Ltd.).
2.11 Analysis of HIV-1 Env neutralizing antibodies
HIV-1 Env specific neutralizing antibodies were quantitated by Dr. David Montefiori at Duke University, as described [34]. Briefly, YU2 (clade B, Tier 2) and SF162.LS (clade B, Tier 1) pseudotyped viruses were incubated with heat inactivated mouse sera and then added to TZM-bl indicator cells. Neutralizing antibody titers were determined as the reciprocal serum dilution at which luciferase expression was reduced by 50%; a negative titer was defined as a titer of <20.
2.12 Note on Statistical Analyses
Most of the in vivo experiments conducted in this manuscript used a group size of 4 animals, and statistical comparisons between groups were generally made using a non-parametric Mann-Whitney test. As a consequence, p values for several experiments are identical. This is because the Mann-Whitney test generates a p-value of 0.0286 when comparing two groups that each contain four data values, if all of the data values in the first group are greater than any of the data values in the second group.
3. Results
3.1. Migration of Ad5 and HSV-1 virus particles in whole saliva
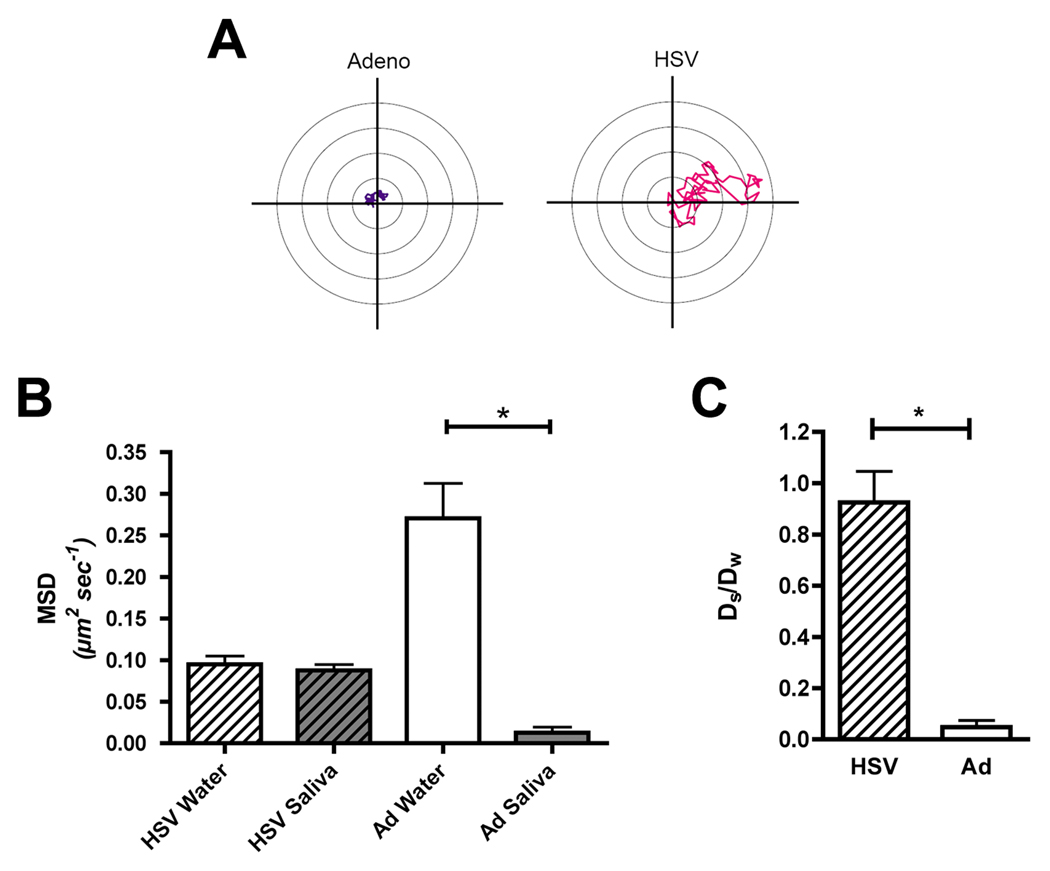
Purified, fluorescent Ad5 and HSV-1 virus particles were suspended in whole saliva or water, and then loaded into custom-made microfluidic chambers in order to analyze their mobility, using fluorescent videomicroscopy. Trajectories were captured from multiple 15 second long movies for each condition for an average of 70 individual particles per condition. Two representative trajectories for HSV-1 and Ad5 virus particles in saliva are presented in Figure 1A, which shows that the Ad5 particle was almost completely immobilized in saliva, while the HSV-1 virion diffused freely.
Figure 1. Adenovirus particles, but not HSV-1 virions, are trapped in saliva.
Fluorescent adenovirus or HSV-1 virions were suspended in either water or saliva, and then loaded into custom-made microfluidic chambers. Virus trajectories were obtained with a 100X oil immersion objective (total magnification of 1,000X) at an imaging speed of 13.44 fps for a duration of 15 seconds. Mean squared displacements (MSD) as a function of time were calculated from trajectories to determine the mobility of individual viral particles and averaged for each viral population (average of 70 particles per condition). (A) Representative trajectories of adenovirus and HSV in saliva. (B) Mobility of HSV and adenovirus in water and saliva. Data represent mean values ± the standard error of the mean (SEM). *: Denotes a statistically significant difference in the MSD value for adenovirus in water, versus saliva (p=0.0028, Mann Whitney test). (C) Ratios of the diffusion coefficients of the viruses in saliva (DS), compared to water (Dw). Data represent mean values ± standard deviation (SD). *: Denotes a statistically significant difference in the Ds/Dw value for adenovirus versus HSV (p<0.0001, unpaired t test).
The trajectories were used to calculate the mean squared displacements (MSD) as a function of time for each condition. These results are presented in Figure 1B. Consistent with their smaller size (90 nm diameter for adenovirus versus 180 nm for HSV; [35, 36]), Ad5 particles exhibited considerably greater mobility in water than HSV-1 virions. However, when Ad5 particles were resuspended in saliva, their mobility was greatly reduced when compared to water (Fig. 1B; p=0.0028, Mann Whitney test). In contrast, HSV-1 virions showed roughly equivalent mobility in either water or saliva.
The diffusion coefficient of the entire virus population analyzed was also calculated, in both water and saliva. The ratio of the diffusion coefficients of Ad5 in saliva compared to water (Ds/Dw) was found to be 0.048, indicating that virus mobility in saliva was reduced by approximately 20-fold compared to its mobility in water. In contrast, the ratio of the diffusion coefficients of HSV-1 in saliva compared to water (Ds/Dw) was 0.93, indicating that virus mobility in saliva was essentially equivalent to its mobility in water (Figure 1C). The difference in the Ds/Dw ratio for Ad versus HSV virions was found to be statistically significant (p<0.0001, unpaired t test).
3.2 Penetration of sublingual epithelium by rAd5 and HSV-1 amplicon virus vectors
We next examined the ability of rAd5 and HSV-1 amplicon vectors to drive expression of an encoded transgene following sublingual delivery in a mouse. To do this, an Ad5 vector expressing firefly luciferase (rAd5:Luc) and a helper-free (hf) HSV-1 amplicon vector encoding the same gene (HSV-1:Luc) were delivered via the SL and IM route to BALB/c mice. Expression of the encoded luciferase transgene was then measured 24 hours later, using In Vivo Imaging System (IVIS) technology.
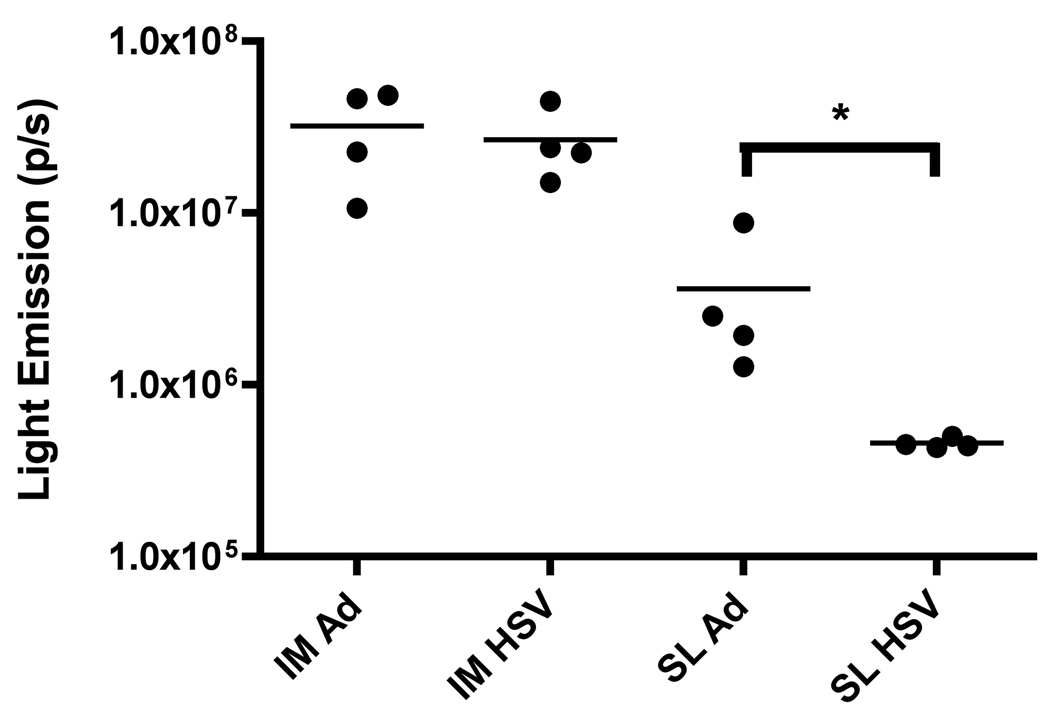
For this experiment, we selected viral inocula that were expected to yield similar levels of gene expression following delivery by the IM route. Thus, we used 1×108 viral particles (VP) for the Ad5:Luc vector and 5×105 VP for HSV-1:Luc. As expected, IM delivery of both vectors resulted in similar, strong levels of luciferase expression in vivo (1.23±0.09 × 107 photons/second [p/s] and 2.66±0.63 × 107 p/s, respectively) (Figure 2). However, SL delivery of the HSV-1:Luc elicited very low levels of luciferase expression, suggesting that this vector was unable to efficiently penetrate the murine sublingual epithelium (Figure 2). In contrast, SL delivery of Ad5:Luc resulted in strong luciferase expression, indicating that the vector was able to effectively penetrate the sublingual epithelium (3.63±1.73 × 106 p/s) (Figure 2). As noted in Figure 2, the difference in the luciferase expression levels for sublingually delivered Ad5:Luc versus sublingually delivered HSV-1:Luc achieved statistical significance (p=0.0286, Mann Whitney test).
Figure 2. An Ad5 encoding firefly luciferase efficiently penetrates the sublingual mucosa following SL instillation, while a HSV-1 amplicon vector encoding a matched insert fails to do so.
An Ad5 vector encoding firefly luciferase (1 × 108 VP), or a HSV-1 amplicon vector (5 × 105 VP) encoding the same insert was administered via the IM or SL route to female BALB/c mice; control animals were inoculated with PBS. 24 hours after administration animals were anesthetized with Avertin (240 mg/kg), and then injected I.P. with D-Luciferin (250 mg/kg), prior to imaging 10 minutes later using a Xenogen IVIS system to quantitate luciferase expression. Data were then analyzed using Living Image Software and normalized based on background luminescence. Results represent mean values from 4 animals/group; bars denote the standard error of the mean. Data are representative of 3 experiments for each vector/condition. *: Denotes a statistically significant difference in luciferase expression levels for sublingually delivered Ad5:Luc versus HSV-1:Luc (p=0.0286, Mann Whitney test).
It can be readily appreciated that the absolute magnitude of measured luciferase expression was considerably greater in animals that received the rAd5:luc vector via the IM route, compared to those that received the same dose of vector via the SL route (Figure 2). The significance of this observation is unclear, however, since in vivo analysis of luciferase expression at the SL site of delivery necessarily requires imaging through the soft palate – likely resulting in diminution of signal intensity. In contrast, muscle tissue can be imaged directly, and may therefore tend to yield higher apparent levels of luciferase activity. Regardless, the data show that the rAd5 vector, but not the hf HSV-1 amplicon vector, was able to effectively penetrate the sublingual epithelium.
3.3 Saliva does not attenuate HSV-1 infectivity
One possible explanation for the very poor gene expression efficiency by sublingually delivered HSV-1:Luc is that the vector may be inactivated by saliva. We therefore tested whether incubation of HSV-1:Luc with whole saliva resulted in a loss of vector infectivity in vitro. For this experiment, HSV-1:Luc vector was incubated with saliva samples from four different human subjects, and then added to HEK 293A target cells. Twenty-four hours later, the cells were collected and lysed, and luciferase activity in the cell lysates was determined.
Both vectors efficiently expressed the reporter gene in HEK293A cells. Luciferase expression levels in cells exposed to control virus stocks (not incubated with saliva) was assigned a level of 100%, and luciferase levels in cells exposed to saliva-treated virus stocks was calculated as a percentage of this baseline. As shown in Figure 3A, incubation with unstimulated whole human saliva had no effect on the infectivity of the HSV-1:Luc vector (99% of baseline expression).
We also conducted an analogous experiment using whole, pilocarpine-stimulated mouse saliva (pilocarpine stimulation being necessary to generate a sufficient volume of saliva to perform the study). In this experiment, a HSV-1 amplicon vector encoding a green fluorescent protein (GFP) reporter gene was used, because available HSV-1:Luc stocks had been depleted. The HSV-GFP vector efficiently transduced HEK293A cells. The percentage of transduced (GFP positive cells) in cultures exposed to control virus stocks (not incubated with saliva) was assigned a level of 100%, and the percentage of transduced cells in cultures exposed to saliva-treated virus stocks was then calculated as a percentage of this baseline. As shown in Figure 3B, incubation with pilocarpine-stimulated whole mouse saliva had no effect on the infectivity of the HSV-1:GFP vector (96% of baseline expression). We conclude that the very poor in vivo gene expression efficiency by sublingually delivered HSV-1:Luc (Figure 2) cannot be attributed to virus inactivation by saliva.
3.4 Humoral immune response to sublingual delivery of rAd5 and hf HSV-1 amplicon vectors encoding HIV-1 gp120
rAd5 and hf HSV-1 vectors encoding an identical human codon-optimized HIV-1 Env gene (HIV-1MN gp120) were delivered to BALB/c mice via either the SL or IM route, using a homologous prime-boost immunization regimen. Animals were boosted on day 21, and sacrificed on day 31. Sera were then collected and HIV-1 Env-specific antibody responses were measured by IgA and IgG ELISA assay.
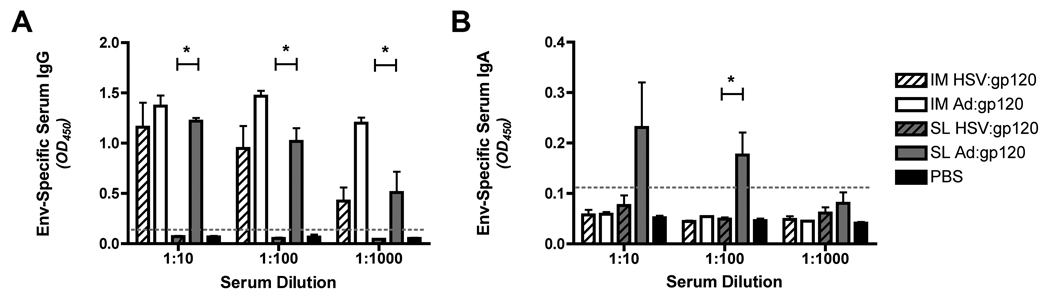
IM delivery of both vectors elicited a robust Env-specific IgG response in the serum of immunized mice (Figure 4A). In contrast, only rAd:gp120 elicited a strong Env-specific IgG response when delivered by the SL route (Figure 4A). The difference in the magnitude of the Env-specific IgG response elicited by sublingual delivery of rAd:gp120 versus HSV-1:gp120 achieved statistical significance at all three serum dilutions tested (p=0.0286; Mann-Whitney test).
Figure 4. Sublingual administration of an Ad5 vector expressing HIV-1 gp120 elicits robust antigen-specific serum IgG and IgA immune responses, while sublingual administration of a matched HSV-1 amplicon vector fails to do so.
An Ad5 vector encoding HIV-1 gp120 (1 × 108 VP), or a HSV amplicon vector (1 × 106 VP) encoding the same antigen was delivered IM or SL to female BALB/c mice; control animals were inoculated with PBS. A homologous boost was performed on day 14 and sera samples were collected on day 28. Sera from immunized mice were diluted 1:10, 1:100, and 1:1000 in PBS and incubated with ELISA plates coated with recombinant HIV-1 gp160 (MN strain, Protein Sciences). Peroxidase conjugated anti-mouse IgG (panel A) or anti-mouse IgA (panel B) were then added, as appropriate, and results were quantitated by measuring absorbance at 450 nm. The gray dotted line represents the background absorbance in this assay (this was set at 2 times the mean background absorbance in samples from PBS-treated control animals). Results represent mean values from 4 animals/group; bars denote the standard error of the mean. Data shown are representative of 2 experiments, performed with similar results. *: Denotes a statistically significant difference in the magnitude of the Env-specific serum IgG or IgA response in mice immunized with Ad:gp120 via the SL route versus the serum IgG or IgA response in mice immunized with HSV-1:Luc via the same route (p=0.0286; Mann-Whitney test).
The Env-specific serum IgA response was also measured in these animals. SL administration of rAd5:gp120 elicited a robust HIV-1 Env specific IgA response in serum, but all other immunization routes/vectors failed to do so (including SL delivery of the HSV:gp120 vector and IM delivery of the rAd5:gp120 and HSV:gp120 vectors) (Figure 4B). The difference in the magnitude of the Env-specific IgA response elicited by sublingual delivery of rAd:gp120 versus HSV-1:gp120 achieved statistical significance at the 1:100 serum dilution level (p=0.0286; Mann-Whitney test), as indicated.
3.5 Robust serum and mucosal IgG and IgA antibody responses to sublingual delivery of an rAd5 vector encoding oligomeric HIV-1 Env
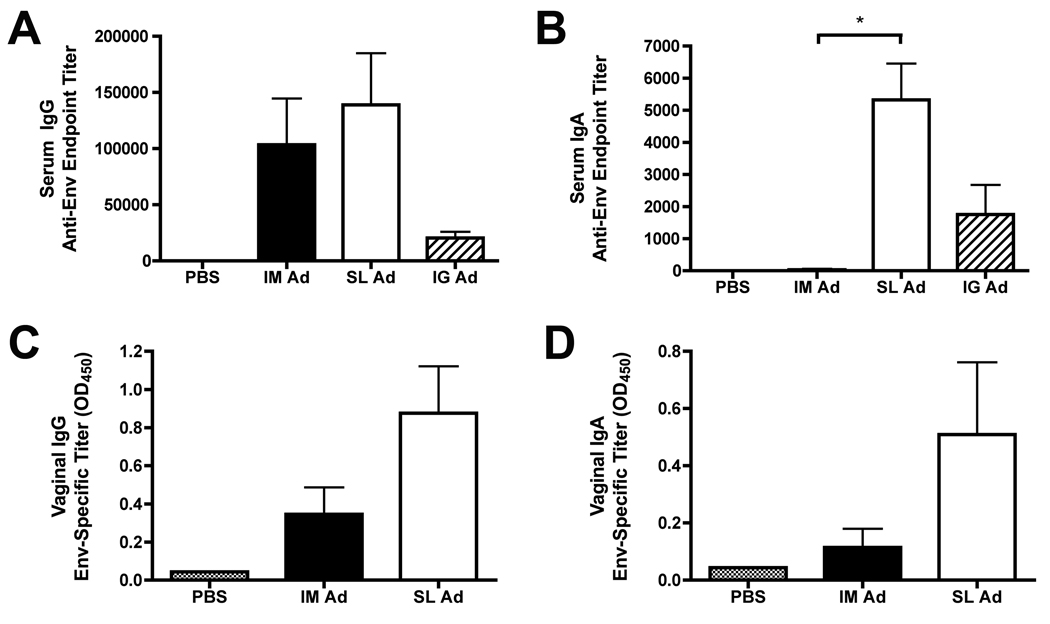
In order to confirm the reproducibility of the results shown in Figure 4, we constructed an E1-deleted, replication-defective rAd5 vector encoding oligomeric HIV-1 Env gp140 (YU2 strain). This vector (rAd5:gp140) was administered to BALB/c mice via the IM, SL or intra-gastric (IG) route. A homologous prime-boost regimen was employed, with a boost at day 28; the animals were sacrificed and samples taken for analysis on day 38. IM or SL delivery of the rAd5:gp140 vector elicited equivalent levels of Env-binding serum IgG antibodies (Figure 5A). In contrast, SL delivery, but not IM delivery, of the rAd5:gp140 vector resulted in a robust HIV-1 Env specific serum IgA response (Figure 5B) - confirming the data generated with the rAd5:gp120 vector (Figure 4B).
Figure 5. Sublingual delivery of an Ad5 vector expressing oligomeric HIV-1 Env (gp140) induces robust, antigen-specific IgG and IgA antibody responses in both serum and in vaginal washes.
An Ad5 vector encoding oligomeric HIV-1 Env (gp140-YU2) was administered to female BALB/c mice via the SL, IM or intragastric (IG) route at a dose of 1×109 VP. At day 28, animals received a booster immunization with the same vaccines/routes, and at day 38, animals were sacrificed and samples collected for analysis. (A, B) Quantitation of endpoint Env-specific serum IgG (A) and IgA (B) antibody titers in sera from groups of immunized mice. Sera were serially diluted, two-fold, in PBS and incubated with ELISA plates coated with recombinant gp140 (YU2 Strain). Peroxidase conjugated anti-Mouse IgG (Sigma) or anti-mouse IgA (Sigma) were then added, as appropriate, and results were quantitated by measuring absorbance at 450 nm. Data represent mean endpoint titers ± SEM, calculated from 4 mice per group. Results shown are representative of 3 experiments, performed with similar results. *: Denotes a statistically significant difference in the magnitude of the Env-specific serum IgA response in mice immunized with Ad:gp140 via the SL route versus the IM route (p=0.0286, Mann-Whitney test). (C, D) Quantitation of Env-specific mucosal IgG (C) and IgA (D) antibody titers in vaginal washes from groups of immunized mice. In this case, antibodies were measured only in undiluted wash samples, and results are presented directly as mean absorbance (A450) values plus or minus SEM calculated from 4 mice per group. Results shown are representative of 2 experiments, performed with similar results.
IG delivery of the rAd5:gp140 vector elicited a considerably weaker Env-specific IgG and IgA serum antibody response, compared with animals that received the same dose of vector via the SL route (Figure 5A,B). These data suggest that swallowing of the rAd5:gp140 vector likely cannot account for the robust HIV-1 specific humoral response elicited by SL vector delivery.
In this experiment, vaginal wash samples were also collected at the time of sacrifice from mice that received the rAd5:gp140 vector via the SL or IM route, as well as from control mice (which received PBS). Env-specific IgG and IgA antibody responses were detected in vaginal washes from animals that received the rAd5:gp140 vector via the SL route (Fig. 5C and 5D respectively). IM administration of the same vector dose resulted in reduced HIV-1 Env specific IgG responses in the vaginal wash, and undetectable Env-specific IgA responses.
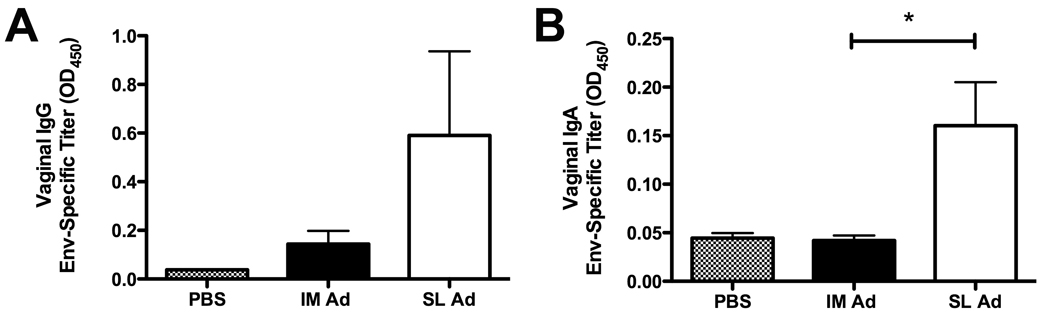
3.6 High-dose sublingual delivery of an Ad5 vector expressing oligomeric HIV-1 Env (gp140) results in enhanced antigen-specific IgA antibody responses in vaginal washes
The data shown in Fig. 5C and 5D revealed a trend towards enhanced Env-specific, vaginal IgA and IgG responses following SL delivery of the rAd5:gp140 vector. Based on these encouraging findings, we performed a followup experiment using a higher dose of the same vector (2×109 VP, or 20-fold higher than the dose used in the experiment shown in Figure 5) and a second boost (i.e., boosts at both day 21 and 42 following initial priming). As shown in Figure 6, this intensified immunization regimen revealed a statistically significant difference in the level of Env-specific IgA in vaginal wash samples from mice immunized with Ad:gp140 via the SL route versus mice immunized via the IM route (p=0.0079, Mann-Whitney test).
Figure 6. High-dose sublingual delivery of an Ad5 vector expressing oligomeric HIV-1 Env (gp140) results in enhanced antigen-specific IgA antibody responses in vaginal washes.
Ad:140 (Fig. 5) was administered to female BALB/c mice via the SL or IM at a dose of 2×109 VP. At day 21 and day 42, animals received a booster immunization with the same vaccines/routes, and at day 56, animals were sacrificed and samples collected for analysis. Env-specific mucosal IgG (A) and IgA (B) antibody levels in vaginal washes from groups of immunized mice were then quantitated. To do this, antibodies were measured only in undiluted wash samples, and results are presented directly as mean absorbance (A450) values plus or minus SEM calculated from 5 mice per group. *: Denotes a statistically significant difference in the level of Env-specific IgA in vaginal wash samples from mice immunized with Ad:gp140 via the SL route versus mice immunized via the IM route (p=0.0079, Mann-Whitney test).
3.7 Quantitation of Env-specific antigen secreting cells and neutralizing antibodies elicited in response to sublingual delivery of a rAd5 vector encoding oligomeric HIV-1 Env
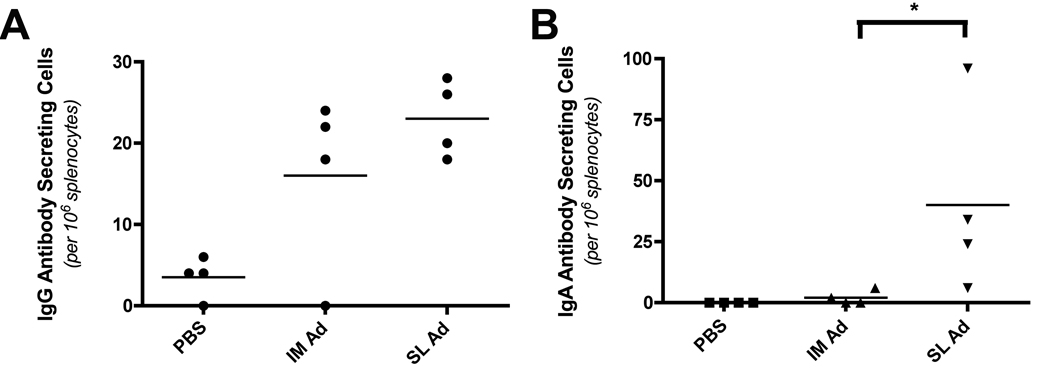
In order to quantitate the magnitude of the B cell response elicited by sublingual delivery of an Env-encoding Ad5 vector, rAd5:gp140 particles were delivered to BALB/c via the SL or IM route at day 0, followed by a homologous boost at days 28 and 56 via the same route. On day 63 (seven days after the final boost), single-cell splenocyte suspensions were prepared and Env-specific antibody secreting cells (ASC) were quantitated using an HIV-1 Env (YU2) specific ELIspot assay. SL and IM delivery of the rAd5:gp140 vector elicited approximately equivalent frequencies of HIV-1 Env specific IgG ASC in the spleens of immunized animals (23.0 +/− 2.4 versus 16.0 +/− 5.5 Env-specific ASC per 106 splenocytes in animals that received vector by the SL and IM routes, respectively) (Figure 7). In contrast, only SL vector delivery elicited HIV-1 specific IgA ASC (40.0 +/− 19.5 versus 2.0 +/− 1.4 Env-specific ASC per 106 splenocytes in animals that received vector via the SL and IM routes, respectively). Statistical data analysis confirmed that the difference in the magnitude of the Env-specific IgA ASC response elicited by SL versus IM delivery of rAd5:gp140 achieved statistical significance (p=0.0286; Mann-Whitney test).
Figure 7. Sublingual delivery of an Ad5 vector expressing oligomeric HIV-1 Env (gp140) elicits Env-specific IgG and IgA antibody secreting cells (ASC) in spleens of immunized mice.
An Ad5 vector encoding oligomeric HIV-1 Env (Ad) was administered to female BALB/c mice via the SL or IM route at a dose of 1×108 VP. At day 28, and again at day 56, animals received a booster immunization with the same vaccines/routes, and at day 63, animals were sacrificed and samples collected for analysis. (A, B) Quantitation of the numbers of ASC producing Env-specific IgG (A) or IgA (B) in spleens from immunized mice. Splenocytes were processed into single cell suspensions and then coated onto ELIspot plates (Millipore) coated with YU2 gp140. AP-conjugated anti-mouse IgG (Southern Biotech) or anti-mouse IgA (Southern Biotech) were then added, as appropriate, and HIV-1 Env specific IgG or IgA secreting cells were detected and quantitated using a colorimetric AP substrate. Data represent the mean number of ASC per 106 splenocytes plus or minus SEM, calculated from 4 mice per group. Results shown are representative of 2 experiments, performed with similar results. *: Denotes a statistically significant difference in the number of ASC producing Env-specific IgA in spleens from mice immunized with Ad:gp140 via the SL route versus mice immunized via the IM route (p=0.0286, Mann-Whitney test).
In addition to quantifying Env-specific ASC, Env-specific neutralizing antibody (Nab) titers were also measured using two clade B strains of HIV-1 (YU2 and SF162.LS). This analysis showed that SL vaccine delivery elicited serum neutralizing antibodies against one or both of the HIV-1 strains tested in 3 of 3 mice that were analyzed, while IM vector delivery elicited detectable Nabs in 2 of 3 animals tested (Table 1).
Table 1.
Neutralizing antibody titers from mice immunized with rAd5:gp140 vector.
| Group | # Mice Positive | YU2 | SF162.LS |
|---|---|---|---|
| PBS | 1 of 3 | (−) (−) 26 |
(−) (−) (−) |
| Sublingual | 3 of 3 | (−) 24 721 |
21 (−) 366 |
| IM | 2 of 3 | 50 (−) (−) |
615 262 (−) |
Single round neutralization assay with YU2 (Clade B, Tier 1) and SF162.LS (Clade B, Tier 2) viruses in TZM-bl cells. TCID50 titers represent the reciprocal dilution at which relative luminescence units (RLU) were decreased by 50% compared to virus control wells. (−) Corresponds to a reciprocal titer of <20.
4. Discussion
This study was designed to compare the ability of two well-characterized virus vectors (rAd5 and hf HSV-1 amplicon) to mediate transgene expression and elicit antigen-specific humoral immune responses following sublingual delivery. We selected these two vectors because of their differing sizes (90 nm versus 180 nm diameter) and surface composition (non-enveloped versus enveloped), and also because we expected that HSV-1 amplicon vectors would be capable of efficiently transducing oral epithelial tissue [37, 38].
Before embarking on in vivo experiments, we first examined whether the mobility of adenovirus and HSV-1 virions was inhibited by saliva. Whole unstimulated human saliva was chosen for these experiments because (i) the troubleshooting and analytical methods required a volume of saliva larger than could be readily obtained from mice and (ii) saliva collection methods from mice typically rely on secretagogues such as pilocarpine to enhance salivary flow. The use of such agents was deemed undesirable because pilocarpine and other secretagogues have been shown to significantly alter salivary protein and mucin content, both in mice and in humans [39, 40].
Previous studies have shown that human cervical mucus, which is considerably more viscous than saliva, can efficiently trap HSV-1 virions but not smaller, virus-like particles [41]. Unexpectedly, we found that saliva almost completely arrested the mobility of the adenovirus particles, but had only a negligible effect on the migration of HSV-1 virions (Figure 1). The most likely explanation is a mucoadhesive interaction with the Ad particles, but not with the HSV-1 virions [42].
Analysis of in vivo gene expression by sublingually delivered rAd5 and hf HSV-1 amplicon vectors revealed that the rAd5 vector, but not the hf HSV-1 amplicon, could drive efficient expression of an encoded luciferase reporter gene in BALB/c mice (Figure 2). This finding was unexpected in that HSV-1 is associated with infections of the oral cavity [43, 44], suggesting that it must be capable of infecting oral epithelial cells. Moreover, it could not be attributed to inhibition of HSV-1 infectivity by either whole unstimulated human saliva or pilocarpine-stimulated murine saliva (Figure 3), which is in agreement with previous reports that HSV-1 is minimally affected by exposure to whole human saliva [45].
We conclude that physicochemical properties that are favorable for efficient gene transfer by viral vectors may not be favorable in terms of promoting interactions that lead to productive virus infection and generation, release and spread of progeny virions. It is conceivable that mucoadhesive trapping of HSV-1 virions in saliva would, for example, interfere with virus spread in oropharyngeal secretions.
In the context of oral gene transfer and immunization using synthetic nanomaterials, however, there is considerable evidence that mucoadhesion leads to higher levels of gene expression/transfer and more robust immune responses [46–49]. This has been attributed to prolonged in vivo residence time of the biomaterials at the mucosal surface [47], and more efficient uptake by local dendritic cells [50]. Our results suggest that mucoadhesion may be similarly associated with more efficient gene transfer and increased immunogenicity of viral vectors – thereby explaining the elevated gene transfer efficiency and enhanced immunogenicity of a sublingually delivered rAd5 vector, as compared to a sublingually delivered HSV-1 vector.
The ability of an rAd5 vector to efficiently penetrate the SL epithelium and to elicit an immune response against an encoded transgene is consistent with work recently reported by Appledorn and colleagues, who showed that sublingual vaccination with an Ad5 vector encoded HIV-1 Gag resulted in a robust mucosal and systemic T cell response to Gag [20]. These investigators did not, however, examine the humoral response to Gag. Thus, our studies complement and extend this earlier work, by demonstrating that SL delivery of an Ad5 vector also leads to robust induction of humoral responses to an encoded HIV-1 transgene (in this case, Env).
SL delivery of Ad5 vectors encoding HIV-1 Env antigens led to a robust induction of both mucosal and serum antibody responses to Env, as well as antigen-specific splenic ASC. Overall, the frequencies of rAd5-induced splenic IgA and IgG ASC were well correlated with the levels of serum antibodies. Thus, splenic IgG ASC frequencies were very similar following either IM or SL vector administration, while IgA ASC frequencies were considerably higher following SL vector delivery.
SL administration of the rAd5 vector was associated with striking Env-specific IgA responses, which were considerably greater in magnitude than those elicited by IM vector delivery, both in serum and in vaginal washes. This may be important at mucosal sites of HIV-1 transmission, where Env-specific secretory IgA antibodies may promote the aggregation of cell-free virus particles and thereby prevent viral transcytosis or transmission [51, 52], due to their multivalent binding antigen capacity [53].
The robust elicitation of antigen-specific antibody responses in the genital tract following sublingual immunization is consistent with other reports showing that SL delivery of adjuvanted human papillomavirus (HPV) L1 protein or virus-like particles (VLP) can elicit vaginal antibody responses [10, 54]. In the present study, however, we were able to successfully elicit such antibodies without the need for a strong mucosal adjuvant [54] or the use of a “self-adjuvanting” immunogen such as the HPV VLP [10]. This suggests that the rAd5 vector, and potentially other adenovirus serotype vectors, may have unique advantages as vaccine delivery vectors for sublingual immunization.
In conclusion, our findings show that salivary trapping of virus vectors is associated with more efficient gene transfer and immunogenicity, following sublingual vector delivery. Our data also show that SL delivery of an Env-encoding rAd5 vector can elicit a potent Env-specific IgA response in the absence of adjuvant, with significant levels of Env-specific antibodies in the female reproductive tract. Overall, these findings support the further exploration of the sublingual delivery route for HIV-1 vaccine delivery.
ACKNOWLEDGEMENTS
This work was supported by the following grants from the National Institutes of Health (NIH): R01AI084111 and R21AI087149 (to S.D.). We gratefully acknowledge Dr. David Montefiori (Duke University) for performing the neutralizing antibody assays, through the Comprehensive Antibody Vaccine Immune Monitoring Consortium. We also thank Dr. Prashant Desai (Johns Hopkins University) for kindly providing the fluorescent HSV virus, K26GFP, and Clark Burris and Louis Lotta (University of Rochester) for helper virus-free amplicon packaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Murrell W. Nitroglycerin as a remedy for angina pectoris. Lancet. 1879;1:80–81. 113- 5, 51-52, 225-7. [Google Scholar]
- 2.Goswami T, Jasti B, Li X. Sublingual drug delivery. Crit Rev Ther Drug Carrier Syst. 2008;25(5):449–484. doi: 10.1615/critrevtherdrugcarriersyst.v25.i5.20. [DOI] [PubMed] [Google Scholar]
- 3.Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008 May 22;358(21):2259–2264. doi: 10.1056/NEJMct0708337. [DOI] [PubMed] [Google Scholar]
- 4.Broide DH. Immunomodulation of allergic disease. Annu Rev Med. 2009;60:279–291. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durham SR. Sublingual immunotherapy: what have we learnt from the 'big trials'? Curr Opin Allergy Clin Immunol. 2008 Dec;8(6):577–584. doi: 10.1097/ACI.0b013e3283196764. [DOI] [PubMed] [Google Scholar]
- 6.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, et al. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J Allergy Clin Immunol. 2008 Feb;121(2):512–518. e2. doi: 10.1016/j.jaci.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006 Aug;118(2):434–440. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Didier A, Malling HJ, Worm M, Horak F, Jager S, Montagut A, et al. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007 Dec;120(6):1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 9.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006 Apr;117(4):802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 10.Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, Holmgren J, et al. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J Immunol. 2009 Dec 15;183(12):7851–7859. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007 Dec 12;25(51):8598–8610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 12.Song JH, Kim JI, Kwon HJ, Shim DH, Parajuli N, Cuburu N, et al. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. J Immunol. 2009 Jun 1;182(11):6851–6860. doi: 10.4049/jimmunol.0803568. [DOI] [PubMed] [Google Scholar]
- 13.Huang CF, Wu TC, Chu YH, Hwang KS, Wang CC, Peng HJ. Effect of neonatal sublingual vaccination with native or denatured ovalbumin and adjuvant CpG or cholera toxin on systemic and mucosal immunity in mice. Scand J Immunol. 2008 Nov;68(5):502–510. doi: 10.1111/j.1365-3083.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Hashizume T, Kurita-Ochiai T, Yamamoto M. Sublingual vaccination with outer membrane protein of Porphyromonas gingivalis and Flt3 ligand elicits protective immunity in the oral cavity. Biochem Biophys Res Commun. 2009 Dec 18;390(3):937–941. doi: 10.1016/j.bbrc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 15.Raghavan S, Ostberg AK, Flach CF, Ekman A, Blomquist M, Czerkinsky C, et al. Sublingual immunization protects against Helicobacter pylori infection and induces T and B cell responses in the stomach. Infect Immun. 2010 Oct;78(10):4251–4260. doi: 10.1128/IAI.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hervouet C, Luci C, Cuburu N, Cremel M, Bekri S, Vimeux L, et al. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine. 2010 Aug 2;28(34):5582–5590. doi: 10.1016/j.vaccine.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, et al. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008 Feb 5;105(5):1644–1649. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennessy M, MacQueen K, McKirnan DJ, Buchbinder S, Judson F, Douglas JM, Jr, et al. A factorial survey study to assess the acceptability of HIV vaccine trial designs. Control Clin Trials. 1996 Jun;17(3):209–220. doi: 10.1016/0197-2456(95)00155-7. [DOI] [PubMed] [Google Scholar]
- 19.Lemiale F, Kong WP, Akyurek LM, Ling X, Huang Y, Chakrabarti BK, et al. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J Virol. 2003 Sep;77(18):10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, Amalfitano A. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin Vaccine Immunol. 2011 Jan;18(1):150–160. doi: 10.1128/CVI.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duke CM, Maguire CA, Keefer MC, Federoff HJ, Bowers WJ, Dewhurst S. HSV-1 amplicon vectors elicit polyfunctional T cell responses to HIV-1 Env, and strongly boost responses to an adenovirus prime. Vaccine. 2007 Oct 16;25(42):7410–7421. doi: 10.1016/j.vaccine.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hocknell PK, Wiley RD, Wang X, Evans TG, Bowers WJ, Hanke T, et al. Expression of human immunodeficiency virus type 1 gp120 from herpes simplex virus type 1-derived amplicons results in potent, specific, and durable cellular and humoral immune responses. J Virol. 2002 Jun;76(11):5565–5580. doi: 10.1128/JVI.76.11.5565-5580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos K, Duke CM, Rodriguez-Colon SM, Dakwar A, Fan S, Keefer MC, et al. Effect of promoter strength on protein expression and immunogenicity of an HSV-1 amplicon vector encoding HIV-1 Gag. Vaccine. 2007 Feb 19;25(9):1634–1646. doi: 10.1016/j.vaccine.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattiacio J, Walter S, Brewer M, Domm W, Friedman AE, Dewhurst S. Dense display of HIV-1 envelope spikes on the lambda phage scaffold does not result in the generation of improved antibody responses to HIV-1 Env. Vaccine. 2011 Mar 21;29(14):2637–2647. doi: 10.1016/j.vaccine.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire CA, Sapinoro R, Girgis N, Rodriguez-Colon SM, Ramirez SH, Williams J, et al. Recombinant adenovirus type 5 vectors that target DC-SIGN, ChemR23 and alpha(v)beta3 integrin efficiently transduce human dendritic cells and enhance presentation of vectored antigens. Vaccine. 2006 Jan 30;24(5):671–682. doi: 10.1016/j.vaccine.2005.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai P, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998 Sep;72(9):7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowers WJ, Howard DF, Brooks AI, Halterman MW, Federoff HJ. Expression of vhs and VP16 during HSV-1 helper virus-free amplicon packaging enhances titers. Gene Ther. 2001 Jan;8(2):111–120. doi: 10.1038/sj.gt.3301340. [DOI] [PubMed] [Google Scholar]
- 29.Sims K, Ahmed Z, Read ML, Cooper-Charles L, Gonzalez AM, Fisher KD, et al. In vitro evaluation of a 'stealth' adenoviral vector for targeted gene delivery to adult mammalian neurones. J Gene Med. 2009 Apr;11(4):335–344. doi: 10.1002/jgm.1306. [DOI] [PubMed] [Google Scholar]
- 30.Bindschadler M, McGrath JL. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J Cell Sci. 2007 Mar 1;120(Pt 5):876–884. doi: 10.1242/jcs.03395. [DOI] [PubMed] [Google Scholar]
- 31.van der Vaart AW. Asymptotic Statistics. Cambridge, U.K.: Cambridge University Press; 1998. [Google Scholar]
- 32.Sheludyakov VD, Tkachev AS, Sheludyakova SV, Kozyukov V, Mironov VF. Synthesis and properties of organic and organosilicon acyl isocyanates. J Gen Chem USSR. 1977:2061–2067. [Google Scholar]
- 33.Fan S, Maguire CA, Ramirez SH, Bradel-Tretheway B, Sapinoro R, Sui Z, et al. Valproic acid enhances gene expression from viral gene transfer vectors. J Virol Methods. 2005 Apr;125(1):23–33. doi: 10.1016/j.jviromet.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Mascola JR, D'Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, et al. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol. 2005 Aug;79(16):10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart PL, Burnett RM, Cyrklaff M, Fuller SD. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991 Oct 4;67(1):145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- 36.Grunewald K, Desai P, Winkler DC, Heymann JB, Belnap DM, Baumeister W, et al. Three-dimensional structure of herpes simplex virus from cryo-electron tomography. Science. 2003 Nov 21;302(5649):1396–1398. doi: 10.1126/science.1090284. [DOI] [PubMed] [Google Scholar]
- 37.Yura Y, Iga H, Terashima K, Yoshida H, Yanagawa T, Hayashi Y, et al. The role of epithelial cell differentiation in the expression of herpes simplex virus type 1 in normal human oral mucosa in culture. Arch Virol. 1987;92(1–2):41–53. doi: 10.1007/BF01310061. [DOI] [PubMed] [Google Scholar]
- 38.Sturn B, Schneweis KE. Protective effect of an oral infection with herpes simplex virus type 1 against subsequent genital infection with herpes simplex virus type 2. Med Microbiol Immunol. 1978 Jul 4;165(2):119–127. doi: 10.1007/BF02122747. [DOI] [PubMed] [Google Scholar]
- 39.Dawes C. The composition f human saliva secreted in response to a gustatory stimulus and to pilocaprine. J Physiol. 1966 Mar;183(2):360–368. doi: 10.1113/jphysiol.1966.sp007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denny PC, Denny PA, Yim MS. The effects of various secretagogues on the mucin content of pure submandibular salivas. J Dent Res. 1987 May;66(5):1011–1015. doi: 10.1177/00220345870660050301. [DOI] [PubMed] [Google Scholar]
- 41.Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001 Oct;81(4):1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci U S A. 2010 Jan 12;107(2):598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hale BD, Rendtorff RC, Walker LC, Roberts AN. Epidemic herpetic stomatitis in an orphanage nursery. JAMA. 1963 Mar 30;183:1068–1072. doi: 10.1001/jama.1963.03700130036008. [DOI] [PubMed] [Google Scholar]
- 44.Buddingh GJ, Schrum DI, Lanier JC, Guidry DJ. Studies of the natural history of herpes simplex infections. Pediatrics. 1953 Jun;11(6):595–610. [PubMed] [Google Scholar]
- 45.Malamud D, Davis C, Berthold P, Roth E, Friedman H. Human submandibular saliva aggregates HIV. AIDS Res Hum Retroviruses. 1993 Jul;9(7):633–637. doi: 10.1089/aid.1993.9.633. [DOI] [PubMed] [Google Scholar]
- 46.Pawar D, Goyal AK, Mangal S, Mishra N, Vaidya B, Tiwari S, et al. Evaluation of mucoadhesive PLGA microparticles for nasal immunization. AAPS J. 2010 Jun;12(2):130–137. doi: 10.1208/s12248-009-9169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slutter B, Bal S, Keijzer C, Mallants R, Hagenaars N, Que I, et al. Nasal vaccination with N-trimethyl chitosan and PLGA based nanoparticles: nanoparticle characteristics determine quality and strength of the antibody response in mice against the encapsulated antigen. Vaccine. 2010 Aug 31;28(38):6282–6291. doi: 10.1016/j.vaccine.2010.06.121. [DOI] [PubMed] [Google Scholar]
- 48.Park JS, Oh YK, Kang MJ, Kim CK. Enhanced mucosal and systemic immune responses following intravaginal immunization with human papillomavirus 16 L1 virus-like particle vaccine in thermosensitive mucoadhesive delivery systems. J Med Virol. 2003 Aug;70(4):633–641. doi: 10.1002/jmv.10442. [DOI] [PubMed] [Google Scholar]
- 49.Florindo HF, Pandit S, Lacerda L, Goncalves LM, Alpar HO, Almeida AJ. The enhancement of the immune response against S. equi antigens through the intranasal administration of poly-epsilon-caprolactone-based nanoparticles. Biomaterials. 2009 Feb;30(5):879–891. doi: 10.1016/j.biomaterials.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 50.Saint-Lu N, Tourdot S, Razafindratsita A, Mascarell L, Berjont N, Chabre H, et al. Targeting the allergen to oral dendritic cells with mucoadhesive chitosan particles enhances tolerance induction. Allergy. 2009 Jul;64(7):1003–1013. doi: 10.1111/j.1398-9995.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 51.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998 Aug;9(2):277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 52.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011 Feb 25;34(2):269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Renegar KB, Jackson GD, Mestecky J. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J Immunol. 1998 Feb 1;160(3):1219–1223. [PubMed] [Google Scholar]
- 54.Cho HJ, Kim JY, Lee Y, Kim JM, Kim YB, Chun T, et al. Enhanced humoral and cellular immune responses after sublingual immunization against human papillomavirus 16 L1 protein with adjuvants. Vaccine. Mar 19;28(14):2598–2606. doi: 10.1016/j.vaccine.2010.01.013. [DOI] [PubMed] [Google Scholar]