Abstract
The role of protective immunity to Plasmodium falciparum (Pf) malaria in Burkitt lymphoma (BL) is unknown. We investigated the association between BL and antibodies reactive to SE36 antigen, a recombinant protein based on Pf-SERA5 gene, targeted by protective malaria immune responses. Cases were children (0–14 years) enrolled at the Korle-Bu Teaching Hospital, Accra, Ghana, during 1965–1994 with BL confirmed by histology or cytology (92% of cases). Controls were healthy appearing children enrolled contemporaneous to the cases from the nearest neighbor house to the case house age- and sex-frequency matched to the cases. Anti-SE36 IgG antibodies were measured using enzyme-linked absorbent immunoassays (ELISA). SE36 titers were estimated by extrapolating ELISA optical density (OD) readings to a standard fitting curve. Anti-SE36 titers were log-transformed for analysis. Odds ratios (OR) and 2-sided 95% confidence intervals (95% CI) were estimated using unconditional logistic regression. The mean log endpoint dilution titers were 0.63 logs lower in cases than in controls (8.26 [SD 1.68] versus 8.89 [SD 1.75], Student’s t-test, P=0.019). Lower titers were observed in cases than controls aged 0–4 years (P=0.05) and in those aged 5–14 years (P=0.06). Low and medium tertiles of anti-SE36 IgG antibodies were associated with increased OR for BL ([OR 1.67, 95% CI 1.21–2.31] and [OR 1.33, 95% CI 0.96– 1.86], respectively, Ptrend =0.002) in analyses adjusting for age, sex, calendar period, and test plate. Our findings suggest that compared to similarly aged children enrolled from the same community, children with BL in Ghana have lower antibodies to SE36 antigen.
Keywords: Plasmodium falciparum malaria, Africa, Epstein-Barr virus, immunity, epidemiology
Background
Burkitt lymphoma (BL) is an aggressive B cell lymphoma first described by Denis Burkitt in African children in 19581. Epidemiological studies conducted shortly after linked BL to Plasmodium falciparum (Pf) malaria 2–4. Specifically, a high incidence of BL was noted in regions where malaria transmission is high and a low incidence was noted in regions where malaria transmission is low3, 5. In addition, a reduction in BL incidence was reported in one community in Tanzania where malaria suppression intervention was applied6, providing further epidemiological support for a role of malaria in BL. Recently, case control studies measuring anti-malaria whole schizont antibodies, which measure the cumulative impact of immune activation from malaria, have reported the odds of BL increase with increasing anti-schizont antibody titers7, 8. Biologically, malaria stimulates polyclonal proliferation of B cells, increases expression of Epstein-Barr virus (EBV) proteins, also linked to BL, and impairs EBV-specific T-cell immune responses 9, which may singly or jointly influence the risk for BL.
Environmental exposure to and subsequent infection with malaria is thought to be the most important determinant of individual risk for BL5. Whether intrinsic differences in level of protective immunity to mild clinical malaria or asymptomatic malaria parasitemic attacks might influence cumulative immunological burden from malaria among individuals from the same community has not been examined before. Lack of well established serological correlates of malaria immunity 10 have hitherto precluded this possibility from being examined. One study conducted in Ghana using age-, sex-, and residence-matched controls in 197911 found lower anti-malaria-specific IgG, IgA, and IgM antibodies. These findings were not replicated in a study conducted in Uganda during the same period12. Both studies were small, evaluated different populations and used assays whose malaria protective and growth inhibitory properties are uncertain. Recent efforts to develop malaria vaccines has led to characterization of several antigen targets for protective malaria immunity13. One of these is SE36; a recombinant protein based on the N-terminal domain of P. falciparum serine repeat antigen 5 (Pf-SERA5) genes. Pf-SERA5 exhibits no antigenic variation14 and limited polymorphism15 compared to other Plasmodium antigens, and aside from Plasmodium Theileria (the causative agent for East Coast fever in cattle) is the only genus to possess a SERA homolog gene16. Biologically, SERA expression appears to play an essential role in the release of invasive malaria parasites from host erythrocytes17, 18. SE36 is currently being evaluated as a blood stage vaccine candidate in clinical trials in Japan and Uganda 19. Measuring SE36 would be a refinement of approaches relying on previously available whole schizont antigen, which has been used in previous case-controls studies that investigated the relationship between BL and malaria7, 8. Thus, we selected SE36 for our initial study to gain some insights on the immune-epidemiology of BL, specifically focusing on antibodies reactive to SERA5 in the Ghana BL case-control study conducted during 1965 to 199420. A better understanding of malaria immunology in BL can provide information on the etiology of BL and help target BL treatment and/or prevention.
Study population
We used residual samples from the Ghana Burkitt lymphoma study conducted at Korle Bu Teaching Hospital in Accra, Ghana, during 1965 to 1994 (29 years)20 to obtain preliminary data for our hypothesis. Briefly, the cases were children (0 through 14 years) enrolled from BL and malaria-endemic rural areas in the southern half of Ghana. Cases were confirmed by histology or cytology (92% of cases). Controls were apparently healthy children from the same community where the case arose. To find the controls, study staff visited the home of the case and starting from there, followed predetermined directions to reach the first home that was nearest to the home of the case and had children eligible to serve as controls. Eligible children were enrolled with frequency matching to the case on age and sex. Controls were enrolled contemporaneous to the case, except during 1980–1984 when it was interrupted leading to lower control numbers during that period. Some controls were members of the extended family of the case, but this group was thought to be small 21. Demographic (age, sex) information was collected from both cases and controls and venous blood was drawn; in the cases this was done before starting BL-specific treatment. Blood was processed within a few hours after collection and separated into sera, which was stored at −70°C until testing. The current study included sera from 657 (84%, of 778) cases and 498 (83% of 599) controls from the original study. Subjects were excluded either because sera were exhausted or cases have paired serum-tumor samples so their sera were preserved for future proteomic biomarker discovery studies. Parents or guardians of the children gave verbal informed consent for the children to participate and for blood samples to be taken and stored for use in future studies. The current study was done using anonymized data and samples that cannot be linked to original personal identifiers. Ethical approval for the current study was obtained from the Office of Human Subject Research at the National Institutes of Health.
Serological methods
Anti-SE36 IgG antibody were measured at the Research Institute for Microbial Diseases, Osaka University, Japan, using an enzyme-linked immunosorbent assay (ELISA) as previously described22, with minor modifications. Sera (x100 dilution) were assayed twice for anti-SE36 IgG antibodies using flat-bottomed 96-well Nunc-Immuno plates (Nunc, Roskilde, Denmark) coated overnight at 4°C with 100 μL of antigen (recombinant SE36 protein) at a concentration of 1 μg/mL in carbonate coating buffer, pH 9.6. The plates were washed 3 times in PBS/0.05% Tween-20 (PBS/T)and blocked overnight at 4°C with 5% skimmedmilk powder in PBS/T. Prior to addition of sera, plates were again washed 3 times with PBS/T. Test sera were added(100 μL per well) at dilutions of 1:100 in 5% skimmedmilk powder inPBS/T and the plates were incubated overnight at 4°C. After washing thrice in PBS/T, horseradish peroxidase conjugated anti-human IgG (Horseradish peroxidase-conjugated rabbit anti-human IgG antibody A8792; Sigma-Aldrich Corp., St. Louis, MO) diluted 1:4000 in 5% skimmedmilk powder in PBS/T was added and the mixture incubated at room temperature for 4 hours. The plates were washed3 times, and color development reaction was done with TMB Microwell Peroxidase Substrate System (KPL, Inc., Gaithersburg, MD) for 1 minute. The reaction was stopped with 50 μL of2 M sulfuric acid and optical density (OD) read at 450 nm. Healthy malaria-naive Japanese serum was used as negative reference. Cutoff for positivity was set from mean OD values in negative controls + 3SD. For quantitation of antibody titers in the test sample, each plate contained a Ugandan high titer pool (made from a pool of 10 individuals from malaria-endemic region in Uganda) that was used as a standard and serially diluted from 1/100, 1/300, 1/900 to 1/218, 7000 (eight points from 100 to 218,700 dilution) to fit as a standard curve (Figure 1). OD values were fit on standard fitting curves to calculate SE36 antibody titers. We included 5% samples as blinded duplicates to measure variability in estimating endpoint dilution titers using this assay system.
Figure 1.
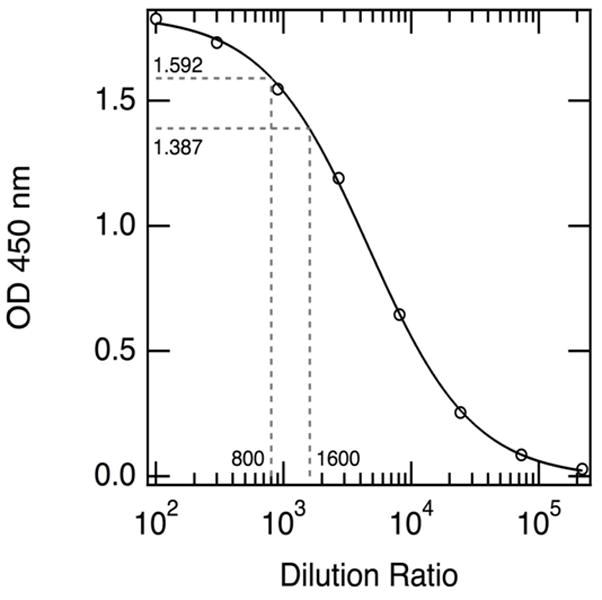
Representative standard fitting curve for extrapolating optical density readings to estimated titers; Table shows standard endpoint titer dilution, and theoretical titers for plate 4. Serum dilution based on a pool of strongly reactive malaria-smear positive sample from 10 Ugandan individuals.
Statistical methods
Endpoint dilution titers of anti-SE36 IgG antibodies were log-transformed (base 2) to obtain a normal distribution to facilitate analysis on a continuous scale. Log-transformed titers were grouped into a three-level ordinal scale (low, medium, and high titer) using cutoffs derived from the values in the control group, which represents, to some extent, the values that would be seen in the ‘normal’ population. The means of log-transformed anti-SE36 IgG antibodies for grouped variables (cases versus controls over all and by age [0–4 years and 5–14 years], early [0–2] versus late stage [3–4] disease among cases) were compared using the Student’s t-test. We used two age groups that reflect extreme differences in malaria-related mortality, which is highest in children aged 0–4 years and substantially lower in children aged 5–14 years. Crude and adjusted odds ratios (OR) and 2-sided 95% confidence interval (CI) for association of BL with anti-SE36 antibodies were estimated using unconditional logistic regression because controls were frequency matched to the cases. ORs were adjusted for the matching variables age, sex, enrollment period, and for test plate, which included to the final model to adjust for imprecision of assay measurement across plates. Chi-squared P values for trend were used to assess for a trend across tertiles of anti-SE36 IgG antibody titers and Chi-squared P values for heterogeneity were calculated to assess whether at least one association for a multi-level category variable was statistically significant from the others. Because we used tertiles agnostically without presuming a biologically relevant cutoff point, we performed sensitivity analyses where individuals with medium anti-SE36 IgG antibody titers were either included as negatives or excluded from analyses. A two-sided P-value <0.05 was considered statistically significant.
Results
Males were more frequent than females among the cases and most cases were aged 5–9 years or 10–14 years (Table 2); cases and controls were enrolled during all calendar periods, but the last 10 years (1984–1994) of the study accounted for the majority (about 50%). The mean log endpoint dilution titer was 8.36 logs (range 1.71–15.2) for cases and controls combined. We noted some within-subject variation in anti-SE36 ELISA measurements, based on a coefficient of variation of 10% and 16%, based on 5% duplicate quality-control samples. The mean log endpoint dilution titer was 0.63 logs lower in cases than in controls (8.26 [SD 1.68] versus 8.89 [SD 1.75], p=0.019) overall (Figure 2A). It was 0.62 logs lower in cases than controls aged 0–4 years (8.25 [SD 1.71] versus 8.87 [SD 1.64], P=0.05) and 0.2 logs lower in those aged 5–14 years (8.26 [1.67] versus 8.46 [1.75], P=0.06). The mean log endpoint dilution titers in cases with early stage disease was similar to that of cases with late stage disease (8.29 [SD 1.54] versus 8.17 [1.63], P=0.47) (not shown). In analyses by calendar-year period, mean log titers were on average 0.2 logs lower in BL cases than in controls for all calendar periods (Figure 2B–D), but the strata-specific differences were not statistically significant.
Table 2.
Characteristics of Burkitt lymphoma cases and controls 0–14 years from Ghana, 1965–1994
| Cases (N=657) | Controls (N=498) | Crude Odds Ratio (95% CI) | Adjusted Odds* Ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Sex | ||||||
| Female | 250 | 38.1 | 227 | 45.6 | Reference | Reference |
| Male | 407 | 61.9 | 271 | 54.4 | 1.36 (1.08, 1.73) | 1.30 (1.02, 1.66) |
| Age Group, years | ||||||
| 0–4 | 96 | 14.6 | 41 | 8.2 | 1.46 (0.98, 2.18) | 1.45 (0.97, 2.18) |
| 5–9 | 391 | 59.5 | 244 | 49.0 | Reference | Reference |
| 10–14 | 170 | 25.9 | 213 | 42.8 | 0.50 (0.39, 0.64) | 0.50 (0.39, 0.65) |
| Enrollment year | ||||||
| 1965–1974 | 200 | 30.5 | 119 | 23.9 | 1.54 (1.17, 2.04) | 1.52 (1.14, 2.02) |
| 1975–1984 | 152 | 23.1 | 99 | 19.9 | 1.41 (1.04, 1.90) | 1.38 (1.01, 1.87) |
| 1985–1994 | 305 | 46.4 | 280 | 56.2 | Reference | Reference |
| Anti-SE36 IgG antibody titers | ||||||
| High tertiles | 168 | 25.6 | 165 | 33.1 | Reference | Reference |
| Medium tertiles | 217 | 33.1 | 168 | 33.7 | 1.27 (0.94–1.70) | 1.33 (0.96–1.86) |
| Low tertiles | 271 | 41.3 | 165 | 33.1 | 1.61 (1.21–2.15) | 1.67 (1.21–2.31) |
Model includes all variables in the table and plate to plate variation.
Figure 2.
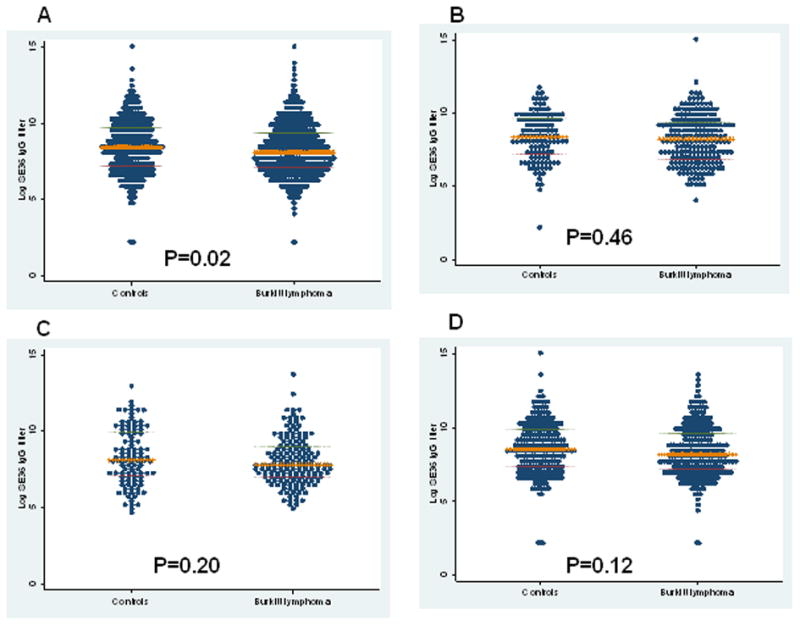
Dot plot of log (base 2) anti-SE36 IgG antibody titers for all cases and controls (panel A) and for cases and controls during 1965–1974 (Panel B), 1975–1984 (Panel C) and 1985–1994 (Panel D). Each dot represents a single subject, the horizontal bar in the middle is the median and the outer bars represent the 25th and 75th inter-quartile ranges.
The cutoff points for anti-SE36 IgG antibody titer tertiles derived from the control group were 7.7 and 9.3 logs. Compared to controls, cases were more likely to have low or medium than high anti-SE36 IgG antibody titers (OR 1.61, 95% CI 1.21–2.15], and [OR 1.27, 95% CI: 0.94–1.70], respectively, Ptrend=0.001) (Table 1). The associations did not change substantially when we adjusted for sex, age, and calendar year of enrollment (results not shown) and it only changed slightly when test plate was included in the model (OR 1.67, 95% CI 1.21–2.31] and [OR 1.33, 95% CI: 0.96–1.86], respectively, Ptrend=0.002 ). The association remained significant in sensitivity analyses where medium tertiles of anti-SE36 IgG antibody titers were coded as low (OR 1.42, 95% CI: 1.11–1.81) and when they were coded as indeterminate and excluded in the analysis (OR 1.61, 95% CI: 1.21–2.15).
Table 1.
Standard fitting curve
| Standard dilution ratio | Optical density readings | Calculated Titer | Theoretical Titer | |
|---|---|---|---|---|
| H1 | 100 | 1.339 | 111721.176 | 218700 |
| H2 | 300 | 1.359 | 157952.877 | 72900 |
| H3 | 900 | 1.08995 | 22121.054 | 24300 |
| H4 | 2700 | 0.74355 | 7875.525 | 8100 |
| H5 | 8100 | 0.39525 | 2992.76 | 2700 |
| H6 | 24300 | 0.10145 | 693.254 | 900 |
| H7 | 72900 | 0.04785 | 347.159 | 300 |
| H8 | 218700 | 0.01445 | 130.911 | 100 |
Discussion
We hypothesized that serological response to SE36, an antigen that has been shown to elicit protective immunity to malaria19, may vary between BL cases and controls enrolled from the same community in Ghana. We found modest but statistically significant lower mean log anti-SE36 titers in cases than in controls enrolled from the same community as the cases. We confirmed this result using categories agnostically grouped according to tertiles calculated from results obtained in the control group. Our results provide the first indication that humoral immune responses to an antigen targeted by protective malaria immunity, in this case SE36, may be lower in children with BL than controls from Ghana. Previous studies relied on the whole schizont anti-malaria antibodies, which measure the cumulative impact of malaria-related immune activation on the risk for BL7, 8, but not protection from severe malaria. Antibodies reactive to SE36 antigen have been shown in previous immunoepidemiological studies to correlate with lower risk for malaria fever or parasitemia and protective immunity in Aotus and squirrel monkeys against parasite challenge 13, 14, 19. Taken together, our study and previous case control studies7, 8 support the role of malaria in BL, but they suggest that the association with BL may differ according to the association between the specific anti- malaria-antigen antibody and severe malaria. Our findings need to be confirmed in hypothesis-driven studies using a larger panel of malaria antigens targeted by humoral and other immune responses to malaria. In addition, proof of concept studies need to be done to define levels of humoral immune response that may significantly impact on the frequency of mild or asymptomatic malaria attacks, malaria parasitemia and to elucidate the role of malaria immunoepidemiology in BL.
Our study has some weaknesses. First, the case-control design raises concern that the findings may be the result, not the cause, of BL. This concern was alleviated, somewhat, by our finding similar mean titers in cases with early disease versus cases with late disease. Second, we evaluated only one malarial antigen targeted by malaria immunity and we did not measure the whole schizont anti-malaria antibody, whose association with BL is well established. This approach was considered reasonable to obtain preliminary data to support future hypothesis-driven studies. We did not have data on use of anti-malarial treatment prior to admission. Recent anti-malaria treatment is not known to suppress antibodies to the whole-schizont anti-malaria antibodies. An increased risk for BL was associated with elevated anti-malarial antibody titers in Uganda7, although children with BL also frequently reported a history of treatment for malaria, suggesting that prior malaria treatment may not be associated with depression of anti-malaria antibodies. Third, samples from cases were collected at the hospital, whereas samples from controls were collected in the community, which may have resulted in slight differences in pre-processing times or conditions of the sera in cases versus controls. Efforts were made to process all samples the same day, usually within 2–8 hours, thus, differences in pre-processing conditions would have been minimal. Finally, the samples were collected over 29 calendar years (1965–1994), which may distort results due to differences in sample handling and storage or in changes in malaria epidemiology over time. Decay of antibodies is a theoretical concern, but is probably of less concern in or study as immunoglobulins are stable under a broad range of pre-analytic and long-term storage conditions23, 24. Long-term changes in malaria epidemiology would not undermine our results because malaria control activities during the study period were largely ineffective and malaria-specific mortality increased in most African countries in Africa 25, including in Ghana. The implementation of effective malaria control measures, including bed nets, insecticide spraying, and combinatorial treatments has only been introduced recently in the 2000s26, so they would not impact our results. Anti-SE36 IgG antibody titers were lower in cases than controls for all calendar year periods, but period-specific analyses were under powered to show significance differences. Readers should consider these caveats when interpreting our results. Our results are valuable for generating new hypotheses, which can be tested in hypothesis-driven studies including adequate sample size and more malaria markers. The strengths of our study include using controls from the same community as the case and enrolling controls contemporaneous to the cases, especially because of data suggesting that environmental malaria in endemic areas can vary by geography and time27.
We are aware of only one other study of BL that evaluated antibody assays that measure protective immunity to malaria in patients with BL28, including the merozoite surface protein-1, which is being evaluated as a vaccine candidate 28. No significant differences were noted in titers of the anti-MSP1 antibodies or the other anti-malarial antibodies evaluated between frequency age- and residence matched BL cases and controls28. Significant differences, however, were noted in anti-Zta and VCA EBV antibodies between BL cases and controls. The inconsistency with our study may be explained by the usage of different malarial antigens as well as study sample size (32 BL cases and 25 controls) which may have lacked power to exclude a positive result of a magnitude observed in our study (657 cases and 498 controls), or to over matching in their study. Because anti-SE36 antibodies were not assessed in that study, their findings are not directly comparable to our study. Large and well designed malariaimmuno-epidemiological studies are needed to confirm or refute our findings.
Although SE36 is an abundantly expressed late-trophozoite to schizont stage protein, we do not know why SE36 titers, which presumably represent more malaria experience, were associated with decreased risk for BL. Anti-SE36 IgG antibodies have been associated with reduced risk of severe clinical malaria in children in Uganda 22 exposed to extraordinarily high environmental malaria (1500 or up to 5 infectious mosquito bites per night29), evidence for malaria parasite growth inhibitory activity in vitro14, and protection of non-human primates vaccinated with SE36 antigen13, 19. Possibly, genetic, nutritional30, or environmental factors predispose some individuals to high anti-SE36 IgG titers resulting in a lower propensity for mild or asymptomatic malaria parasitemia. Conversely, other individuals lacking those genetic or environmental exposures may be predisposed to low anti-SE36 antibody titers resulting in a greater propensity for mild or asymptomatic malaria parasitemia, higher cumulative exposure to malaria-induced immune-activation. These explanations would be consistent with both the malaria hypothesis and with immunoepidemiological studies showing a correlation between BL and high titers of whole schizont malaria antibodies7, 8.
To conclude, we found that compared to controls from the same community in rural Ghana, children with BL were more likely to have lower mean or low and medium anti-SE36 IgG antibody titers. Our findings suggest that serological reactivity to SE36 may be lower in children with BL in Ghana and suggest that it might be worth investigating these patterns in other settings or using a larger panel of antigens.
Acknowledgments
We are grateful to Dr. Paul H. Levine at George Washington University School of Public Health-Department of Epidemiology (Washington, D.C.) for his comments on this report and Dr. Myhanh Dotrang at Computer Services Corporation (Rockville, Maryland) for preparing data analysis files.
Financial support: The study was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services; Grant number: N01-CO-12400. The study was also funded by The Biomedical Kansai project supported by the Regional Innovation Cluster Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Footnotes
Disclaimers: The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the United States Department of Health and Human Services.
References
- 1.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–23. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 2.Burkitt D. A children’s cancer dependent on climatic factors. Nature. 1962;194:232–4. doi: 10.1038/194232a0. [DOI] [PubMed] [Google Scholar]
- 3.Morrow RH., Jr Epidemiological evidence for the role of falciparum malaria in the pathogenesis of Burkitt’s lymphoma. IARC Sci Publ. 1985;60:177–86. [PubMed] [Google Scholar]
- 4.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt’s lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3:182–7. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 5.Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R. Spatial distribution of Burkitt’s lymphoma in Kenya and association with malaria risk. Trop Med Int Health. 2007;12:936–43. doi: 10.1111/j.1365-3156.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 6.Geser A, Brubaker G, Draper CC. Effect of a malaria suppression program on the incidence of African Burkitt’s lymphoma. Am J Epidemiol. 1989;129:740–52. doi: 10.1093/oxfordjournals.aje.a115189. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter LM, Newton R, Casabonne D, Ziegler J, Mbulaiteye S, Mbidde E, Wabinga H, Jaffe H, Beral V. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: a case-control study in Uganda. Int J Cancer. 2008;122:1319–23. doi: 10.1002/ijc.23254. [DOI] [PubMed] [Google Scholar]
- 8.Mutalima N, Molyneux E, Jaffe H, Kamiza S, Borgstein E, Mkandawire N, Liomba G, Batumba M, Lagos D, Gratrix F, Boshoff C, Casabonne D, et al. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS ONE. 2008;3:e2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Wijesinghe RS, Meshnick SR. Host immunity as a determinant of treatment outcome in Plasmodium falciparum malaria. Lancet Infect Dis. 10:51–9. doi: 10.1016/S1473-3099(09)70322-6. [DOI] [PubMed] [Google Scholar]
- 10.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–32. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 11.Nkrumah FK, Sulzer AJ, Maddison SE. Serum immunoglobulin levels and malaria antibodies in Burkitt’s lymphoma. Trans R Soc Trop Med Hyg. 1979;73:91–5. doi: 10.1016/0035-9203(79)90137-8. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler JL, Bluming AZ, Morrow RH, Jr, Cohen MH, Fife EH, Jr, Finerty JF, Woods R. Burkitt’s lymphoma and malaria. Trans R Soc Trop Med Hyg. 1972;66:285–91. doi: 10.1016/0035-9203(72)90159-9. [DOI] [PubMed] [Google Scholar]
- 13.Inselburg J, Bzik DJ, Li WB, Green KM, Kansopon J, Hahm BK, Bathurst IC, Barr PJ, Rossan RN. Protective immunity induced in Aotus monkeys by recombinant SERA proteins of Plasmodium falciparum. Infect Immun. 1991;59:1247–50. doi: 10.1128/iai.59.4.1247-1250.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoki S, Li J, Itagaki S, Okech BA, Egwang TG. Serine repeat antigen (SERA5) is predominantly expressed among the SERA multigene family of Plasmodium falciparum, and the acquired antibody titers correlate with serum inhibition of the parasite growth. J Biol Chem. 2002;277:47533–40. doi: 10.1074/jbc.M207145200. [DOI] [PubMed] [Google Scholar]
- 15.Pratt-Riccio LR, Sallenave-Sales S, de Oliveira-Ferreira J, da Silva BT, Guimaraes ML, Santos F, de Simone TS, Morgado MG, de Simone SG, Ferreira-Da-Cruz Mde F, Daniel-Ribeiro CT, Zalis MG, et al. Evaluation of the genetic polymorphism of Plasmodium falciparum P126 protein (SERA or SERP) and its influence on naturally acquired specific antibody responses in malaria-infected individuals living in the Brazilian Amazon. Malar J. 2008;7:144. doi: 10.1186/1475-2875-7-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arisue N, Kawai S, Hirai M, Palacpac NM, Jia M, Kaneko A, Tanabe K, Horii T. Clues to evolution of the SERA multigene family in 18 Plasmodium species. PLoS ONE. 6:e17775. doi: 10.1371/journal.pone.0017775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SK, Good RT, Drew DR, Delorenzi M, Sanders PR, Hodder AN, Speed TP, Cowman AF, de Koning-Ward TF, Crabb BS. A subset of Plasmodium falciparum SERA genes are expressed and appear to play an important role in the erythrocytic cycle. J Biol Chem. 2002;277:47524–32. doi: 10.1074/jbc.M206974200. [DOI] [PubMed] [Google Scholar]
- 18.Yeoh S, O’Donnell RA, Koussis K, Dluzewski AR, Ansell KH, Osborne SA, Hackett F, Withers-Martinez C, Mitchell GH, Bannister LH, Bryans JS, Kettleborough CA, et al. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131:1072–83. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Horii T, Shirai H, Jie L, Ishii KJ, Palacpac NQ, Tougan T, Hato M, Ohta N, Bobogare A, Arakaki N, Matsumoto Y, Namazue J, et al. Evidences of protection against blood-stage infection of Plasmodium falciparum by the novel protein vaccine SE36. Parasitol Int. 59:380–6. doi: 10.1016/j.parint.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Nkrumah FK, Olweny CL. Clinical features of Burkitt’s lymphoma: the African experience. IARC Sci Publ. 1985:87–95. [PubMed] [Google Scholar]
- 21.Nkrumah FK, Perkins IV. Sickle cell trait, hemoglobin C trait, and Burkitt’s lymphoma. Am J Trop Med Hyg. 1976;25:633–6. doi: 10.4269/ajtmh.1976.25.633. [DOI] [PubMed] [Google Scholar]
- 22.Okech B, Mujuzi G, Ogwal A, Shirai H, Horii T, Egwang TG. High titers of IgG antibodies against Plasmodium falciparum serine repeat antigen 5 (SERA5) are associated with protection against severe malaria in Ugandan children. Am J Trop Med Hyg. 2006;74:191–7. [PubMed] [Google Scholar]
- 23.Gislefoss RE, Grimsrud TK, Morkrid L. Stability of selected serum proteins after long-term storage in the Janus Serum Bank. Clin Chem Lab Med. 2009;47:596–603. doi: 10.1515/CCLM.2009.121. [DOI] [PubMed] [Google Scholar]
- 24.Hart J, Miller C, Tang X, Vafai A. Stability of varicella-zoster virus and herpes simplex virus IgG monoclonal antibodies. J Immunoassay Immunochem. 2009;30:180–5. doi: 10.1080/15321810902782871. [DOI] [PubMed] [Google Scholar]
- 25.White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, Kokwaro G, Ouma J, Hien TT, Molyneux ME, Taylor TE, Newbold CI, et al. Averting a malaria disaster. Lancet. 1999;353:1965–7. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- 26.Snow RW, Marsh K. Malaria in Africa: progress and prospects in the decade since the Abuja Declaration. Lancet. 376:137–9. doi: 10.1016/S0140-6736(10)60577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardiner C, Biggar RJ, Collins WE, Nkrumah FK. Malaria in urban and rural areas of southern Ghana: a survey of parasitaemia, antibodies, and antimalarial practices. Bull World Health Organ. 1984;62:607–13. [PMC free article] [PubMed] [Google Scholar]
- 28.Asito AS, Piriou E, Odada PS, Fiore N, Middeldorp JM, Long C, Dutta S, Lanar DE, Jura WG, Ouma C, Otieno JA, Moormann AM, et al. Elevated anti-Zta IgG levels and EBV viral load are associated with site of tumor presentation in endemic Burkitt’s lymphoma patients: a case control study. Infect Agent Cancer. 5:13. doi: 10.1186/1750-9378-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D’Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–25. [PubMed] [Google Scholar]
- 30.Sumba PO, Kabiru EW, Namuyenga E, Fiore N, Otieno RO, Moormann AM, Orago AS, Rosenbaum PF, Rochford R. Microgeographic variations in Burkitt’s lymphoma incidence correlate with differences in malnutrition, malaria and Epstein-Barr virus. Br J Cancer. 103:1736–41. doi: 10.1038/sj.bjc.6605947. [DOI] [PMC free article] [PubMed] [Google Scholar]


