Abstract
Cell invasion through basement membrane (BM) is a specialized cellular behavior critical to many normal developmental events, immune surveillance and cancer metastasis. A highly dynamic process, cell invasion involves a complex interplay between cell-intrinsic elements that promote the invasive phenotype, and cell-cell and cell-BM interactions that regulate the timing and targeting of BM transmigration. The intricate nature of these interactions has made it challenging to study cell invasion in vivo and model in vitro. Anchor cell invasion in C. elegans is emerging as an important experimental paradigm for comprehensive analysis of BM invasion, revealing the gene networks that specify invasive behavior and the interactions that occur at the cell-BM interface.
Introduction
The basement membrane (BM) is a dense, highly cross-linked sheet of extracellular matrix that structurally underlies all epithelia and endothelia [1]. During normal development and immune surveillance, specialized cells cross through BM in a process known as cell invasion or BM transmigration [2]. Examples include gastrulation [3]; trophoblast implantation [4,5]; epicardial, myoblast and neural crest cell migrations [6–8]; and leukocyte trafficking [9]. Cell invasion is also key to the pathology of many human diseases, including asthma, rheumatoid arthritis, pre-eclampsia and, most notably, metastatic cancer [10–14]. Cell invasion involves dynamic interactions between the invading cell(s), the tissue that is invaded, and the BM separating them. An inability to recapitulate these interactions in vitro and the difficulty of experimentally accessing them in vivo has limited our understanding of this fundamental cell biological process.
Recent advances using ex vivo and in vivo systems, however, are beginning to yield significant new insights into cell invasion. The chick chorioallantoic membrane (CAM) and rat peritoneal BM invasion assays, which allow direct visualization of cell-BM interactions, are uncovering the cellular and molecular mechanisms used to breach a native BM [15–17]. Studies modeling tumor development in Drosophila imaginal discs have revealed signaling interactions between wild-type and oncogenic neighbors that initiate invasive behavior [18–22]. In vertebrates, studies of leukocyte transmigration have identified regions of the perivascular BM that contain reduced levels of the BM components laminin and type IV collagen, sites that may present invading cells less resistance [23]. Further, intravital imaging studies in murine tumor models have found remarkable plasticity in invasive cell motility, including collective, mesenchymal and amoeboid migration, as well as lymphatic and hematogenic mechanisms of dispersal [24,25]. In spite of the different strengths of each of these models, none allow for the observation of events at the cell-BM interface in vivo. Furthermore, these models lack the predictability required for a rigorous examination of dynamic events that occur during BM transmigration. Thus, despite considerable advances, our understanding of cell invasion through BM remains incomplete.
Anchor cell invasion: a simple model of cell invasion in vivo
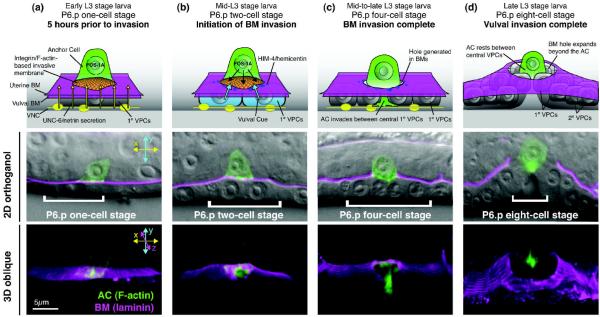
Anchor cell (AC) invasion in C. elegans is unique amongst models of cell invasion in that it combines predictability, tractable genetics and subcellular visual resolution [26]. The molecular organization of BM in C. elegans is highly conserved with vertebrate BM and contains orthologs of the major structural components, including type IV collagen, laminin, perlecan and nidogen [27]. Moreover, these BM components have been tagged with GFP, which, paired with AC-specific expression of fluorescent protein fusions, allows the cell-BM interface to be easily visualized [28–30] (Figure 1). The AC is a specialized uterine cell that invades through the juxtaposed uterine and ventral epidermal BMs and then moves between the central vulval precursor cells (VPCs) to initiate uterine-vulval connection during hermaphrodite development [26]. AC invasion is highly stereotypical, occurring in tight synchrony with the divisions of the underlying 1° VPC P6.p and its descendants (Figure 1A–D). BM transmigration, which occurs over a 90-minute period (Figure 1B–C), is controlled by a combination cell-intrinsic factors that promote BM removal [31–33] and extracellular cues that control the timing and targeting of AC invasion [26,29,34]. Many genes regulating AC invasion have been implicated in other developmental invasion events and metastasis [29,31,34,35], suggesting the AC's tissue-invasive program is conserved. As we discuss in this review, the AC's extensive experimental toolkit is allowing for a comprehensive analysis of cell invasion: from identifying the factors that specify the invasive phenotype to understanding the role of the microenvironment and visualizing the dynamic cellular events that orchestrate BM removal.
Figure 1. Anchor cell invasion in the nematode C. elegans.
Panels depict four time points over the course of anchor cell (AC) invasion. Schematic diagram (top), single confocal z-section of AC (F-actin probe mCherry::moeABD, green) and basement membrane (BM; laminin::GFP shown in magenta) fluorescence overlaid on DIC micrograph (middle; anterior is left, ventral is down in this and subsequent figures; brackets denote 1° vulval precursor cell (VPC) P6.p and its descendants), and 3D reconstructions of a confocal z-stacks of the AC and BM fluorescence, rotated 45° forward (bottom). (A) Approximately five hours prior to invasion, during the early L3 larval stage (P6.p one-cell stage), secretion of UNC-6/netrin (yellow arrows) from the ventral nerve cord (VNC) acts in conjunction with integrin signaling to establish an F-actin-based invasive membrane domain (orange) within the AC at the interface with the BM. (B) During the mid-L3 larval stage, the 1°VPC P6.p cell daughters (P6.p two-cell stage) secrete a chemotactic vulval cue (blue arrows) that stimulates protrusions from the AC's invasive membrane domain that breach the BM. The transcription factor FOS-1A controls the expression of target genes with in the AC (black arrows), including the BM protein hemicentin (purple spots), that promote BM removal. (C) By the mid-to-late L3 stage (P6.p four-cell stage) the AC has generated a large hole in the BMs and has invaded between the central 1°VPCs, initiating uterine-vulval connection. (D) During the late L3 larval stage, the hole in the BM expands beyond the AC as the underlying vulval cells invaginate. The AC invades between the central vulval cells (out of the plane of focus), and sits at the apex of vulval.
Transcriptional control of AC invasion: specifying the invasive phenotype
Cell invasion through BM is a transcriptionally acquired behavior [36,37]. In embryonic development and tumor progression, cell invasion is strongly associated with epithelialto-mesenchymal transitions (EMTs), wherein individual epithelial cells downregulate cell-cell adhesions, lose polarity and acquire mesenchymal-like motility and invasive capabilities [38,39]. Several transcription factors are known to promote EMT, including Snail, Slug, Twist and Zeb1/2 [36]. The known links between these transcription factors and the invasive cellular machinery (e.g., proteases and F-actin-based structures called invadosomes), however, are indirect, suggesting these EMT transcription factors do not themselves specify invasion [40,41]. Furthermore, BM invasion is not always tied to EMT, as cells can invade through BM without adopting a full mesenchymal phenotype. These include collective invasions during normal morphogenetic processes and tumor progression, in which groups of cells assume mesenchymal-like migratory properties at an invasive front, while maintaining cell-cell junctions [38,42,43]. AC invasion most closely resembles this type of invasion [26,29] (Figure 1C–D; Figure 2A). In addition, leukocytes and some cancers adopt an amoeboid-type of motility and are invasive [44]. Based on these observations it is unclear whether distinct invasion programs are used in different contexts (e.g., EMT, collective or amoeboid invasion) or if EMT regulators and other cell-specific factors intersect with transcriptional regulators of a shared BM invasion program.
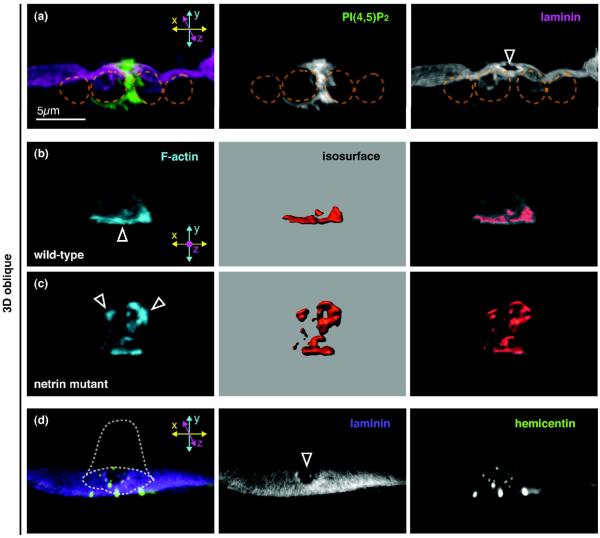
Figure 2. Dissecting AC invasion at single cell resolution.
3D reconstructions of confocal z-stacks rotated 45° forward (A and D) or viewed laterally (B–C). (A) AC-specific expression of a PI(4,5)P2 probe (mCherry::PLCδPH, green) reveals a dynamic protrusion from the AC's invasive membrane domain breaching the BM (laminin::GFP shown in magenta, arrowhead points to BM breach) and extending between the central VPCs (outlined by dotted orange line) at the early P6.p four-cell stage. Of note, the AC maintains cell-cell junctions with neighboring uterine cells (not shown), throughout the course of AC invasion. (B–C) Images show the AC at the P6.p two-cell stage, prior to the normal time of BM invasion. 3D isosurface renderings (red, center) are used to quantify the amount, volume, and polarity of F-actin (mCherry::moeABD, cyan, left) within the AC (overlay, right). (B) In wild-type animals, the F-actin network of the invasive membrane domain is polarized towards the BM (arrowhead) prior to invasion. (C) In unc-6 (netrin) mutants, which fail to invade at the appropriate time, F-actin and other components of the invasive membrane domain are no longer polarized towards the BM but localize throughout the cortex of the AC (arrowheads). (D) Prior to invasion, the AC secretes HIM-4 (hemicentin::GFP, green) into the BM (laminin::mCherry shown in purple) at the site through which the AC invades (arrowhead points to small breach in the BM). The dotted white line depicts the position of the AC.
One of the first genes found to regulate AC invasion was fos-1a, the C. elegans ortholog of the transcription factor Fos [31]. Vertebrate Fos proteins have been associated with a wide range of cell invasions, from trophoblast implantation to many types of metastatic cancers [45–47]. Furthermore, Fos proteins are a component of the AP-1 transcription factor complex, which in vertebrates is the major regulator of matrix metalloproteinases (MMPs), a class of proteases whose expression is strongly correlated with normal BM invasion and tumor progression [20,48–50]. In fos-1a null mutants, the AC extends polarized cellular protrusions that flatten at an intact BM, indicating that targeting and polarization to the site of invasion are normal but the ability to breach the BM barrier is compromised. FOS-1A is upregulated in the AC prior to invasion and regulates the expression of the Evi-1 transcription factor ortholog EGL-43L, the protocadherin CDH-3, the conserved extracellular matrix protein hemicentin (HIM-4) and, strikingly, the MMP ortholog ZMP-1 [31–33]. Considering the conservation of Fos regulation of MMP expression from C. elegans to humans and the strong association of Fos and MMPs with diverse BM invasion events, we suspect that Fos is a component of a conserved BM transmigration program that intersects with cell-specific transcriptional elements. Consistent with this idea, vertebrate Fos proteins have recently been shown to function with EMT transcription factors in multiple contexts [39,51,52]. Importantly, in addition to FOS-1A, other transcriptional regulators appear to contribute to the AC's invasiveness. ACs in fos-1a null mutants maintain expression of several genes required for BM invasion, including the Rac GTPase MIG-2, the integrin heterodimer INA-1/PAT-3 and the guanylate kinase GUK-1 [29,34,35]. Identifying and examining how these additional factors function, as well as how FOS-1A is regulated during AC invasion, will advance our understanding of the transcriptional regulation of BM invasion and likely help elucidate how diverse cell types become invasive.
Integrating cell-intrinsic factors and extracellular cues at the AC-BM interface
How extracellular cues are integrated with cell-intrinsic pathways to control BM transmigration is poorly understood. Studies on AC invasion have shown that a combination of cell-intrinsic and extrinsic signals coordinate the precise timing and targeting of BM invasion and that these pathways intersect at the AC-BM interface. Approximately five hours prior to invasion, the guidance factor UNC-6/netrin, which is secreted from the ventral nerve cord (VNC; Figure 1A), polarizes its receptor UNC-40/DCC to the basal membrane of the AC (i.e., the AC-BM interface) [29]. Here, netrin signaling, in combination with the putative laminin-binding integrin INA-1/PAT-3, is thought to act as a scaffolding to localize actin regulators. These include the phospholipid phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), the Rac proteins MIG-2 and CED-10, and the Ena/VASP ortholog UNC-34 [29,34] (Figure 2B–C). By establishing this invasive membrane domain at the AC-BM interface, the first two signals (integrin and netrin) prepare the AC to respond to a third, currently unidentified, chemotactic cue secreted from the underlying vulval cells. In response to this cue, the AC extends protrusions from its invasive membrane domain that breach the BM (Figure 1C, 2A). Illustrating how extrinsic and cell-intrinsic pathways integrate at the AC-BM interface, the proper deposition of the FOS-1A transcriptional target hemicentin (HIM-4; Figure 2D), which promotes BM removal by the AC, is also dependent on integrin and netrin signaling [29,34]. Convergence of distinct pathways at the invasive cell-BM interface is likely a key principle of all cell invasions.
BM remodeling during AC invasion: breaching the barrier
To transit through BM, it is thought that cells must disassemble, displace or proteolytically break down the BM [2,53], or alternatively, find regions with reduced BM components or cross-linking [23,54]. A lack of in vivo models and visual markers to monitor the fate of the BM and the corresponding activity of invading cells over time has hindered our ability to determine the relative contributions of each of these processes.
During AC invasion, the transcription factor FOS-1A plays a key role in regulating the expression of genes within the AC that enable BM removal, including the MMP ZMP-1. Animals harboring null mutations in zmp-1, however, show only a mild AC invasion defect, suggesting ZMP-1 functions redundantly, possibly with other MMPs. Consistent with this idea, a recent screen of the predicted proteases in the C. elegans genome failed to identify a single protease that alone was required for AC invasion [30]. In vertebrate development and tumor progression, MMPs are strongly expressed within or near invading cells [50,55] and have been functionally implicated in BM transmigration in ex vivo assays [15]. Similar to AC invasion, however, mouse knockouts of MMPs show only mild phenotypes and there is to date no clear in vivo evidence demonstrating a requirement for MMPs in cell invasion through BM [2]. Furthermore, therapies targeting MMPs have been unsuccessful in a wide range of cancers, though the reasons for these failures are unclear. [56]. Thus, the role of MMPs in BM transmigration remains a crucial gap in our understanding of cell invasion. Given that C. elegans has only six MMP family proteins, compared to more than 20 in vertebrates, a functional analysis of potentially redundant roles should be more feasible.
The unbiased genetic approaches in C. elegans have also facilitated the identification of new, unexpected players that regulate BM removal. An example of this is HIM-4, the C. elegans ortholog of the conserved matrix protein hemicentin [31,57]. Hemicentin is paradoxically secreted by the AC into the BM through which the AC later invades (Figure 2D). Hemicentin might promote BM removal by providing increased adhesion for the AC or by biophysically changing the BM, which has been hypothesized as a mechanism for the repeated non-destructive transmigration of leukocytes [2]. Suggesting a conserved role in invasion, hemicentin has been shown to localize to the surface of pre-implantation trophoblasts [58].
Considering the essential role of BM in cellular physiology and tissue integrity, it seems likely that BM removal is more complex and tightly regulated than previously recognized. Supporting this idea, we recently developed a BM optical highlighting technique and found that a combination of bulk BM movement followed by targeted cell-BM adhesion contributes to the formation of the large BM gap during the later stages of uterine-vulval development [30] (Figure 1D). Combining more advanced microscopy methods and BM markers with genetic analysis of AC invasion promises to better resolve the initial stages of BM removal during cell invasion.
AC invasion as a gene discovery tool: a comprehensive approach
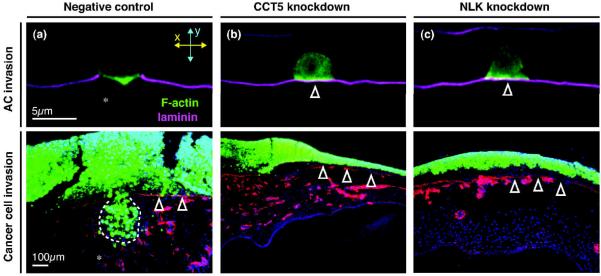
The ability to identify functionally relevant genes using powerful genetic screens and functional genomics has been a great strength of AC invasion. For example, both integrin and netrin signaling were identified in screens for regulators of AC invasion [29,34]. More recently, Matus et al., 2010 conducted a whole-genome RNA interference (RNAi) screen that identified 99 genes that promote AC invasion [35]. The identified genes included chaperone complexes, transcriptional regulators, kinases and conserved molecules with unknown functions. Notably, most of these genes have clear human orthologs but have never been implicated in cell invasion. To investigate potential functional homology in vertebrate cells, two of the genes that had single orthologs in humans, the TCP-1-like chaperonin cct-5 and the NEMO-like kinase (NLK) ortholog lit-1, were knocked down by siRNA in metastatic breast and colon cancer cells. Depletion of either CCT-5 or NLK abrogated the ability of these cells to breach the BM in an in vivo CAM assay (Figure 3A–C), providing strong evidence for the conservation of invasive programs in development and disease.
Figure 3. AC invasion screens identify genes required for human cancer cell invasion.
Top panels show the C. elegans AC (expressing F-actin probe mCherry::moeABD, green) and BM (laminin∷GFP, magenta) in RNAi fed animals at the P6.p four-cell stage, the time by which wild-type BM invasion is complete. Bottom panels show siRNA transfected MDA-MB-231 human breast cancer cells (green) plated on a chick chorioallantoic membrane (CAM; nuclei, blue; type IV collagen in BM, red). Arrowheads point to intact BM. (A) In empty vector RNAi controls, the AC invades through the BM (asterisk; top panel). In scrambled siRNA controls, the breast cancer cells breach the chorioallantoic epithelial BM (asterisk, bottom panel) and invade into the extraembryonic intermediate mesenchyme (invading cells outlined by dotted white line). (B) RNAi and siRNA-mediated knockdown of the TCP1-like chaperonin CCT-5 blocked BM invasion by the AC (top) and breast cancer cells (bottom), respectively. (C) Knockdown of NEMO-like kinase (NLK) similarly blocked BM transmigration by the AC and human breast cancer cells.
As this was the first genome-wide functional screen of BM invasion in vivo, it will be important to further characterize the identified genes to determine where each gene functions during AC invasion and to examine whether these genes have conserved roles in promoting cell invasion in other contexts. Ultimately, the human orthologs of genes identified in screens for AC invasion might provide novel therapeutic targets to modulate invasive behavior in disease states such as metastatic cancer.
Future directions: major unanswered questions at the invasive front
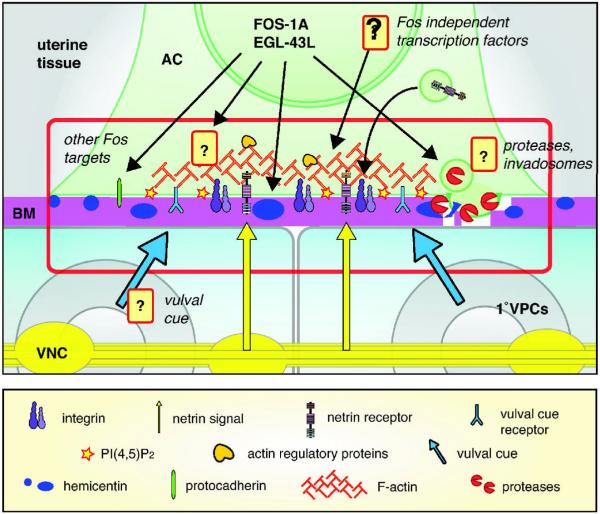
Although AC invasion has begun to yield important new insights into BM transmigration, several major questions remain (summarized in Figure 4). The full complement of FOS-1A targets that enable BM removal remains a critical gap in our understanding of AC invasion, as does the nature of the FOS-1A-independent transcriptional pathway(s). The molecular identity of the vulval cue and its receptor are also unknown. Identifying these molecules will be particularly important to understand how this cue stimulates the formation of the AC's invasive membrane protrusions that breach the BM. Notably, many of the molecules that localize to these invasive protrusions are also components of invadosomes, F-actin-based membrane protrusions found in numerous invasive cell types when cultured in vitro [59]. It will thus be of interest to investigate whether the structures in the AC might be the in vivo equivalent of invadosomes. Finally, though protease expression is associated with AC invasion and protease activity has been strongly implicated in in vitro and ex vivo models of cell invasion, a defined role for proteases in AC invasion, as in other in vivo contexts, remains a major unanswered question.
Figure 4. Current understanding of AC invasion and major unanswered questions.
Schematic diagram depicts the integration of multiple extrinsic (integrin, netrin and the vulval cue) and cell-intrinsic (FOS-1A, EGL-43L, and hemicentin) factors at the visually accessible AC-BM interface (highlighted by red box). Questions for future research are labeled in italics and include identifying additional targets of the transcription factor FOS-1A, other transcription factors, the molecular nature of the vulval cue and its receptor, as well as investigating the potential roles of proteases and invadosomes.
Currently, there is no unifying model for how cells, in either normal or pathological contexts, breach BM. As BM can have tissue-specific properties and compositions, different strategies might be employed to transmigrate distinct BMs [1,2]. In general, however, BMs have a highly conserved composition that is shared throughout the animal kingdom, particularly regarding the core structural components. Thus, it seems equally plausible that cells use a common but adaptable invasion program to breach BM. Supporting this idea, cancer cells and leukocytes can invade BMs of diverse tissues. Further, many of the genes associated with AC invasion (Fos, integrin, MMPs) are thought to regulate cell invasion in diverse contexts in vertebrates and Drosophila. Finally, invasive cancer cells appear to have adaptive properties, as they have been found to match the strength of their invasive machinery to the rigidity of the matrix [60]. We thus favor the notion that a conserved but flexible cell invasion program underlies BM transmigration in most cell types. We anticipate that studies on AC invasion will help to elucidate this conserved BM invasion program and bring about a deeper, more unified understanding of the mechanisms controlling this fundamental and clinically important cellular behavior.
Acknowledgements
We thank A. Schindler and M. Morrissey for helpful comments on the manuscript, S. Johnson of the Duke University LMCF for imaging advice and all current and former members of the Sherwood Laboratory for performing much of the work reviewed in this article. Research in our lab was funded by a Howard Temin Award (K01CA098316-01), Basil O'Connor Scholars Research Award, The Pew Scholars Program in the Biomedical Sciences, and NIH grants GM079320 and GM079320-03S1 to D.R.S. We apologize to colleagues whose work was not included for the sake of brevity.
References
- 1.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 2.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–775. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 4.Genbacev OD. Trophoblast L-Selectin-Mediated Adhesion at the Maternal-Fetal Interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y. Trophoblasts acquire a chemokine receptor, CCR1, as they differentiate towards invasive phenotype. Development. 2003;130:5519–5532. doi: 10.1242/dev.00729. [DOI] [PubMed] [Google Scholar]
- 6.Craig EA, Austin AF, Vaillancourt RR, Barnett JV, Camenisch TD. TGFβ2-mediated production of hyaluronan is important for the induction of epicardial cell differentiation and invasion. Experimental Cell Research. 2010;316:3397–3405. doi: 10.1016/j.yexcr.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes SM, Blau HM. Migration of myoblasts across basal lamina during skeletal muscle development. Nature. 1990;345:350–353. doi: 10.1038/345350a0. [DOI] [PubMed] [Google Scholar]
- 8.Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Delpech B, Girard N, Christ B. The extracellular matrix during neural crest formation and migration in rat embryos. Anat Embryol (Berl) 1990;182:29–39. doi: 10.1007/BF00187525. [DOI] [PubMed] [Google Scholar]
- 9.Madsen CD, Sahai E. Cancer dissemination--lessons from leukocytes. Dev Cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Ingram JL, Huggins MJ, Church TD, Li Y, Francisco DC, Degan S, Firszt R, Beaver DM, Lugogo NL, Wang Y, et al. Airway Fibroblasts in Asthma Manifest an Invasive Phenotype. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201009-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, Fisher SJ. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol. 1997;151:1809–1818. [PMC free article] [PubMed] [Google Scholar]
- 12.Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 2003;24:640–647. doi: 10.1016/j.tips.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 14.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 15.Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20:2673–2686. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this study, the authors use a native BM dissected from a rat peritoneum and show how, in a three step process, cancer cells use invasive membrane structures called invadosomes to breach BM. Proper formation and maturation of the invadosomes in this study required all three elements of the cytoskeleton: actin, microtubules, and intermediate filaments).
- 17.Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci U S A. 2009;106:20318–20323. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this study, Ota and colleagues adopt a previously established model, the chick chorioallantoic membrane (CAM) assay, to examine the cell-BM interactions that occur during the early stages of cancer cell BM transmigration in vivo. The authors find that the classic EMT transcription factor Snail induces cancer cells to transmigrate BM by mobilizing proteases.
- 18.Cordero JB, Macagno JP, Stefanatos RK, Strathdee KE, Cagan RL, Vidal M. Oncogenic Ras Diverts a Host TNF Tumor Suppressor Activity into Tumor Promoter. Developmental Cell. 2010;18:999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this study, Cordero and colleagues report that interactions between tumor cells and wild-type cell drive cell oncogenic cell invasion in Drosophila.
- 19.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proceedings of the National Academy of Sciences. 2007;104:2721–2726. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidal M, Salavaggione L, Ylagan L, Wilkins M, Watson M, Weilbaecher K, Cagan R. A Role for the Epithelial Microenvironment at Tumor BoundariesEvidence from Drosophila and Human Squamous Cell Carcinomas. The American Journal of Pathology. 2010;176:3007–3014. doi: 10.2353/ajpath.2010.090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463:545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Using genetic clones in Drosophila imaginal discs, Wu and coworkers demonstrate that heterotypic interactions between neighboring oncogenic and wild-type cells is required for the tumor cells to become invasive.
- 23.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is an interesting intravital imaging study that highlights how different modes of cancer cell invasion, specifically collective versus single-cell migration, depend on TGFβ signaling. The authors further demonstrate that these distinct modes of invasion differentially use hematogenic or lymphatic routes of dispersal.
- 25.Beerling E, Ritsma L, Vrisekoop N, Derksen PWB, van Rheenen J. Intravital microscopy: new insights into metastasis of tumors. Journal of Cell Science. 2011;124:299–310. doi: 10.1242/jcs.072728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell. 2003;5:21–31. doi: 10.1016/s1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 27.Kramer JM. Basement membranes. WormBook. 2005:1–15. doi: 10.1895/wormbook.1.16.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kao G, Huang CC, Hedgecock EM, Hall DH, Wadsworth WG. The role of the laminin beta subunit in laminin heterotrimer assembly and basement membrane function and development in C. elegans. Dev Biol. 2006;290:211–219. doi: 10.1016/j.ydbio.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol. 2009;11:183–189. doi: 10.1038/ncb1825. [DOI] [PMC free article] [PubMed] [Google Scholar]; • One of the first studies to demonstrate a functional requirement of netrin signaling in cell invasion through BM, the authors show how this pathway functions to orient a specialized F-actin-based invasive membrane domain at the AC-BM interface.
- 30.Ihara S, Hagedorn EJ, Morrissey MA, Chi Q, Motegi F, Kramer JM, Sherwood DR. Basement membrane sliding and targeted adhesion remodels tissue boundaries during uterine-vulval attachment in C. elegans. Nat Cell Biol. 2011 doi: 10.1038/ncb2233. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Using a new BM optical highlighting technique, Ihara and colleagues, find a new mechanism contributes to BM removal-a combination of bulk BM movement followed by targeted cell-BM adhesion drives the formation of a large breach in the BM during the later stages of uterine-vulval development in C. elegans.
- 31.Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Hwang BJ, Meruelo AD, Sternberg PW. C. elegans EVI1 proto-oncogene, EGL-43, is necessary for Notch-mediated cell fate specification and regulates cell invasion. Development. 2007;134:669–679. doi: 10.1242/dev.02769. [DOI] [PubMed] [Google Scholar]
- 33.Rimann I, Hajnal A. Regulation of anchor cell invasion and uterine cell fates by the egl-43 Evi-1 proto-oncogene in Caenorhabditis elegans. Dev Biol. 2007;308:187–195. doi: 10.1016/j.ydbio.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell's invasive membrane in C. elegans. Dev Cell. 2009;17:187–198. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this study the authors demonstrate that the AC's invasive membrane domain at the AC-BM interface is an integration hub for cell-intrinsic pathways and extracellular cues. Specifically, they find that integrin acts upstream of netrin signaling to form the AC's invasive cell membrane, which primes the AC to respond to a stimulatory chemotactic cue from the underlying vulval cells.
- 35.Matus DQ, Li XY, Durbin S, Agarwal D, Chi Q, Weiss SJ, Sherwood DR. In vivo identification of regulators of cell invasion across basement membranes. Sci Signal. 2010;3:ra35. doi: 10.1126/scisignal.2000654. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• As the first genome-wide functional screen for regulators of BM invasion in vivo, this study identified 99 genes that promote BM transmigration during AC invasion. The authors went on to knockdown orthologs of two of the identified genes in breast and colon cancer cells, which blocked the ability of these cells to invade the BM in an in vivo CAM assay, suggesting a conserved invasion program operates in development and disease
- 36.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Ozanne BW, Spence HJ, McGarry LC, Hennigan RF. Invasion is a genetic program regulated by transcription factors. Curr Opin Genet Dev. 2006;16:65–70. doi: 10.1016/j.gde.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jorda M, Olmeda D, Vinyals A, Valero E, Cubillo E, Llorens A, Cano A, Fabra A. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005;118:3371–3385. doi: 10.1242/jcs.02465. [DOI] [PubMed] [Google Scholar]
- 42.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 43.Gray RS, Cheung KJ, Ewald AJ. Cellular mechanisms regulating epithelial morphogenesis and cancer invasion. Curr Opin Cell Biol. 2010;22:640–650. doi: 10.1016/j.ceb.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 45.Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41:2449–2461. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Young MR, Colburn NH. Fra-1 a target for cancer prevention or intervention. Gene. 2006;379:1–11. doi: 10.1016/j.gene.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Bischof P, Campana A. A putative role for oncogenes in trophoblast invasion? Hum Reprod. 2000;15(Suppl 6):51–58. [PubMed] [Google Scholar]
- 48.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]; • This review presents the current understanding of BM transmigration during leukocyte trafficking. In particular it discusses recently identified regions of the vascular BM that contains reduced levels of laminin and type IV collagen. Sites that might provide invading cells a path of less resistance.
- 50.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Zhu G, Li Y, Padia RN, Dong Z, Pan ZK, Liu K, Huang S. Extracellular signal-regulated kinase signaling pathway regulates breast cancer cell migration by maintaining slug expression. Cancer Res. 2009;69:9228–9235. doi: 10.1158/0008-5472.CAN-09-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin S, Dimitri CA, Yoon SO, Dowdle W, Blenis J. ERK2 but not ERK1 induces epithelial-to-mesenchymal transformation via DEF motif-dependent signaling events. Mol Cell. 2010;38:114–127. doi: 10.1016/j.molcel.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabinovitz I, Gipson IK, Mercurio AM. Traction forces mediated by alpha6beta4 integrin: implications for basement membrane organization and tumor invasion. Mol Biol Cell. 2001;12:4030–4043. doi: 10.1091/mbc.12.12.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gligorijevic B, Kedrin D, Segall JE, Condeelis J, van Rheenen J. Dendra2 photoswitching through the Mammary Imaging Window. J Vis Exp. 2009 doi: 10.3791/1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 57.Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development. 2001;128:883–894. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- 58.Xu X, Dong C, Vogel BE. Hemicentins assemble on diverse epithelia in the mouse. J Histochem Cytochem. 2007;55:119–126. doi: 10.1369/jhc.6A6975.2006. [DOI] [PubMed] [Google Scholar]
- 59.Saltel F, Daubon T, Juin A, Ganuza IE, Veillat V, Genot E. Invadosomes: intriguing structures with promise. Eur J Cell Biol. 2011;90:100–107. doi: 10.1016/j.ejcb.2010.05.011. [DOI] [PubMed] [Google Scholar]; • This work reviews the latest research on F-actin-based invasive membrane structures termed `invadosomes'. Though mostly studied in vitro, invadosomes are found in diverse invasive cells types and are thought to mediate the BM invasion events in developmental and disease states such as cancer.
- 60.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–1299. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]