Abstract
Background:
Detection of extended spectrum β-lactamase (ESBL) production among uropathogens is an important marker of endemicity.
Aim:
Intervention of this endemic transmission is important for the control of initial outbreak of ESBL producing organisms in a hospital or specialized unit of hospital.
Materials and Methods:
During the study period of one and a half months, 1,551 urine samples were processed for significant bacteriuria. Two hundred gram negative bacterial isolates were tested for ESBL production. Antimicrobial sensitivity pattern was ascertained for ESBL producing isolates.
Results:
ESBL production was seen in 36% of isolates. All the isolates were multidrug resistant with uniform sensitivity to imipenem.
Conclusion:
This study reveals the significant prevalence of ESBL producing organisms in this north Indian tertiary care hospital. Constant revision of antibiotic policies with infection control interventions is suggested.
Keywords: ESBL, endemicity, infection, uropathogens
INTRODUCTION
Extended spectrum β-lactamase (ESBL) isolates were first detected in western Europe in the mid-1980s. Since then, their incidence has been increasing steadily. A large number of outbreaks of infections due to ESBL producing organisms have been described on every continent of the globe. In some hospitals, initial outbreaks of infections have been supplanted by endemicity of the ESBL producing organisms. This may lead to increased patient mortality when antibiotics inactive against ESBL producers are used. Therefore, control of the initial outbreak of ESBL producing organisms in a hospital or specialized unit of a hospital is of critical importance.[1–3]
Special efforts should be undertaken by clinical microbiology laboratories as recommended by the Clinical and Laboratory Standards Institute (CLSI) for ESBL detection. Additional use of ESBL detection methods has originated because some ESBL producing organisms appeared susceptible to cephalosporins, using conventional breakpoints. It has been recommended that physicians should avoid all penicillins, aztreonam, and cephalosporins if an ESBL producing organism is present.[1–3]
Detection of ESBL producing organism from samples such as urine may be important because this represents an epidemiologic marker of colonization, and therefore there is potential for transfer of such organisms to other patients.[1] Hence, the present study was designed to detect ESBL production among uropathogens.
MATERIALS AND METHODS
The present study was conducted in the Department of Microbiology which is a part of 1,300 bedded teaching tertiary care hospital in India. During one and a half months study period, a total of 1,551 urine samples received in the microbiology laboratory were processed following standard protocol.[4] All the gram negative bacilli (GNB) isolated in significant numbers were identified by standard microbiological procedures and sensitivity to third generation cephalosporins (3GC, ceftazidime, ceftriaxone, and cefotaxime) was determined by disc diffusion technique.[5] Results were interpreted according to CLSI guidelines.[6] Escherichia coli ATCC 25922 was used as control strain.
ESBL detection
The ESBL detection in all 200 urinary isolates was done using double disc synergy test as described by Jarlier et al.[6] Synergy was determined between a disc of amoxiclav (20 μg amoxicillin and 10 μg clavulanic acid) and 30 μg disc of each third generation cephalosporin (3GC) test antibiotic placed at a distance of 15 mm apart.
Antimicrobial susceptibility of ESBL producing urinary isolates was done by disc diffusion method and results were interpreted according to CLSI guidelines using E. coli 25922 ATCC as control strain.[5,6] Antimicrobials used were cephalothin (30 μg), cefamandole (30 μg), cephalexin (30 μg), cefoxitin (30 μg), cefoperazone (75 μg), azlocillin (30 μg), ticarcillin (75 μg), imipenem (10 μg), ticarcillin/clavulanate (75/10 μg), ampicillin/sulbactum (10/10 μg), netilmicin (10 μg), tobramycin (10 μg), lomefloxacin (30 μg), ofloxacin (5 μg), cotrimoxazole (25 μg), nalidixic acid (30 μg), nitrofurantoin (50 μg), and norfloxacin (10 μg). Relevant demographic data was also collected. An isolate was considered multidrug resistant if it was resistant to ≥3 antimicrobial agents.[7] All ESBL detection and drug susceptibility tests were done on Mueller Hinton Agar. Mueller Hinton Agar and the susceptibility discs were procured commercially from Hi, Media (Mumbai, INDIA).
RESULTS
A total of 1,551 urine samples from both outdoor and indoor patients were included during one and a half months study period. Out of these 1,551 samples, 226 (14.5%) urine samples yielded significant growth. Amongst these 226 isolates, 26 (11.5%) were gram positive bacteria and yeast isolates. These were excluded from the study. Amongst the remaining 200 (88.49%) gram negative bacterial isolates, 100 were E. coli (50%), 33 were Klebsiella spp. (16.50%), 23 were Pseudomonas aeruginosa (11.50%), 19 were Enterobacter spp. (9.50%), 13 were Proteus spp. (6.50%), nine were Citrobacter spp. (4.50%), and three were Acinetobacter spp. (1.50%). Maximum ESBL production was observed inKlebsiellaspp. with 18 (54.54%) strains being ESBL producers. Though E. coli was the most commonly isolated organism from the urine but ESBL production was lower (40%) than Klebsiella spp. Out of only nine Citrobacter spp. tested for ESBL production, four (44.44%) were found to be enzyme producers [Table 1].
Table 1.
Prevalence of ESBL production among GNB isolates from urine (n=200)
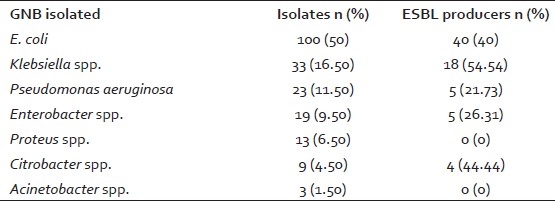
Extended spectrum β-lactamase production was observed in both third generation cephalosporin susceptible and resistant groups. In susceptible group, 12 (15.38%) out of 78 isolates produced ESBL whereas 60 (49.18%) out of 122 resistant isolates produced ESBL [Table 2].
Table 2.
ESBL production among 3GC resistant and susceptible group

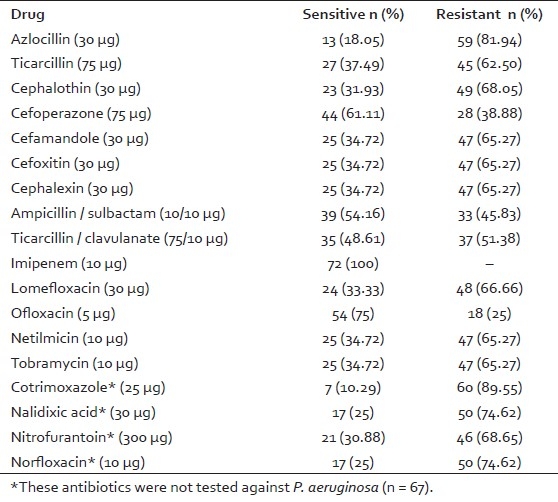
All the ESBL positive urinary isolates were multidrug resistant (drug resistance to ≥ 3 drugs). All the isolates were uniformly sensitive to imipenem. Another effective drug was ofloxacin with 54 (75%) strains being susceptible [Table 3]. Amongst the 72 ESBL producing urinary isolates, 52 (72.72%) were obtained from hospitalized patients, 12 (16.66%) of these patients had been catheterized with Foley's catheter, prior surgery had been performed in eight (11.11%) of these patients and five (6.94%) of these patients had stayed in ICU.
Table 3.
Antimicrobial susceptibility pattern of ESBL producing urinary isolates (n = 72)
DISCUSSION
Since their appearance in Germany in the early 1980s, ESBL producing bacteria have increased both in variety and number.[2] Earlier they were isolated from hospitalized patients only but now reports of isolation from outdoor patients have started coming.[8,9]
In the present study, 200 GNB isolates from urine were included. It was observed that 72 (36%) isolates were ESBL producers. A higher incidence, i.e. 58% ESBL production amongst GNB isolates has been reported by other workers.[10] However, the incidence of 39.90% ESBL production reported by other investigators is in accordance with the present study.[11] Other workers have reported lower rates of ESBL production.[12,13] This geographical difference may be due to different patterns of antibiotic use and differences in the selection of organisms for the study.
Earlier, in vitro resistance to third generation cephalosporin was considered to be suomotto evidence of ESBL production. In our study, 15.38% of susceptible isolates were found to be ESBL producers. Similar findings have also been reported by other Indian workers.[14,15] These studies highlighted the fact that some ESBL producing isolates show false susceptibility to third generation cephalosporin in in- vitro testing. These patients are at a risk of poor outcome if they are treated with antimicrobials to which the organisms exhibit high level of resistance. Mortality rates in these susceptibility - treatment mismatched patients has ranged from 42 to 100%. Therefore, it is recommended that specific tests should be undertaken for detection of ESBL where suspicion for such isolates is high.[1,3]
In this study, 100% sensitivity to imipenem was observed. This finding is in concordance with the studies conducted by other workers.[11,13,16–18] Ofloxacin was also found to be a highly effective drug in vitro. Carbapenems and fluoroquinolones can be alternative to the 3GC for the treatment of serious infections due to ESBL positive GNB.[2,3,19,20]
In earlier studies, prolonged hospitalization, Foley's catheterization, prior surgery, and ICU stay were found to be risk factors.[1–3] Good infection control practices and antibiotic management interventions are instrumental in preventing the emergence of outbreaks due to ESBL producing isolates, especially in high risk areas such as the medical ICU, the neonatal ICU, and oncology units. Educational programs for medical staff to increase awareness of ESBLs should also be developed.
Our study confirms the global trend toward increased resistance to β-lactam antibiotics. It is emphasized that institutions with high prevalence of third generation cephalosporin resistant organisms should employ appropriate tests for their detection and avoid indiscriminate use of third generation cephalosporins. Moreover, the prevalence and antibiotic susceptibility pattern of ESBL producers differs geographically. Hence, such institutional studies will help in the formulation of antibiotic policy for a particular geographical area.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Paterson DL, Bonomo RA. Extended spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathisuwan S, Burgess DS, Lewis JS., 2nd Extended Spectrum β-lactamases: Epidemiology, Detection and treatment. Pharmacotherapy. 2001;21:920–8. doi: 10.1592/phco.21.11.920.34529. [DOI] [PubMed] [Google Scholar]
- 3.Rupp ME, Fey PD. Extended Spectrum β-lactamase (ESBL) – Producing Enterobacteriaceae. Drugs. 2003;63:353–65. doi: 10.2165/00003495-200363040-00002. [DOI] [PubMed] [Google Scholar]
- 4.Duguid JP, Collee JG, Fraser AG. Laboratory strategy in the diagnosis of infective syndromes. In: Collee JG, Duguid JP, Fraser AG, Marmion BP, editors. Mackie and MacCartney: Practical Medical Microbiology. 13th ed. Singapore: Longman Singapore Publishers; 1989. pp. 600–49. [Google Scholar]
- 5.Bauer AW, Kirby W, Sherris J, Turck M. Antibiotic susceptibility testing by a standard single disc method. Am J Clin Pathol. 1996;45:493–6. [PubMed] [Google Scholar]
- 6.Performance standards for antimicrobial susceptibility: sixteenth informational supplement. Wayne, PA, USA: CLSI; 2006. Clinical and Laboratory Standards Institute; pp. M100–S16. [Google Scholar]
- 7.Gupta N, Yadav A, Choudhary U, Arora DR. Citrobacter bacteremia in a tertiary care hospital. Scand J Infect Dis. 2003;35:765–8. doi: 10.1080/00365540310016376. [DOI] [PubMed] [Google Scholar]
- 8.Ananthkrishnan AN, Kanungo R, Kumar A, Badrinath S. Detection of extended spectrum beta lactamase producers among surgical wound infections and burn patients in JIPMER. Ind J Med Microbiol. 2000;18:160–5. [Google Scholar]
- 9.Brigante G, Luzzaro F, Perilli M, Lombardi G, Colì A, Rossolini GM, et al. Evolution of CTX-M type beta -lactamasees in isolates of Escherichia coli infecting hospital and community patients. Int J Antimicrob Agents. 2005;25:157–62. doi: 10.1016/j.ijantimicag.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Mathur P, Kapil A, Das B, Dhawan B. Prevalence of extended spectrum β–lactamase producing gram negative bacteria in a tertiary care hospital. Indian J Med Res. 2002;115:153–7. [PubMed] [Google Scholar]
- 11.Babypadmini S, Appalaraju B. Extended spectrum β lactamases in urinary isolates of Escherichia coli and Klebsiella pneumoniae - Prevalence and susceptibility pattern in a tertiary care hospital. Indian J Med Microbiol. 2004;22:172–4. [PubMed] [Google Scholar]
- 12.Subha A, Ananthan S. Extended spectrum beta lactamase (ESBL) mediated resistance to third generation cephalosporins among Klebsiella pneumoniae in Chennai. Indian J Med Microbiol. 2002;20:92–5. [PubMed] [Google Scholar]
- 13.Shukla I, Tiwari R, Agrawal M. Prevalence of extended spectrum β lactamases producing Klebsiella pneumoniae in a tertiary care hospital. Indian J Med Microbiol. 2004;22:87–91. [PubMed] [Google Scholar]
- 14.Revathi G. Detection of extended spectrum β–lactamases using E–test ESBL strip. Indian J Med Microbiol. 1997;15:69–71. [Google Scholar]
- 15.Uma A, Mehta A, Ayyagari A, Kapil A, Shahnvi A, Rodrigues C, et al. Prevalence of β–lactamase producing strains among clinical isolates obtained from hospital in patients across India and comparison of anti–bacterial susceptibility using disc diffusion method. EGAST 2000. Hospital Today. 2004;9:70–80. [Google Scholar]
- 16.Peña C, Pujol M, Ardanuy C, Ricart A, Pallares R, Liñares J, et al. Epidemiology and successful control of a large outbreak due to Klebsiella pneumoniae producing extended spectrum β-lactamases. Antimicrob Agents Chemother. 1998;42:53–8. doi: 10.1128/aac.42.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winokur PL, Canton R, Casellas JM, Legakis N. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin Infect Dis. 2001;32:S94–103. doi: 10.1086/320182. [DOI] [PubMed] [Google Scholar]
- 18.Hansotia JB, Agarwal V, Pathak AA, Saoji AM. Extended spectrum β-lactamase mediated resistance to third generation cephalosporins in Nagpur, Central India. Indian J Med Res. 1997;105:153–61. [PubMed] [Google Scholar]
- 19.Paterson DL. Recommendations for treatment of severe infections caused by Enterobacteriaceae producing extended spectrum β-lactamase (ESBL) Clin Micriobiol Infect. 2000;6:460–3. doi: 10.1046/j.1469-0691.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- 20.Karas JA, Pillay DG, Muckart D, Sturm AW. Treatment failure due to extended spectrum β-lactamase. J Antimicrob Chemother. 1996;37:203–4. doi: 10.1093/jac/37.1.203. [DOI] [PubMed] [Google Scholar]


