Abstract
AIM:
Nonfermenting gram-negative bacilli (NFGNB), which are saprophytic in nature, have emerged as important healthcare-associated pathogens. They exhibit resistance not only to beta lactam and the other groups of antibiotics, but also to carbapenems. This study was undertaken to identify the nonfermenters isolated from various clinical samples, to assess their clinical significance, to know the type of healthcare-associated infections they caused, and to know their anti-microbial sensitivity pattern.
Materials and Methods:
The nonfermenters were identified using a standard protocol that included tests for motility, oxidase production, oxidation-fermentation test for various sugars, gelatin liquefaction, and growth on 10% lactose agar. The clinical significance was assessed by using various criteria and susceptibility testing was performed with the help of the Kirby-Bauer disc diffusion method.
Results:
A total of 193 NFGNB were isolated from 189 clinical specimens. Pseudomonas aeruginosa was the most common nonfermenter, accounting for 53.8%, followed by Acinetobacter baumannii (22.2%), and Pseudomonas fluorescens (10.8%). Other significant NFGNB isolated were: Sphingobacterium species (5.2%), Acinetobacter lwoffii (3.1%), and Stenotrophomonas maltophilia (2.6%). P. aeruginosa showed good sensitivity to imipenem (94%), cefoperazone (70%), amikacin (69%), and ticarcillin (63%). A. baumannii showed 100% sensitivity to imipenem and 70% sensitivity to piperacillin.
Conclusion:
P. aeruginosa and A. baumannii were the common NFGNB isolated in our study from patients of, urinary tract infection, bacteremia, surgical site infections, and ventilator associated pneumonia. P. aeruginosa showed good sensitivity to imipenem, amikacin, and cefoperazone while A. baumannii showed good sensitivity to imipenem and piperacillin.
Keywords: Acinetobacter baumannii, imipenem, clinical significance, nosocomial pathogens, Pseudomonas aeruginosa
INTRODUCTION
Nonfermenting gram-negative bacilli (NFGNB) are a taxonomically diverse group of aerobic, nonsporing, bacilli that either do not utilize glucose as a source of energy or utilize it oxidatively.[1] They occur as saprophytes in the environment and some are also found as commensals in the human gut.[2,3]
NFGNB are known to account for about 15% of all bacterial isolates from a clinical microbiology laboratory.[4] In recent years, due to the liberal and empirical use of antibiotics, NFGNB have emerged as important healthcare-associated pathogens. They have been incriminated in infections, such as, septicemia, meningitis, pneumonia, urinary tract infections (UTI), and surgical site infections (SSI).[3] NFGNB are innately resistant to many antibiotics and are known to produce extended spectrum ß-lactamases and metallo ß-lactamases.[3,4]
There are very few studies from India wherein the various NFGNB, isolated from patients’ samples, have been identified and their clinical significance assessed. Hence, this study was undertaken to identify the various nonfermenters isolated from patients admitted to our hospital, a tertiary care center, at Kolar. The study was also done to assess their clinical significance and antimicrobial susceptibility pattern, and to identify the various healthcare-related infection they cause.
MATERIALS AND METHODS
A total of 4248 clinical specimens were received in the laboratory during November 2005 and July 2006, which included 1428 urine, 1124 pus, 1035 blood cultures, collected in a brain-heart infusion broth, 365 sputum and respiratory samples, and 296 body fluids. These samples were plated on blood agar, chocolate agar, and Mac Conkey's agar, and incubated at 37°C for 18-24 hours. The organisms isolated were identified using the appropriate biochemical tests.[1] All the organisms that grew on Triple Sugar Iron agar and produced an alkaline reaction were provisionally considered to be NFGNB and identified further by using a standard protocol for identification.[1] The characters assessed included morphology on Gram's stain, motility, pigment production, oxidase production, OF test (Hugh-Leifson's medium) for glucose, lactose, sucrose, maltose, mannitol, and xylose, growth on 10% lactose agar, lysine decarboxylase test, and gelatin liquefaction test. The scheme for identification of NFGNB is shown in Figure 1.
Figure 1.
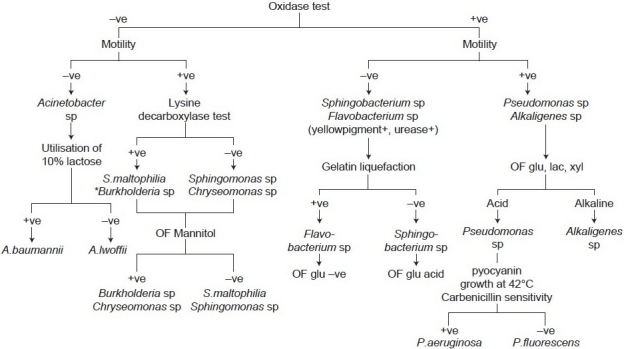
Scheme used in the study for identification of nonfermenting gram-negative bacilli * Burkholderia species vary in their oxidase reaction: Burkholderia gladioli is oxidase -ve, B. cepacia complex is weak +ve and B. pseudomallei is oxidase +ve, OF: Oxidation-fermentation
The clinical significance of the isolated NFGNB was assessed retrospectively by analyzing the case sheets for a combination of relevant laboratory and clinical criteria. The laboratory criteria included: The presence of pus cells along with gram-negative bacilli in the stained smear from the sample, monomicrobial infection, isolation of the same organism from a repeat sample, leukocytosis, and relevant radiological evidence (in cases of VAP).[5] The clinical criteria included the presence of risk factors, such as, bronchiectasis, cystic fibrosis, underlying pneumonia, any malignancy, on mechanical ventilation, and other immunosuppressive conditions.[6,7]
The sensitivity test was performed with the help of the Kirby-Bauer disc diffusion method using commercially available discs (Hi-media). The different antimicrobials tested were imipenem, piperacillin, ticarcillin, amikacin, ciprofloxacin, ceftazidime, cefepime, cefoperazone, ceftriaxone and cotrimoxazole. The results were interpreted as per the Clinical and Laboratory Standards Institute (CLSI) guidelines.[8]
E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as the control strains.
RESULTS
NFGNB were isolated from 189 out of 4248 clinical specimens accounting for an isolation rate of 4.5%. A total of 193 NFGNB were isolated from 189 clinical specimens as four samples yielded two NFGNB. Ninety-eight specimens (51.8%) showed polymicrobial infection where nonfermenters were isolated along with other organisms, of which E. coli and S.aureus were commonly associated. The remaining 91 specimens (48.2%) showed monomicrobial infection. A total of 150 isolates were considered significant by the criteria mentioned, which accounts for about 79% of the nonfermenters causing infection. Analyzed by specimens, NFGNB were isolated from 117 pus, 24 sputum, 23 urine, 22 blood culture, and three body fluid samples.
The NFGNB isolated from various clinical samples are presented in Table 1. Pseudomonas aeruginosa was the most common isolate, accounting for 104 (53.8%), followed by Acinetobacter baumannii 43 (22.2%), and Pseudomonas fluorescens 21 (10.8%). Sphingobacterium species, Acinetobacter lwoffii, Stenotrophomonas maltophilia, and Alkaligenes faecalis were rarely isolated NFGNB, together accounting for 11.4% of the isolates. One isolate each of Pseudomonas stutzeri, Sphingomonas species, and Chryseomonas species was found to be a contaminant. The majority of the nonfermenters were isolated from pus and urine samples.
Table 1.
Nonfermenting gram-negative bacilli isolated from various clinical samples
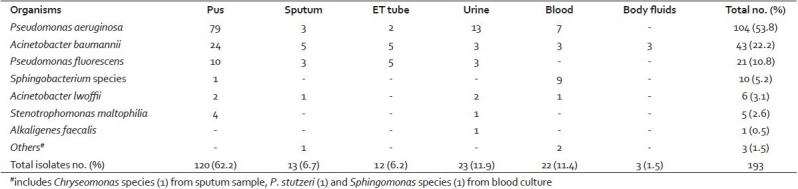
Analysis of the case records of patients with these infections revealed that 120 (62.2%) isolates of NFGNB were isolated from 117 pus samples. P. aeruginosa and A. baumannii were the common isolates from pus samples. Three patients had co-infection of P. aeruginosa and A. baumannii. Four pus samples yielded S. maltophilia. These NFGNB were isolated from cases of burn wounds, nonhealing ulcers, bedsores, deglove injuries, fracture wounds following road traffic accidents (RTA), and postoperative wound infections.
A total of 23 urine samples yielded 23 NFGNB. Repeat isolations were found in 19 cases associated with significant bacteriuria, and presence of inflammatory cells. P. aeruginosa was the major urinary pathogen. This included cases of ureteric calculi, urinary incontinence, pelvic fracture with ruptured urethra, stricture urethra, benign prostatic hyperplasia (BPH), and prolonged catheterization.
Among the NFGNB isolated from blood, except for the Sphingobacterium species, all the other isolates were found to be contaminants, as they could not be re-isolated. The Sphingobacterium species were isolated from an outbreak of septicemia among neonates in the Sick Neonatal Intensive Care Unit (SNICU). Of the nine isolates of Sphingobacterium species, isolated from the blood culture of these neonates, six isolates were found to be significant. Out of these six neonates, three recovered when treated with a combination of imipenem and ofloxacin, but the other three succumbed to the illness despite therapy.
Out of 24 samples from the respiratory tract, 13 sputum and 11 endotracheal tube tips yielded NFGNB. Isolates from seven sputum samples were found to be clinically significant. A. baumannii was the major respiratory pathogen. It was obtained from patients with aspiration pneumonia following organo-phosphorous compound poisoning, ventilator associated pneumonia (VAP) following laparotomy, secondary infection in an asthmatic patient, and pyopneumothorax. The significance of the isolates from the endotracheal tube was difficult to assess; they could have been just colonizers.
Three samples of body fluids, one each of CSF, pleural fluid, and peritoneal fluid yielded A. baumannii. It was found that A. baumannii was significant only in the case of pleural effusion.
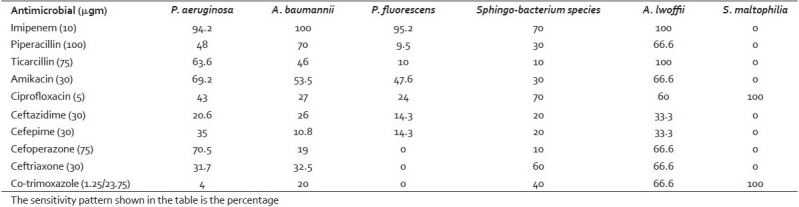
The sensitivity pattern of the NFGNB isolated is presented in Table 2. Most of the isolates of P. aeruginosa were sensitive to imipenem (94.2%), cefoperazone (70.5%), amikacin (69%), and ticarcillin (63%). Comparatively P. aeruginosa was more sensitive to all the antibiotics tested than P. fluorescens; 5.8% of P. aeruginosa and 4.8% of P. fluorescens were imipenem resistant. A. baumannii showed 100% sensitivity to imipenem followed by 70% to piperacillin, and 53.5% to amikacin. A. baumannii showed a higher rate of resistance than A. lwoffii. Seventy percent of the Sphingobacterium species were sensitive to imipenem and ciprofloxacin, 60% to ceftriaxone, and 30% to amikacin. All the isolates of S. maltophilia (100%) were sensitive to cotrimoxazole and ciprofloxacin and were resistant to other antibiotics. The single isolate of A. faecalis was sensitive to imipenem and piperacillin only.
Table 2.
Sensitivity pattern of the nonfermenting gram-negative bacilli isolated
DISCUSSION
NFGNB that were considered to be contaminants in the past have now emerged as important healthcare-associated pathogens.[9] P. aeruginosa and Acinetobacter species are known to be the common nosocomial pathogens.[3,9] Similar observations have also been made by us. NFGNB belonging to Pseudomonas species: P. aeruginosa and P. fluorescens, along with the Acinetobacter species accounted for 87% of the isolates.
In our study most of the isolates of NFGNB were from pus samples, similar to the observation made by others.[10,11] NFGNB were commonly involved in wound infections following RTA and in chronic nonhealing ulcers. Antibiotics such as amikacin, gentamicin, and ciprofloxacin were used to treat these infections, which most often were caused by the two pathogens: P. aeruginosa and A. baumannii.
The-clinical conditions in which NFGNB were isolated in our study included SSI, UTI, VAP, and septicemia. P. aeruginosa and A. baumannii were common organisms incriminated in SSI and UTI. VAP was due to A. baumannii.
There were nine isolates of Sphingobacterium species from the blood culture, in our study. This was due to an outbreak of septicemia in the SNICU caused by the Sphingobacterium species. An environmental survey was done to identify the source, and we could isolate the Sphingobacterium species from the wet soap cakes used for hand washing in the SNICU and also from the dust on the baby weighing machine. Replacement of the soap cakes with liquid soap and institution of isopropyl alcohol hand rub for disinfection of hands before and after patient contact brought the outbreak to an end. Bacteremia due to Sphingobacterium species was reported in literature, which was treated with co-trimoxazole and fluoroquinolones.[2,12] A combination of imipenem and ofloxacin was used to treat our cases.
Five strains of S. maltophilia were isolated during the study. Four of them were from wound infections and one from UTI. Administration of co-trimoxazole promptly brought about a cure in these patients. S. maltophilia is inherently resistant to aminoglycosides and β-lactam agents, including carbapenems, but consistently sensitive to cotrimoxazole.[2]
Resistance patterns among nosocomial bacterial pathogens may vary from country to country and also within the same country, over time.[13] P. aeruginosa isolates in our study were highly susceptible to imipenem (94%), cefoperazone (71%), and amikacin (69%). This contrasts with the antibiotic sensitivity pattern of isolates from Bangalore[14] and Chandigarh.[15] In a study from Bangalore P. aeruginosa showed 60-70% resistance to amikacin, ceftazidime, and ciprofloxacin.[14] In a study from Chandigarh 42% of P. aeruginosa isolates were found to be resistant to imipenem. [15] Similarly Acinetobacter species showed higher rate of resistance to ciprofloxacin, amikacin, ceftazidime, and piperacillin in a study at Bangalore when compared to the present study.[16] We attribute these differences in the susceptibility of strains to differences in the patient population studied by us. Most of our patients were from surgical wards and not from ICU settings. Furthermore, our patients came from rural areas without much exposure to antibiotics.
To conclude, P. aeruginosa and A. baumannii are the most common NFGNB isolated in our study. Their role as healthcare-associated pathogens is well-established and they have caused UTI, septicemia, SSI, and VAP. P. aeruginosa has shown good sensitivity to imipenem, amikacin, and cefoperazone. A. baumannii shows good sensitivity to imipenem and piperacillin. The different species of NFGNB have shown a varied sensitivity pattern in our study. Therefore, identification of NFGNB, and monitoring their susceptibility patterns, are important for the proper management of the infections caused by them. Our study highlights the fact that it is essential to establish the clinical relevance of the isolated NFGNB, before they are considered as pathogens. This would avoid unnecessary usage of antibiotics and emergence of drug-resistant strains.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Winn W Jr, Allen S, Janda W, Koneman E, Procop G, Schreckenberger P, et al., editors. In: Koneman's Color Atlas and textbook of Diagnostic Microbiology. 6th ed. USA: Lippincott Williams and Wilkins Company; 2006. Nonfermenting Gram negative bacilli; pp. 305–91. [Google Scholar]
- 2.Steinberg JP, Rio DC. Gram negative and Gram variable bacilli. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious diseases. 6th ed. Vol. 2. Philadelphia, USA: Elsevier Publication; 2005. pp. 2751–68. [Google Scholar]
- 3.Gales AC, Jones RN, Forward KR, Linares J, Sader HS, Verhoef J. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: Geographic patterns, Epidemiological features, and trends in the SENTRY antimicrobial surveillance program (1997-1999) Clin Infect Dis. 2001;32:104–13. doi: 10.1086/320183. [DOI] [PubMed] [Google Scholar]
- 4.Rubin SJ, Granato PA, Wasilauskas BL. Glucose nonfermenting Gram negative bacteria. In: Lennette EH, Balows A, Hausler WJ Jr, Shadomy HJ, editors. Manual of Clinical Microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 330–49. [Google Scholar]
- 5.Meherwal SK, Taneja N, Sharma SK, Sharma M. Complicated nosocomial UTI caused by nonfermenters. Indian J Urol. 2002;18:123–8. [Google Scholar]
- 6.Hill EB, Henry DA, Speert DP. Pseudomonas. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. 9th ed. Vol. 1. Washington, D.C: American Society for Microbiology; 2007. pp. 734–48. [Google Scholar]
- 7.Quinn JP. Clinical problems posed by multiresistant nonfermenting gram negative pathogens. Clin Infect Dis. 1998;27:S117–24. doi: 10.1086/514912. [DOI] [PubMed] [Google Scholar]
- 8.NCCLS document M100-S11. 1. Vol. 21. Wayne, PA: NCCLS; 2001. National Committee for Clinical Laboratory Standards. Performance Standards for antimicrobial susceptibility testing: Eleventh informational supplement. [Google Scholar]
- 9.Troillet N, Samore MH, Carmeli Y. Imipenem-Resistant Pseudomonas aeruginosa: Risk factors and Antibiotic susceptibility patterns. Clin Infect Dis. 1997;25:1094–8. doi: 10.1086/516092. [DOI] [PubMed] [Google Scholar]
- 10.Yashodara P, Shyamala S. Identification and characterization of nonfermenters from clinical specimens. Indian J Med Microbiol. 1997;15:195–7. [Google Scholar]
- 11.Mishra B, Bhujwala RA, Shriniwas Nonfermenters in human infections. Indian J Med Res. 1986;83:561–6. [PubMed] [Google Scholar]
- 12.Marinella MA. Cellulitis and sepsis due to Sphingobacterium. JAMA. 2002;288:1985. doi: 10.1001/jama.288.16.1985-a. [DOI] [PubMed] [Google Scholar]
- 13.Prashanth K, Badrinath S. In vitro susceptibility pattern of Acinetobacter species to commonly used cephalosporins, quinolones and aminoglycosides. Indian J Med Microbiol. 2004;22:97–103. [PubMed] [Google Scholar]
- 14.Veenakumari HB, Nagarathna S, Chandramuki A. Antimicrobial resistance pattern among aerobic Gram negative bacilli of lower respiratory tract specimens of intensive care unit patients in a neurocentre. Indian J Chest Dis Allied Sci. 2007;49:19–22. [PubMed] [Google Scholar]
- 15.Taneja N, Maharwal S, Sharma M. Imipenem resistance in nonfermenters causing nosocomial urinary tract infections. Indian J Med Sci. 2003;57:294–9. [PubMed] [Google Scholar]
- 16.Sinha M, Srinivasa H, Macaden R. Antibiotic resistance profile and extended spectrum beta- lactamase (ESBL) production in Acinetobacter species. Indian J Med Res. 2007;126:63–7. [PubMed] [Google Scholar]


