Abstract
The culture results of 4,807 blood and 383 sterile body fluid specimens received in our laboratory during a 54-month period, were analyzed to determine the time required for culture to become positive, time at which a culture could safely be considered negative, and the spectrum of isolated organisms. The specimens were processed by automated BACTEC 9120 culture system. A total of 1,677 clinically significant microorganisms were isolated. Gram positive and negative bacterial isolation rates were found to be 62.55% and 32.20%, respectively. Yeasts were recovered in 5.24%. False positivity rate was 1.5%. Clinically significant isolates recovered on day four and five were 97.81% and 99.88%, respectively. At day five, the sensitivity was 99.94% and negative predictive value 99.96%. Our data support a five-day incubation protocol for recovery of all clinically significant organisms with sensitivity reduced by 0.06%, when compared with a six-day protocol.
Keywords: Automated blood culture system, BACTEC, time to detection
INTRODUCTION
Bloodstream infections are a threat to every organ in the body and can have serious immediate consequences, including shock, multiple organ failure, disseminated intravascular coagulation (DIC), and death (mortality rates from 20-50%). As a result, timely detection and identification of blood-borne pathogens is one of the most important functions of the microbiology laboratory.[1] Recently, many advanced techniques such as nucleic acid probes and polymerase chain reaction (PCR) have been developed for the diagnosis of bloodstream infections, but blood culture still remains the most practical and reliable method. [2] Conventional blood culture methods involve visual examination of blood culture bottle once a day for the evidence of growth for two days and blind subculture on the second day on solid media. Culture negative bottle are further re-incubated for 5-7 days before reporting. Over the past few years, dramatic improvement has taken place in blood culture methods, media, and systems. Most of the technologically advanced blood culture systems are fully automated continuously monitored blood culture systems.[3] These systems electronically monitor blood culture bottles every 8-10 minutes and detect algorithms based on assessments of changes associated with microbial growth. Currently, four systems are available: Becton Dickinson Microbiology systems, Sparks, Md. (BACTEC®), Organon Teknika, Durham, N.C. (BacT/Alert®), Trek Diagnostic systems Inc., Westlake, Ohio (ESP®), and bioMerieux, Inc.Hazelwood, Mo. (Vital).[1] There is no major differences in the performances of these systems and all are highly reliable. The primary difference lies in the method used to detect growth.[3] These systems can be programmed by the user to incubate specimen for various time periods; recommended range is from 5-7 days.
The aim of this study was to determine the spectrum of bacteria and yeast isolated from blood and sterile body fluids, their time to detection by BACTEC 9120®, and to analyze the data to decide which incubation protocol would practically be more suitable.
As recommended by the manufacturer, we instituted a six day protocol of incubation as there is lack of published data regarding the optimal length of incubation for the system from this part of the country.
MATERIALS AND METHODS
This study was conducted from July 2003 to December 2007. The automated continuously monitored blood culture system used in our laboratory is BACTEC 9120. Over the course of study, a total of 4,807 blood specimens from patients of suspected septicemia and 383 sterile body fluids suspected to be infected were received.
Blood and sterile body fluids were collected by aseptic procedure. A volume of 1-5 ml of blood specimen was inoculated into BACTEC Peds Plus/F and 10 ml into BACTEC Aerobic/F culture vials. Approximately, 5-10 ml of sterile body fluid was inoculated in BACTEC Aerobic/F culture vials. Anaerobic blood cultures were not done in our laboratory.
After inoculation the culture vials were loaded into BACTEC 9120 instrument as per the manufacturer's instructions. All the study culture vials were incubated for six days. Each culture vial contained enriched Soybean-Casein Digest broth with CO2 and resin (nonionic adsorbing resin and cationic exchange resin) to neutralize a wide variety of antibiotics. The culture vials also have a chemical sensor, which can detect increase in CO2 produced by growth of microorganisms. The sensor is monitored by the instrument every ten minutes for an increase in its fluorescence units, which is proportional to the amount of CO2 produced. A positive reading indicates the presumptive presence of viable microorganisms in the culture vial. Whenever there was a sign of microbial growth, the detection time was documented by BACTEC 9120 instrument software. Days were calculated as full 24-hour periods. For example, isolates detected between 72 and 96 hours were considered as detected on day four. Positive culture vials were sub-cultured on Blood agar and MacConkey agar plates. Smear from positive culture vials were stained by Gram's stain and a preliminary report was intimated to the physician. Growth obtained on culture plates were identified by standard biochemical techniques. Terminal subcultures of negative culture vials were not performed, as it has been shown to be unnecessary.[4,5]
RESULTS
A total of 5,190 specimens (4,807 blood and 383 sterile body fluids) were received for culture over a period of 54 months from July 2003 to December 2007.
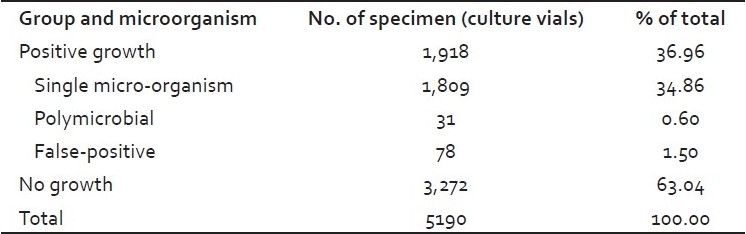
Over the course of study, 1,918 (36.96%) culture vials were flagged positive by BACTEC 9120. Microorganisms were isolated from 1,840 positive culture vials. Single organism was recovered from 1,809 (34.86%) culture vials and two organisms from 31 (0.60%) culture vials. A total of 78 positive vials (1.5%) were taken as false positive, as they showed no organism on Gram stain and no growth on subculture [Table 1].
Table 1.
Culture isolation results from blood and sterile body fluids by BACTEC 9120 system
Microorganisms recovered from positive culture vials and their time to detection is shown in Table 2. A total of 1,871 microorganisms were isolated, out of which 1,677 (32.31%) were clinically significant pathogens and 194 (3.73%) were contaminants. Isolation rate of Gram-positive and negative microorganisms were 62.55% (1,049/1,677) and 32.20% (540/1,677), respectively. Candida spp., were isolated from 5.24% (88/1677) culture vials. Members of Enterobacteriaceae family were the most frequent isolates among the gram-negative bacteria.
Table 2.
Time to detection of microorganisms isolated in BACTEC culture vials
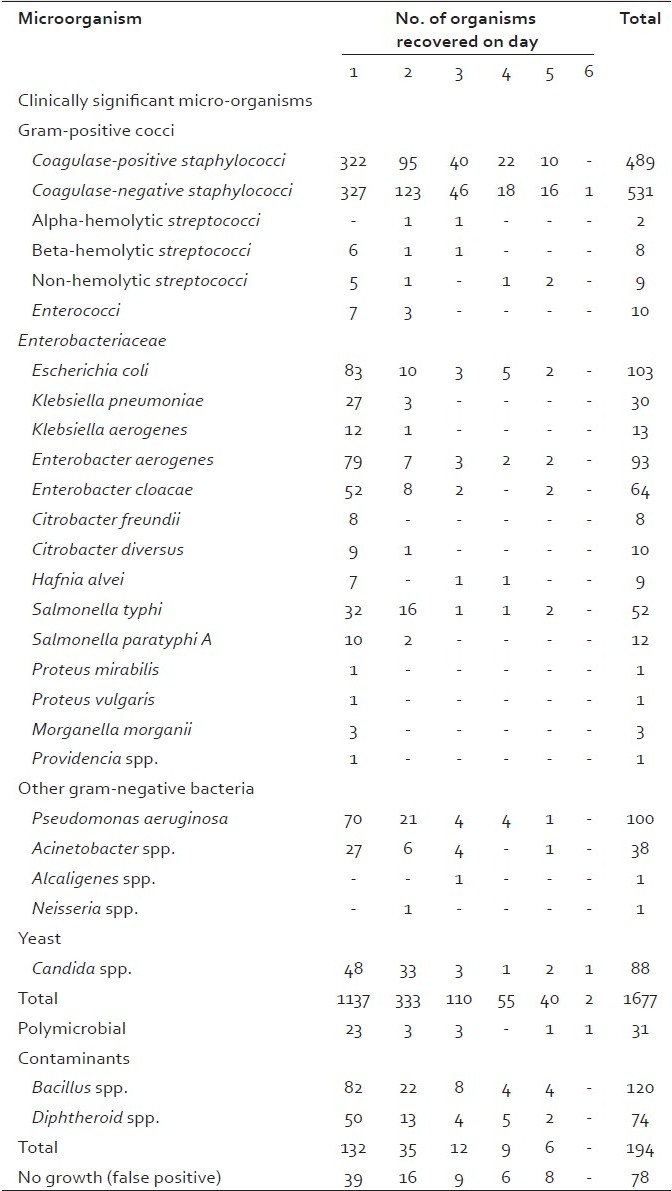
In respect to the time to positivity of clinically significant isolates: 1,137 (67.79%) cultures turned positive on day one; 333 (19.85%) additional isolates were recovered on day two; 110 (6.59%) on day three, 55 (3.28%) on day four, and 40 (2.38%) on day five. Only two isolates detected on day six were Candida and Coagulase negative Staphylococci (CNS).
In the first four days of incubation BACTEC 9120 detected, 1,637 (97.61%) of clinically significant isolates, and 188 (96.90%) of contaminants. After five days of incubation, 1,675 (99.88%) clinically significant isolates and 194 (100%) contaminants were detected.
The sensitivities on days four and five were 97.50% and 99.94%, respectively; the specificity of the culture was same (97.78%) on both days. The negative predictive value of blood culture on day four was 98.61% and day five 99.96%. Thus, a blood culture negative for growth on day five would have 99.96% probability of being negative after six days of incubation.
DISCUSSION
Six to seven days incubation period was generally recommended with the continuous monitoring automated blood culture instruments when they were first introduced. But with longer incubation period of seven days, there would be delay in reporting negative cultures and additional instruments would be required to accommodate the increased number of bottles, so we followed a six day incubation protocol in our institution.
In our study, we recovered 1,637 (97.61%) clinically significant bacterial and yeast isolates within the first four days of incubation and 1,675 (99.88%) by five days of incubation. Only two clinically significant isolates (Candida spp. and CNS) were recovered on sixth day of incubation. Similar studies have been performed with other automated blood culture systems to determine the incubation period required for these systems. Culture positivity reported after four days of incubation by Reisner, et al.[3] was 97.35% and Baka, et al.[6] 98.5%, whereas Kara, et al.,[7] reported a low culture positivity of 77%. Durmaz, et al., recovered most of the pathogens within five days.[2] Some investigators have reported 96-98% positive cultures within three days of incubation.[5,8–12]
In our study, clinically significant Gram-positive bacterial isolates were 62.55% and Gram-negative 32.20%, similar isolation rates were reported by most workers, but Durmaz, et al.,[2] reported more gram-negative isolates. In our study, Enterobacteriaceae were found to be the most frequent isolates among the Gram-negative bacteria, which correlates with other studies.
In most studies, CNS was the most frequently isolated Gram-positive bacteria, which was similar to our study (50.61% CNS).[2,6,7,13,14] Blood cultures yielding CNS, in critically ill febrile patient, is a diagnostic dilemma regarding whether it is a real pathogen or a contaminant. In our study, we considered CNS as pathogen, as these organisms are being increasingly recognized as important organism causing bloodstream infection, especially in hospital settings.
Out of the 88 Candida spp., we isolated 96.59% within four days of incubation, similar results were also observed by other investigators,[15–17] although six days of incubation was recommended by some.[3,5] The isolation rate of Candida spp. was different in all studies, as the isolation rate differs with respect to different clinics from which samples were obtained, for example, specimens obtained from intensive care unit would have a higher isolation rate.
Using BACTEC 9120 false positive rate reported by Durmaz, et al.,[2] was 0.3% and Smith., et al.,[17] 0.5%, while we recorded a slightly higher false positive rate of 1.5% as also reported by Cockerill III.,[16] (1.3%) and Nolte.[18] (2.2%).
In the literature, longer incubation period has been recommended for isolation of fastidious organisms. Durmaz, et al., found mean detection time for 20 isolates of Brucella melitensis to be 63.87 hour, which is significantly short and isolated 65% of the Brucella strains within 72 hours of incubation, but we did not isolate any fastidious organisms.[2]
The overall contamination rate of blood culture was 3.73%, which is slightly higher compared to other studies.[17,18] This may be due to the fact that in our study, nursing staff were responsible for obtaining blood for culture rather than specifically trained phlebotomists. If trained phlebotomists are employed in such settings, a reduced contamination could be achieved as observed by Weinbaum et al.[19]
Our study had two possible limitations. Firstly, no attempt was made to control the volume of specimen inoculated in each bottle as our aim was to find the results with ongoing routine daily practice in our institution. Secondly, previous antibiotic administration was not taken into consideration. BACTEC blood culture media used contained resin, which can neutralize a variety of antibiotics. Kara, et al.,[7] showed in their study that the time to detection of the pathogens from blood samples of patient receiving antibiotics did not differ from the preantibiotic samples.
In conclusion, our data support a five-day incubation protocol for recovery of routine bacteria and yeast with BACTEC 9120 culture system with overall sensitivity reduced by only 0.06% and negative predictive value of 99.96%. This is supported by similar observations by other investigators.[3–9]
This information on time to detection of positive cultures can be used in conjunction with clinical status of patient to assist clinicians in making important patient management decisions regarding the ongoing antibiotic therapy or duration of hospitalization. Studies in other institutions should be conducted to decide their own incubation protocol, which is more appropriate for them as these parameters will vary from institution to institution, in different geographical areas with different patient population.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Forbes BA, Sahm DF, Weissfeld AS, editors. Bailey and Scott's Diagnostic Microbiology. 11th ed. Missouri: Mosby, Inc; 2002. Bloodstream infections; pp. 865–83. [Google Scholar]
- 2.Durmaz G, Tercan US, Aydinli A, Kiremitci A, Kiraz N, Akgun Y. Optimum detection times for bacteria and yeast species with the BACTEC 9120 aerobic blood culture system: Evaluation for a 5-year period in a Turkish University Hospital. J Clin Microbiol. 2003;41:819–21. doi: 10.1128/JCM.41.2.819-821.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reisner BS, Woods GL. Times to detection of bacteria and yeast in 9240 Blood culture bottles. J Clin Microbiol. 1999;37:2024–6. doi: 10.1128/jcm.37.6.2024-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doern GV, Brueggemann AB, Dunne WM, Jenkins SG, Halstead DC, McLaughlin JC. Four-day incubation period for blood culture bottles processed with the Difco ESP blood culture system. J Clin Microbiol. 1997;35:1290–2. doi: 10.1128/jcm.35.5.1290-1292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy DJ, Hulbert BB, Migneault PC. Time to detection of positive BacT/Alert cultures and lack of need for routine subculture of 5- to 7- day negative cultures. J Clin Microbiol. 1992;30:2743–5. doi: 10.1128/jcm.30.10.2743-2745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baka S, Logginidis I, Efstratiou V, Panagiotopoulou E, Kaparos G, Gerolymatos K, et al. In Abstracts of the 17th European Congress of Clinical Microbiology and Infectious Diseases ICC. Munich, Germany: 2007. Time to positivity of BACTEC blood culture bottles, abstr. No. 1733_441. [Google Scholar]
- 7.Kara A, Kanra G, Cengiz AB, Apis M, Gür D. Pediatric blood culture: Time to positivity. Turk J Pediatr. 2004;46:251–5. [PubMed] [Google Scholar]
- 8.Bourbeau PP, Pohlman JK. Three days of incubation may be sufficient for routine blood cultures with BacT/Alert FAN blood culture bottles. J Clin Microbiol. 2001;39:2079–82. doi: 10.1128/JCM.39.6.2079-2082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourbeau PP, Foltzer M. Routine incubation of BacT/Alert FA and FN blood culture bottles for more than 3 days may not be necessary. J Clin Microbiol. 2005;43:2506–9. doi: 10.1128/JCM.43.5.2506-2509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan KL, Foster JA, Coffin SE. Outpatient pediatric blood cultures: Time to positivity. Pediatrics. 2000;106:251–5. doi: 10.1542/peds.106.2.251. [DOI] [PubMed] [Google Scholar]
- 11.Endimiani A, Tamborini A, Luzzaro F, Lombardi G, Toniolo A. Epidemiology of bloodstream infections and time to detection of positive blood culture: An evaluation of the automated BacT/Alert and BACTEC 9240 systems. New Microbiol. 2002;25:9–16. [PubMed] [Google Scholar]
- 12.Johnson AS, Touchie C, Haldane DJ, Forward KR. Four-day incubation for detection of bacteremia using BACTEC 9240. Diagn Microbiol Infect Dis. 2000;38:195–9. doi: 10.1016/s0732-8893(00)00199-1. [DOI] [PubMed] [Google Scholar]
- 13.Pauli I, Jr, Shekhawat P, Kehl S, Sasidharan P. Early detection of bacteremia in the neonatal intensive care unit using the new BACTEC system. J Perinatol. 1999;19:127–31. doi: 10.1038/sj.jp.7200124. [DOI] [PubMed] [Google Scholar]
- 14.Gray J, Brockwell M, Das I. Experience of changing between signal and BACTEC 9240 blood culture systems in a children's hospital. J Clin Pathol. 1998;51:302–5. doi: 10.1136/jcp.51.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurlat I, Stoll BJ, McGowan JE. Time to positivity for detection of bateremia in neonates. J Clin Microbiol. 1989;27:1068–71. doi: 10.1128/jcm.27.5.1068-1071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cockerill FR, 3rd, Reed GS, Hughes JG, Torgerson CA, Vetter EA, Harmsen WS, et al. Clinical comparison of BACTEC 9240 Plus Aerobic/F resin bottles and the Isolator Aerobic culture system for detection of blood stream infections. J Clin Microbiol. 1997;35:1469–72. doi: 10.1128/jcm.35.6.1469-1472.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JA, Bryce EA, Ngui-Yen JH, Roberts FJ. Comparison of BACTEC 9240 and BacT/Alert blood culture system in an adult hospital. J Clin Microbiol. 1995;33:1905–8. doi: 10.1128/jcm.33.7.1905-1908.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolte FS, Williams JM, Jerris RC, Morello JA, Leitch CD, Matushek S, et al. Multicenter clinical evaluation of a continuous monitoring blood culture system using fluorescent-sensor technology (BACTEC 9240) J Clin Microbiol. 1993;31:552–7. doi: 10.1128/jcm.31.3.552-557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SS. Doing it right the first time: Quality improvement and the contaminated blood culture. J Clin Microbiol. 1997;35:563–5. doi: 10.1128/jcm.35.3.563-565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


