Abstract
Background:
Liquid based cytology (LBC) has been reported to increase the sensitivity of cervical cytology, in comparison with conventional cytology Pap smear (CPS). Most LBC systems though require expensive automated devices.
Aims:
To evaluate the efficiency of a new and inexpensive LBC system - LPT cytology system.
Materials and Methods:
Cervical screening was performed on 31500 patients utilizing the LPT cytology system test from January 2006 to May 2007. A similar number (n = 31500) of CPS were performed from January 2004 to July 2006. All cytology positive patients underwent colposcopy and cervical biopsy with histopathology examination. Fifty cases positive both on cytology and biopsy were submitted to the high-risk human papillomavirus (HPV) L1 protein (HR-HPV L1) tests.
Results:
The LPT cytology system adequately preserved cellular structure for morphologic evaluation. There was a significant difference of the histology/cytology diagnosis concordant rate between that of the CPS and LPT systems [93.6 vs. 78.4%, p=0.001]. The significant higher concordant rate was also seen in the low grade intraepithelial lesion (LSIL) (95.4 vs. 78.9%, p=0.001) and in high grade intraepithelial lesion (HSIL) (90.2 vs. 76.1%, p=0.001) cytology diagnosis. There was no statistical difference in rate in atypical glandular cells (AGC) (61.5 vs. 60%) and glandular cell carcinoma (GCC) (83.3 vs. 80%). LPT resulted in a marked increased global detection over the CPS. Nuclear expression of HPV L1 was seen in 34% (17/50) of cases.
Conclusions:
LPT showed an increase in detection rate compared to CPS (P = 0.001) and a significantly higher histological versus cytological concordant referral rate.
Keywords: Cervical carcinoma, cytology screening, liquid-based cytology, Liqui-PREP™ cytology system
Introduction
For more than 35 years, screening for cervical cancer has used the conventional Papanicolaou (Pap) smear (CPS). Despite limited accuracy of the test,[1,2] the incidence of cervical cancer has fallen substantially.[3] Liquid-based cytology (LBC) involves rinsing the sampling tool into a vial of liquid to produce a suspension of cells, from which a layer of cells on a slide is prepared. Mucus, blood and debris often obscuring cells on a CPS are removed. Slides produced in this way can be read more quickly than CPS slides.[4,5] The main advantages of this LBC techniques are to reduce the number of inadequate smears and to provide enough cells for the detection of infectious agents such as human papillomavirus (HPV), Chlamydia trachomatis, N. gonorrhea, etc., through molecular biology techniques.[6–8] This remaining liquid sample can also be used for HPV DNA testing negating the need for another pelvic exam and providing additional clinical information in cervical screening triage management. LBC has been compared with CPS in many studies, most report increased sensitivity for detecting pathological changes and a higher proportion of slides that are adequate for assessment.[9–11] At the moment, the majority of these techniques are using expensive automated devices leading to a significant increase in the price of LBC.[12,13]
The UK National Institute for Clinical Excellence reported that LBC improved sensitivity slightly and that, in a pilot study in England of 178 000 slides,[14] percentages of unsatisfactory slides decreased from 9.1% to an average of 1.6% after conventional cytology was replaced with LBC. However, Sulik[15] and Saville[16] reported little difference in performance between LBC and CPS. Some countries, including the USA, Canada and the UK, are incorporating LBC into national screening program. Many countries have been reluctant to adopt LBC without definitive evidence of higher or at least equivalent accuracy. If equivalence can be shown, other characteristics such as greater reproducibility, lower cost or the capacity for HPV DNA testing could make LBC more desirable than CPS in screening program.
The quality of studies that assess tests can affect conclusions,[17] and low-quality studies consistently overestimate the accuracy of tests.[18] Furthermore, the accuracy of many tests is a trade-off between sensitivity and specificity. Thus, even if LBC does improve sensitivity (true-positive rate) for high-grade abnormalities, it could simultaneously increase the number of low-grade abnormalities (false positives), which are less likely to represent serious disease but might trigger clinical investigation. These false positives are undesirable in a screening program. Standards have been established for the design and reporting of medical tests.[19]
Although LBC is now recommended for cervical cancer screening, it requires expensive automated devices and has a high cost per test, especially in developing country such as China. Therefore, to evaluate a clinical utility of inexpensive LBC system – Liqui-PREP™ (LPT) became an urgent issue to fit Chinese women public health systems. For the purpose of improving the quality of the cervical sample analysed and diagnostic rate of primary cervical cancer, we evaluated the efficiency of a new and inexpensive LBC system – LPT Cytology System.
Materials and Methods
A total of 63 000 cervical samples were collected from 63 000 women for a retrospective analysis study beginning from January 2004 through May 2007 in a routine gynecologic setting at the Department of Gynecology (Jiangxi Province Women and Children Health Care Hospital). 31 500 patients were screened utilizing the LPT System (LGM International Inc., Melbourne, FL, USA) test in our outpatient center from January 2006 to May 2007. The same number (31 500) of patients were screened by CPS from January 2004 through July 2006. To avoid patient selection bias and to achieve a masking (blinding), every sample was randomized by either the LPT or the CPS procedure on patient's consent; none of the samples were processed by both techniques. All patients who resulted positive for cytology, underwent further examinations including colposcopy and cervical biopsy with histopathology studies.
The average age of the LPT group was 35.56 ± 12.8 years, and of the CPS group was 38.32 ± 10.78 years. For inclusion in the primary studies was the direct comparison of LBC as a replacement for CPS, with both techniques done by manual reading (not an automated screening system). Study design was based on independent (direct-to-vial) samples. Unlike independent studies, paired (split-sample) design might disadvantage LBC because residual cells are used to prepare the LBC slide.
Fifty cases, positive both on cytology and biopsy were submitted to the High-Risk HPV L1 protein (HR-HPV L1) test. One immunocytochemistry (ICC) HPV L1 kit was donated by Cytoimmun Diagnostics GmbH, (Pirmasens, Germany).
Specimens collection method: For LPT sample collection, excess cervical mucus was gently wiped off using a cotton swab; the cervical brush was then inserted into the endocervical canal, maintaining gentle pressure and rotated 5–7 times in a clockwise direction; the brush head was then separated and put into the preservative vial for further slides preparation steps.
The CPS sample were collected by spatula, sample were smeared on the slide and then stained by Papanicolaou (Pap) stain. The slide were examined microscopically for adequacy, if inadequate, the gynecologist re-sampled the cervix. The slides were routinely made at the bedside often with multiple sample collections until the pathologists was satisfied of the adequacy.
LPT cytology system method: The preservative vial, centrifuge tube and the slides were uniquely labeled. The specimen contained on the cervical brush in the preservative vial was mixed with the vortex (30 s), and then 4 mL cleaning solution was pipetted into the centrifuge tube. The entire content of the preservative vial was poured onto the cleaning solution in the centrifuge tube and then centrifuged at 1000 g for 10 min. Supernatant was decanted with the cell pellet remaining intact at the bottom of the centrifuge tube. Cellular base at 4-5 times volume of the epithelial cell pellet was added into the centrifuge tube and fully suspended on the vortex; 50 uL cellular base with cervical cells mixture solution was pipetted onto the slide in circular motion (15-17 mm). The prepared slides can be dried at room temperature or in an oven (indirect heat) at temperature up to 50°C. The slides were fixed by dipping in 95% denatured alcohol and stained with Pap stain. For both conventional cytology and LPT cytology diagnosis, the Bethesda system 2001 was used; within normal limits (WNL), atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H), atypical squamous cells of undetermined significance (ASC-US) and low and high-grade squamous intraepithelial lesions (LSIL and HSIL).[20] The histology diagnoses were classified in four groups: WNL, cervical intraepithelial neoplasia grade 1 (CIN 1), grade 2 (CIN 2) and grade 3 (CIN3).
Immunostaining for HPV L1 capsid protein: For immunostaining of L1 capsid protein, endogenous peroxidase activity was blocked for 30 min at room temperature and antigen retrieval was performed by boiling in 0.01 M citric acid phosphate buffer (pH 8.0) for 20 min. Nonspecific staining was eliminated by incubating the tissues with normal bovine serum for 30 min at room temperature. The specimens were then reacted with mouse monoclonal antibodies against HPV L1 capsid protein for 30 min at room temperature in a humidified chamber, slides then stained by AEC chromogen solution. The mouse monoclonal antibody recognizes the major L1 capsid proteins of high-risk HPV types 16, 18, 33, 35, 39, 45, 56 and 58. The specimens could then be observed after subjecting them to benzidine reaction and light counterstaining with Mayer's hematoxylin.
The results were analysed statistically by using the Fisher exact test and P-value. Immunostaining results were evaluated as positive when the smear nuclear staining was interpreted for L1 capsid protein.
Results
A panel of seven morphologic parameters was evaluated: cellularity, clean background, uniform distribution, artifacts, cellular overlapping, architectural and cellular morphologic change (includes cytoplasmic distortion, cytoplasmic vacuolization, cellular shrinkage/elongation, imprecise cytoplasmic borders and folded cytoplasmic borders). Cellularity was adequate in all LPT samples whereas it was the major cause of multiple re-samples in CPS. Clean background was observed in the vast majority of samples with all Liqui-PREP systems whereas it was second major cause of multiple re-samples in CPS. Uniform distribution was commonly found in CPS samples but not in LPT. Artifacts were present in most of CPS samples but rare in LPT. Inflammatory infiltrate and cellular overlapping was observed in all but two LPT samples. Architectural and cellular morphological changes were present in most of CPS samples but rare in samples of LPT [Table 1].
Table 1.
Comparison of morphologic parameters
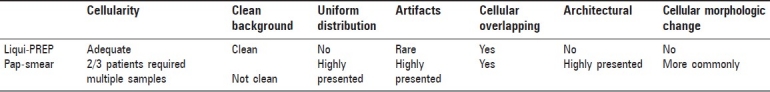
In order to evaluate the concordant rate of the cytology performed by LPT from our clinical samples, we compared the results of two methods of cytology diagnosis with the histological biopsy diagnosis. According to the histology diagnosis, there was a significant difference of the histology/cytology overall diagnosis concordant rate between the CPS and LPT systems (93.6 vs. 78.4%,P=0.001). The significant higher concordant rate was also seen in the LSIL (95.4 vs. 78.9%,P=0.001) and HSIL (90.2 vs. 76.1%,P=0.001) cytology diagnosis. A slightly higher rate was seen in ASC-H (93.9 vs. 80%) and SCC (94.7 vs. 89.5) cytology diagnosis. There was no statistical difference rate in atypical glandular cells (AGC) (61.5 vs. 60%) and glandular cell carcinoma(GCC) (83.3 vs. 80%) cytology diagnosis [Table 2].
Table 2.
Comparison of cytological and histological (biopsy) diagnosis concordant rate to Liqui-PREP with Pap-Smear
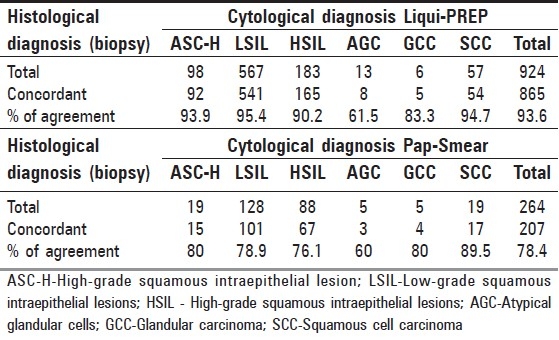
According to Bethesda 2001, LPT resulted in a remarkable increased global detection over the conventional CPS, which was ASCUS (6.8 vs. 2.3%), ASC-H (0.31 vs. 0.06%), AGC (0.41 vs. 0.06%), LSIL (1.80 vs. 0.41%), HSIL (0.58 vs. 0.28%) and SCC (0.18 vs. 0.06%). There was no statistical difference in the detection of AGC (0.04 vs. 0.02%) and GCC (0.02 vs. 0.02%) [Table 3].
Table 3.
Comparison of cytology results by TBS 2001 reportin
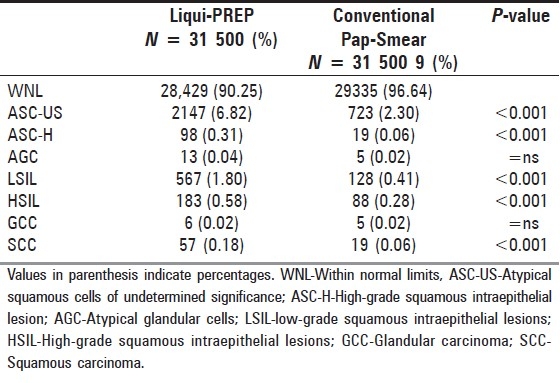
The false-positives of LPT-prepared slides consisted of six ASC-H (6/92; 6.5%), 26 LSIL (26/541; 4.8%), 18 HSIL (18/165;10.9%), one GCC (1/5; 20%), three SCC (3/54; 5.6%) with the total being 54 (54/857; 6.3%).
The false positives of CPS-prepared slides consisted of four ASC-H (4/15; 26.7%), 27 LSIL (27/101; 26.7%), 21 HSIL (21/67; 31.3%), one GCC (1/4; 25%), two SCC (2/17; 11.8%) with the total being 55 (55/207; 26.6%). LPT results significantly decreased the false-positive detection over the CPS [Table 4].
Table 4.
Comparison of cytological and histological (biopsy) diagnosis false-positive rate to Liqui-PREP with Pap-Smear
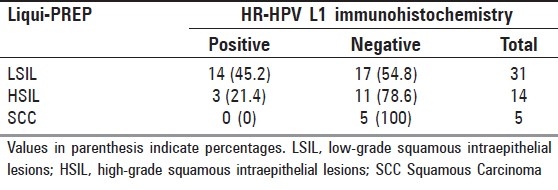
The objective of testing the LPT-prepared LBC slides was to evaluate the suitability for further immunocytochemistry (ICC) analysis on the slides and also to evaluate the HPV L1 ICC detection of an epitope of L1 protein common to High-Risk HPV by mouse monoclonal antibody T16VAHP (HPV types 16, 18, 33, 35, 39, 45, 56 and 58) from Cytoimmun Diagnostics GmbH, (Pirmasens, Germany). Fifty, both cytology and biopsy, positive slides were submitted for the High-Risk HPV L1 protein (HR-HPV L1) test. Positive reaction was characterized by a strong staining of the whole nucleus, surrounded by a cytoplasm with no background. Nuclear expression of HPV L1 has been seen in 34% (17/50) of cases, which included 14 (45.2%) of LSIL, three (21.4%) of HSIL and none of SCC. Approximately 66% (33/50) were L1 negative. Among them, 17 (54.8%) were LSIL, 17 (78.6%) were HSIL and 5 (100%) were SCC [Table 5].
Table 5.
HR-HPV L1 immunocytochemical detection
Discussion
The adequacy of the LBC has been already described in the literature of past 10 years. These authors demonstrated the superiority of the quality of LBC in comparison with those of CPS. [12–20] However, few authors addressed a manual LBC systems regarding the high cost of machine and its material supplies.[21,24] These authors concluded that manual LBC-LPT are cost-effective and provide an alternative method to the currently automated technique of LBC. As with these previous studies, our result further confirm them as the second generation of LBC, the advantage of LPT cytology system is not only relative to its environmental friendly solutions, low cost general laboratory equipments and simple 1-2-3 preparation procedures, but that the LPT test significantly increases the diagnostic rate and reduces the unsatisfactory rate of CPS test slides with its unique thin-layer cervical cells on the slides [Table 1].
This study clearly indicated that in spite of the different methodologies, the LPT systems adequately preserved cellular structure for morphologic evaluation and yields high-quality slides. However, we cannot draw any statistical conclusion on adequacy because of the difference in patient sampling done in the CPS compared with a single use cervical brush used in LPT. In order to assure adequate cell density, repeated sampling by spatula results in some 2/3 of patient being subjected to repeated scrapings for the CPS method to assure sample adequacy. However, re-sampling is not performed in the LPT system due to the use of cervical brushes, which improve overall sampling techniques and does not subject the patient to repeated sampling that might involve some discomfort.
In our study, in the LPT slides it was easier to find small numbers of abnormal cells due to clearer morphology of the individual cells and the removal of cellular debris, blood and mucus. Therefore, the rate of ASCUS was increased, which was welcomed by gynecologists concerned about receiving false-negative results. The number of WNL diagnoses was as expected for LBC system. It was; however, lower for LPT than for the CPS, because of higher global detection of LPT systems.
There was no statistical difference between cytological and histological diagnostic concordant rate in AGC (61.5 vs. 60%) and GCC (83.3 vs. 80%) cytology diagnosis [Tables 2 and 3]; this may be related to the physiological distribution on the LPT-prepared slides. Since the well-differentiated abnormal glandular cells or glandular tumor cells often have papillary pattern, the endophytic ones show a tubular and glandular pattern. Poorly differentiated glandular tumors are largely composed of solid sheets of tumor cells with only occasional evidence of gland formation even after LBC preparation.
The “quality” of the cervical sample analysed play the key role during cytology diagnosis procedures. The basic techniques of sampling and interpreting the CPS have remained essentially unchanged in the past 50 years. A large amount of cells were lost from the discarded collection device; interference from noncellular materials (blood, mucus, debris) and multilayer cells on the slide are the major cause of false-negative CPS results and false-positive results. However, LPT represents a significant advance over CPS for cervical cancer screening, and it has shown all its advantages including full cells components availability, highly improved slides screening quality, monolayer of cells with broad cells distribution, clarity of the cells structure and background. These advantages provide a much better cells screening environment for the reading cytologist and therefore increased the positive detection rate than the CPS method. In our study, LPT resulted in a significant decrease in the false-positive detection over the CPS, which was ASC-H (6.5 vs. 26.7%), LSIL (4.8 vs. 26.7%), HSIL (10.9 vs. 31.3%), SCC (5.6 vs. 11.8%) and the total false-positive rate (6.3 vs. 26.6%) [Table 4]. Slight decreased false-positive detection was also seen in the group of GCC (20 vs. 25%). Since the biopsy was only performed in the patients with positive cytology slides, we were not able to detect the true negative rate in this study.
The HPV L1 capsid protein is the main antigen used in producing the HPV vaccine. Expression of L1 is closely linked to HPV episomal and infectious stage, but is gradually lost during the viral integration into the host genome. Recently, this immunocytochemistry (ICC) test of HPV L1 nucleus expression became a cervical dysplasia predictive biomarker, and it was also helpful in risk assessment in HPV-infected women.[22,23] In our ICC study, nuclear expression of HPV L1 has been seen in 34% (17/50) of cases, which included 14 (45.2%) of LSIL, 3 (21.4%) of HSIL and 0% of SCC. Approximately 66% (33/50) were L1 negative. Among them, 17 (54.8%) were LSIL, 17 (78.6%) were HSIL and 5 (100%) were SCC [Table 5]. This result was similar to two other studies.[22,23]
Conclusions
Thus, from our study it becomes clear that the LPT system is the latest LBC technology and the basis for the next generation LBC product. When compared to similar LBC products, LPT advantages of simple working procedure, standard lab equipment, more representative cervical sampling cells, clear slide background and high diagnostic accuracy offers great promise and potential in laboratories looking to adopt LBC technology. The LPT system will significantly increase the number of positive cases detected when compared to CPS results, reduce the number of unsatisfactory specimens and offer higher accuracy at a lower cost than previous LBC system, making LPT an especially attractive approach in China. From our 63 000 cases studied, the LPT cytology system represents a significant improvement of referral ratios over CPS and also provides that the LPT cytology system is a reliable and a ‘gold standard’ LBC technique and a practical solution for many laboratories in China seeking access to LBC technology.
Acknowledgments
We wish to thank Qingze Wu, M.D., PhD. for his assistance in preparing English translation of our manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Walter SD, Irwig L, Glasziou PP. Meta-analysis of diagnostic tests with imperfect reference standards. J Clin Epidemiol. 1999;52:943–51. doi: 10.1016/s0895-4356(99)00086-4. [DOI] [PubMed] [Google Scholar]
- 2.Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995;141:680–9. doi: 10.1093/oxfordjournals.aje.a117485. [DOI] [PubMed] [Google Scholar]
- 3.Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: Epidemiology, prevention and the role of human papillomavirus infection. CMJ. 2001;164:1017–25. [PMC free article] [PubMed] [Google Scholar]
- 4.Ferenczy A, Robitaille J, Franco E, Arseneau J, Richart RM, Wright TC. Conventional cervical cytologic smears vs ThinPrep smears: A paired comparison study on cervical cytology. Acta Cytol. 1996;40:1136–42. doi: 10.1159/000333971. [DOI] [PubMed] [Google Scholar]
- 5.Laverty CR, Farnsworth A, Thurloe JK, Grieves A, Bowditch R. Evaluation of the CytoRich slide preparation process. Anal Quant Cytol Histol. 1997;19:239–45. [PubMed] [Google Scholar]
- 6.Sherman ME, Shiffman MH, Lorenz AT, Manos M, Scott DR, Kurman R, et al. Toward objective quality assurance in cervical cytopathology: Correlation of cytopathology diagnosis with detection of high risk human papillomavirus types. Am J Clin Pathol. 1994;102:182–7. doi: 10.1093/ajcp/102.2.182. [DOI] [PubMed] [Google Scholar]
- 7.Sherman ME, Mendoza M, Lee KR, Ashfaq R, Birdsong GG, Corkill ME, et al. Performance of liquid-based thin layer cervical cytology: Correlation with reference diagnose and human papillomavirus testing. Mod Pathol. 1998;1:837–43. [PubMed] [Google Scholar]
- 8.Darwin LH, Cullen AP, Arthur PM, Long CD, Smith KR, Girdner JL, et al. Comparison of Digene Hybrid Capture 2® and conventional culture for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in cervical specimens. J Clin Microbiol. 2002;40:641–4. doi: 10.1128/JCM.40.2.641-644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein SJ, Sanchez-Ramos L, Ndubisi B. Liquid-based cervical cytologic smear study and conventional Papanicolaou smears: A meta-analysis of prospective studies comparing cytologic diagnosis and sample adequacy. Am J Obstet Gynecol. 2001;185:308–17. doi: 10.1067/mob.2001.116736. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Gurley AM, Thurloe JK, Bowditch R, Laverty CR. Evaluation of the ThinPrep Pap test as an adjunct to the conventional Pap smear. Med J Aust. 1997;167:466–9. doi: 10.5694/j.1326-5377.1997.tb126672.x. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter AB, Davey DD. ThinPrep Pap Test: Performance and biopsy follow-up in a university hospital. Cancer. 1999;87:105–12. doi: 10.1002/(sici)1097-0142(19990625)87:3<105::aid-cncr2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytology abnormalities: A systematic review. Ann Intern Med. 2000;132:810–9. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 13.Obwegeser JH, Brack S. Does liquid-based technology really improve detection of cervical neoplasia? A prospective, randomized trial comparing the ThinPrep Pap Test with the conventional Pap Test, including follow-up of HSIL cases. Acta Cytol. 2001;45:709–14. doi: 10.1159/000328292. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Clinical Excellence. Guidance on the use of liquidbased cytology for cervical screening. Technology Appraisal Number 69. [last accessed on 2004 Sept 10]. Available from: http://www.nice.org.uk/pdf/TA69_LBC_review_FullGuidance.pdf .
- 15.Sulik SM, Kroeger K, Schultz JK, Brown JL, Becker LA, Grant WD. Are fluid-based cytologies superior to the conventional Papanicolaou test.A systematic review? J Fam Pract. 2001;50:1040–6. [PubMed] [Google Scholar]
- 16.Davey E, Barratt A, Irwig L, Chan SF, Macaskill P, Mannes P, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: A systematic review. Lancet. 2006;367:122–32. doi: 10.1016/S0140-6736(06)67961-0. [DOI] [PubMed] [Google Scholar]
- 17.Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: A systematic review. Ann Intern Med. 2004;140:189–202. doi: 10.7326/0003-4819-140-3-200402030-00010. [DOI] [PubMed] [Google Scholar]
- 18.Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999;282:1061–6. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]
- 19.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–4. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon D, Nayar R. 2nd ed. New York: Springer-Verlag; 2004. The Bethesda system for reporting cervical cytology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J, Jung EH, Kim C, Choi YH. Direct-to-vial comparison of a new liquid-based cytology system, liqui-PREP versus the conventional pap smear. Diagn Cytopathol. 2007;35:488–92. doi: 10.1002/dc.20665. [DOI] [PubMed] [Google Scholar]
- 22.Melsheimer P, Kaul S, Dobeck S, Bastert G. Immunocytochemical detection of HPV high-risk type L1 capsid proteins in LSIL and HSIL as compared with detection of HPV L1 DNA. Acta Cytol. 2003;47:124–8. doi: 10.1159/000326491. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T, Sano T, Kanuma T, Owada N, Sakurai S, Fukuda T, et al. Immunochemical analysis of HPV L1 capsid protein and p16 protein in liquid-based cytology samples from uterine cervical lesions. Cancer. 2008;114:83–8. doi: 10.1002/cncr.23366. [DOI] [PubMed] [Google Scholar]
- 24.Behtash N. Liqui-PREP™, a new liquid based cervical cytology in comparison with conventional pap smear 2008;3:627-30. Res J Biol Sci. 2008;3:627–30. [Google Scholar]


