Abstract
Background:
Cytological grading (CG) on aspirates of breast carcinoma is a useful tool for surgical maneuver and prognosis.
Aims:
An endeavor was made to use CG on aspirates of breast carcinoma using Robinson's grade and to correlate it with Bloom Richardsons’ histopathological grading.
Materials and Methods:
A total of 59 patients of breast carcinoma, aged 28-57 years, were aspirated and the smears were graded using Robinson's criteria. All the cases were correlated with Bloom Richardson's grade on histopathology in mastectomy specimens. Lymphadenopathy in 38 cases was aspirated and stained with Papanicolaou and Romanowsky stain.
Results:
Robinson's CG correlated well with Bloom Richardson's histopathological grading. For grade I and II tumors, there was substantial strength of agreement between cytology and histopathology, while in grade III, the concordance was nearly perfect. Lymph node metastasis was found in 27 of 32 axillary nodes, three of five cervical nodes and the only palpable supraclavicular node. Lymph node metastasis was observed in three with cytological grade II, 28 of grade III and none of grade I. All grade I had stage A, two of grade II had stage B, while all grade III had either stage B or stage C disease.
Conclusions:
Thus, CG of breast carcinoma correlates well with histopathological grading and may well be useful as a prognostic marker.
Keywords: Breast carcinoma, Bloom Richardson's grading, Robinson's grading, fine needle aspiration cytology
Introduction
Histological grading of breast carcinoma using the Nottingham method described by Elston and Ellis (also called Elston's modified Bloom and Richardson method) is a widely accepted tumor grading system and has been found to have good prognostic correlations.[1] In recent years, fine needle aspiration (FNA) cytology is increasingly being used for the pre-operative diagnosis of breast cancer. Attempts have been made to determine various prognostic parameters on FNA material for the management in a given case.[2,3] The National Cancer Institute (NCI), Bethesda, sponsored conference had also recommended that tumor grading on FNA material should be incorporated in FNA reports for prognostication.[4] It was also emphasised that the cytologic grading system on FNA specimens should correspond to the grading system used in the histologic material. Of the different cytologic grading methods corresponding to Elston's modified Bloom Richardson grading, the method described by Robinson et al.[5] was found to be useful in grading breast carcinoma in FNA.[6]
In this study, an endeavor was made to use cytological grading (CG) on aspirates of breast carcinomas and to correlate them with Elston's modified Bloom Richardsons’ grading and histopathological findings. Palpable lymph nodes were also assessed for metastatic deposits to decide regarding the level of clearance to be undertaken at surgery.
Materials and Methods
A total of 59 female patients of breast carcinoma in the age range of 28–57 years, seen between January 2006 and June 2007, were included in this study. FNA was performed using a 20ml disposable syringe and 23-gauge needle. No local anesthesia was given during the procedure. Papanicolaou (Pap) and May-Grünwald-Giemsa (MGG)-stained FNA smears and hematoxylin and eosin (H and E)-stained tissue sections (obtained from mastectomy specimens) were evaluated for cytological and histological grading, respectively. The cases were graded cytologically using Robinson's criterion on Pap-stained smear and histological grading was performed using Scarff Bloom Richardsons’ criteria. Only the cases having six or more epithelial cell clusters (ECCs) on cytology were subjected to grading as this is the minimum criteria for specimen adequacy.[7] In the Robinson's grading system for breast carcinoma, six different cytological parameters, namely cell dissociation, cell size, cell uniformity, nucleolus, nuclear margin and nuclear chromatin, were used to grade the tumors.[8] An ocular micrometer was used to study these morphological parameters. The cytological analysis was performed in a blinded manner by three different pathologists. The results were reproducible in the majority of cases. In cases of difference in opinion, the grade on which two of the pathologists agreed was recorded. A score of 1–3 was given to each of these parameters and the tumor was graded by adding up the scores. Cancers that scored in the range of 6–11 were graded I, scores of 12–14 were graded II and grade III was given for a score ranging from 15 to 18.[8] Histological grading was performed on formalin-fixed paraffin-embeded sections from mastectomy specimens using Elston's modified Bloom Richardsons’ scoring.[1] Mitotic figures were scored using a Leitz Ortholux microscope with a field diameter of 0.59 mm. Kappa (k) coefficient was calculated for each grade to compare the agreement.
Results
In the present study, of the total of 59 cases of invasive duct carcinomas, 55 could be diagnosed as such on cytology. Of the remaining four, three were diagnosed as atypical ductal hyperplasia and one as ductal carcinoma in situ. The cytological grade correlated well with that of histological grade. Table 1 shows the comparison of CG with that of histopathological grading along with the strength of agreement for each grade. Of the total of 59 cases, seven tumors were grade I on histopathology and five tumors were grade I on cytology. Four tumors showed concordance of assessment as grade I by both the methods [Figure 1]. Thus, three of seven cases diagnosed as grade I on histopathology and one of five cases diagnosed on cytology did not show concordance between the two methods. These three cases were diagnosed as atypical ductal hyperplasia on cytology. Again, of the 19 cases graded as grade II on histopathology, 14 showed concordance on cytology [Figure 2] while the other five did not. One of these cases was diagnosed as ductal carcinoma in situ on cytology. Four cases in grade II on cytology turned out to be grade III on histology. The K value for grade I and II tumors was 0.63 and 0.656, respectively, signifying substantial agreement between cytological and histopathological grading systems. Finally, 30 cases were diagnosed as grade III on both histopathology and cytology [Figure 3]. Three of 33 cases diagnosed on histopathology and two of 32 cases diagnosed on cytology as grade III did not show concordance. The K value of grade III tumors was 0.829, signifying an almost-perfect strength of agreement. Thus, grading systems showed stronger correlations in higher grades.
Table 1.
Comparison of cytological and histopathologic grades with statistical analysis
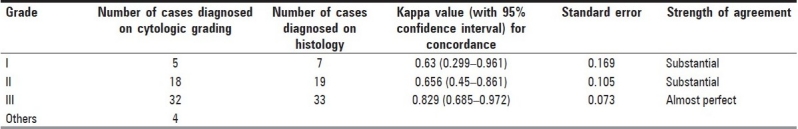
Figure 1.
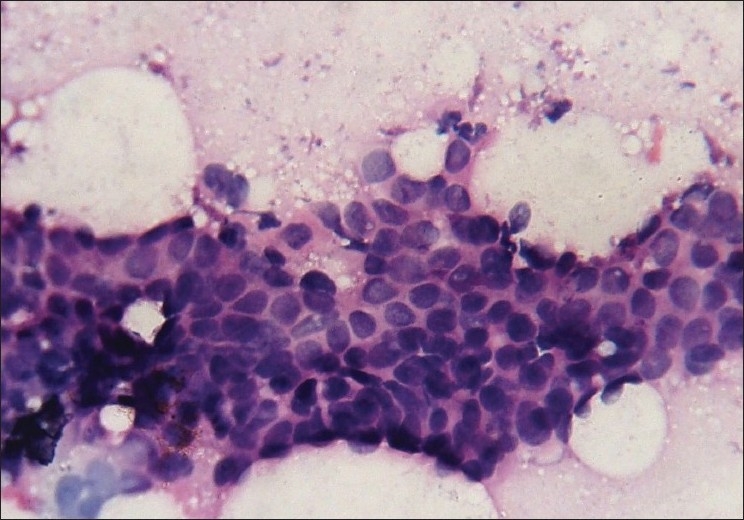
Photomicrograph of nuclear grade I duct carcinoma. The cells are in loosely cohesive clusters and the nuclei are enlarged, with mild pleomorphism, smooth nuclear margin and inconspicuous nucleoli (Pap, ×400)
Figure 2.
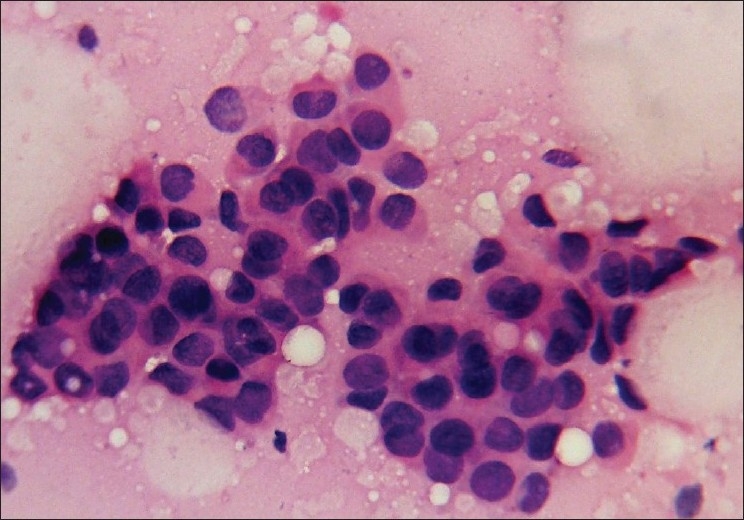
Photomicrograph showing nuclear grade II duct carcinoma. The cells are in loose clusters with many dispersed cells. Nuclei are three to four times the erythrocytes, with granular nuclear chromatin, smooth contour and single prominent nucleoli (Pap, ×400)
Figure 3.
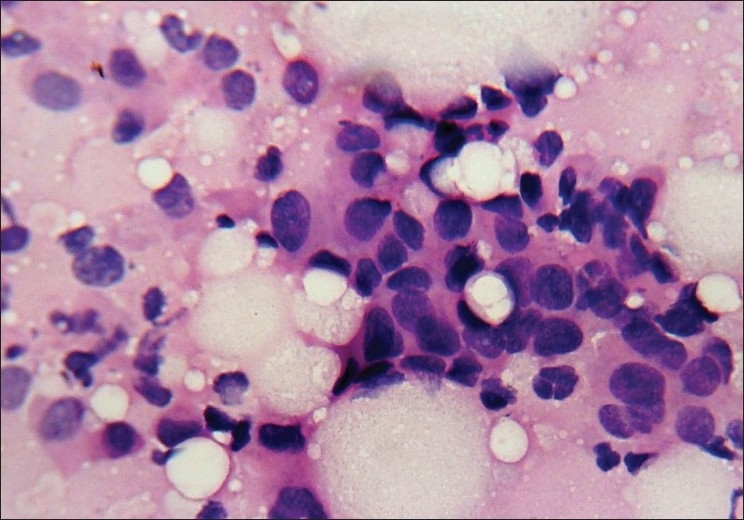
Photomicrograph showing duct carcinoma grade III. The cells are predominantly dispersed with coarsely granular chromatin, irregular nuclear margin and prominent nucleoli (Pap, ×400)
The lymph node metastasis was found in 27 of 32 axillary nodes, three of five cervical nodes and in the only examined supraclavicular node. Lymph node metastasis was observed in three with cytological grade II, 28 of grade III and none of grade I. All grade I had stage A, two of grade II had stage B while all grade III had stage B or C disease. Aspirated lymph nodes showed two false-positive results.
Reviewing the discordant cases (total 18) for cytological adequacy, we found that eight of the cases had less than eight ECC while none of the concordant cases (total 41) had ECC below this value. Those with low ECC either had a large amount of necrosis or dense fibrosis on histopathology.
Discussion
CG of breast carcinoma is feasible and reproducible. Its application, in combination with mammography, can provide important information on tumor type, grade and size before surgery so that the most appropriate medical regimen can be selected.[9] Nuclear grading on cytological specimens has been shown to correlate well with that of histological sections unlike other parameters like tubule formation and mitotic count.[10] CG on aspirates of breast carcinomas are useful tools for assessing prognosis. Correct diagnoses of benign and malignant breast lesions is important for further treatment protocols and also to guide the surgeons to plan the type of operation and level of lymph node resection beforehand. Robinson's cytological grade corresponded well with the established histological grades (Elston's modified Bloom Richardson method).[1] Sinha et al.,[11] in their study, could not reliably distinguish in situ carcinoma from invasive disease based on cytology. However, in 73% of the cases, the CG correlated with Bloom Richardsons’ grading on histopathology. Pandit and Parekh[6] graded 75 breast carcinomas by Robinson's method and showed 64% concordance with Bloom Richardsons’ grading on histopathology. They concluded that cytologic grade could be used to predict the histologic grade as a significant relationship exists between them.
In the present study, the overall concordance of Robinson's grading with histological grading was highest in stage III compared to stages I and II. The overall concordance was 69.5%, which is comparable with other published data.[6,11] Discordant CG were found in 18 cases. Of these, four were in grade I, nine in grade II and five in grade III. However, atypical ductal hyperplasia and CG I was often difficult to differentiate.
Aspirated lymph nodes showed two false-positive results. All grade I had stage A disease (LN 0) while stage B (axillary lymph nodes up to 3/1 internal mammary) and stage C (axillary lymph nodes ≥3) were mostly restricted to CG III. Aspiration of any palpable lymph node is also useful as level 3 clearance is carried out if the nodes contain metastatic tumor.
The optimal number of cell clusters necessary to define an adequate FNA of a palpable breast mass is not clear, although many authors have used six as the definitive number of ECCs.[7] Our study shows that a better level of concordance between grading systems may be achieved by increasing the cut-off value of ECC in breast aspirates to eight.
Pre-operative typing and grading of breast cancers permits the surgeon to plan the type of operation and the level of lymph node resection beforehand. Our findings suggest that Robinson's method of CG is a reasonably reliable method of grading breast carcinoma in FNA smears. Beside its role in early diagnosis, FNA also helps to assess the prognosis, providing information on tumor grading and the lymph node status. It allows the judicious use of neoadjuvant therapy, which can then be monitored by repeating the fine needle aspirate and also gauging the response clinically and mammographically. Wittekind and Schulte[12] have shown morphometry to be far superior with respect to accuracy and reproducibility of the results. They also suggested that nuclear perimeter can be used as an additional parameter not only for the FNA cytologic diagnosis of breast cancer but also for the estimation of patients’ prognosis.
Conclusions
Robinson's grading system of breast carcinoma is simple, quick and correlates well with histological grading and typing. Together with lymph node status, it may help determine the prognosis. CG may be helpful to decide level of resection.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer: I: The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 2.Masood S. Prognostic factors in breast cancer: Use of cytologic preparations. Diagn Cytopathol. 1995;13:388–95. doi: 10.1002/dc.2840130507. [DOI] [PubMed] [Google Scholar]
- 3.Bozzetti C, Nizzoli R, Naldi N, Guazzi A, Camisa R, Manotti L, et al. Nuclear grading and flow cytometric DNA pattern in fine-needle aspirates of primary breast cancer. Diagn Cytopathol. 1996;15:116–20. doi: 10.1002/(SICI)1097-0339(199608)15:2<116::AID-DC6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Abati A, Abele J, Bacus SS, Bedrossian C, Beerline D, Bibbo M, et al. The uniform approach to breast fine-needle aspiration biopsy. Diagn Cytopathol. 1997;16:295–311. doi: 10.1002/(sici)1097-0339(1997)16:4<295::aid-dc1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Robinson IA, McKee G, Kissin MW. Typing and grading of breast carcinoma on fine-needle aspiration: Is this clinically useful information? Diagn Cytopathol. 1995;13:260–5. doi: 10.1002/dc.2840130315. [DOI] [PubMed] [Google Scholar]
- 6.Pandit AA, Parekh HJ. Cytologic grading of breast carcinoma: Comparison of four grading systems. J Cytol. 2000;17:39–44. [Google Scholar]
- 7.Layfield LJ, Mooney EE, Glasgow B, Hirchowitz S, Coogan A. What constitutes an adequate smear in needle aspiration cytology of the breast? Cancer. 1997;81:16–21. doi: 10.1002/(sici)1097-0142(19970225)81:1<16::aid-cncr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Grace T, Mckee G. Prognostic features and research: In diagnostic cytopathology of breast. Hain Zakhour Cliv Well. 1999:205–14. [Google Scholar]
- 9.Robinson IA, McKee G, Nicholson A, D’Arcy J, Jackson PA, Cook MG, et al. Prognostic value of cytological grading of fine needle aspirates from breast carcinomas. Lancet. 1994;343:947–9. doi: 10.1016/s0140-6736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 10.Dabbs DJ, Silverman JF. Prognostic factors from the fine needle aspirate: Breast carcinoma nuclear grade. Diagn Cytopathol. 1994;10:203–4. doi: 10.1002/dc.2840100302. [DOI] [PubMed] [Google Scholar]
- 11.Sinha SK, Bandyopadhyay R, Mitra RB, Chatterjee U. FNAC of breast with reference to topography and nuclear grading in malignant lesions. J Cytol. 2002;19:187–92. [Google Scholar]
- 12.Wittekind C, Schulte E. Computerized morphometric image analysis of cytologic nuclear parameters in breast cancer. Anal Quant Cytol Histol. 1987;9:480–4. [PubMed] [Google Scholar]


