Abstract
Introduction:
The HIV epidemic has posed major, almost insurmountable, challenges to tuberculosis control efforts across the world. This study analyzes the prevalence and disease profile of HIV/AIDS coinfection in Vadodara, Gujarat, India.
Materials and Methods:
This study was conducted in the HIV Referral Clinic at Vadodara, India. Using convenience sampling method, 246 HIV-positive patients coinfected with tuberculosis were enrolled. A detailed history of every case was taken followed by a thorough physical examination. Baseline and follow up laboratory and radiological investigations were carried out as appropriately warranted.
Results:
Out of 500 HIV positive patients who presented to the clinic during the study period, 246 (49.2%) were coinfected with tuberculosis. Out of 246 coinfected cases, 35(14.2%) presented with demonstrable and documented tuberculosis whereas in 211(85.8%) cases, tuberculosis was extemporaneously detected by actively screening the patients. Sixty nine percent of patients were males, while 10.5% of cases were below fifteen years of age. The majority (68%) of patients had manifestations of extrapulmonary tuberculosis; but pulmonary tuberculosis, which is a more common presentation in HIV-negative cases, was present in only fifty five percent of this segment of the population. Abdominal tuberculosis was the most common site (74%) amongst extrapulmonary tuberculosis involvement, followed by clinically palpable lymph nodes (22%) and pleural effusion (17%).
Conclusion:
The prevalence of tuberculosis in HIV-positive patients in this study (49%) was substantially higher than that reported in previous studies. However, this could be attributed to a selection and/or a diagnosis bias. This study used abdominal ultrasound for the diagnosis of tuberculosis which might have obviously increased the prevalence. Moreover, these cases were not confirmed by biopsy or other definitive TB diagnostic methods.
Keywords: Coinfection, HIV, HIV/AIDS, India, TB, tuberculosis
INTRODUCTION
Concomitant Human Immunodeficiency Virus (HIV) infection and Tuberculosis (TB) is a lamentable medical phenomenon with dreadful social and economic impact across the globe, aptly described as the “accursed duet”. In India alone, 2.5 million people are currently infected with HIV, of whom 40% are also coinfected with TB.[1,2] The HIV epidemic has posed major and almost insurmountable challenges to TB control efforts across the world. Conjoined HIV and TB infection is also intricately and causally associated with malnutrition, unemployment, alcoholism, drug abuse, poverty, homelessness and illiteracy.[3] The two diseases represent a deadly combination, since they are more clinically devastating together than either disease presenting alone.[4,5]
The risk of eventually developing active TB from latent TB infection is about 10% per year in HIV-positive patients in contrast to 10% lifetime risk in HIV-negative patients. HIV-positive patients with TB are more likely to have extrapulmonary, atypical and unique clinical and radiological presentations. Moreover, TB is more difficult to diagnose and, therefore, progresses more rapidly in the HIV-positive population.[6,7] As a corollary, the presence of the TB agent itself accelerates HIV disease progression concurrently. Prompt diagnosis and appropriate treatment of TB will demonstrably and significantly abate HIV disease progression.[8]
Study objectives
To determine the prevalence of tuberculosis in both asymptomatic and symptomatic HIV-positive and AIDS patients.
To analyze the disease pattern and clinical scenario of TB in asymptomatic and symptomatic HIV-positive and AIDS patients.
MATERIALS AND METHODS
This study was conducted in the HIV Referral Clinic, Department of Skin and Venereal Diseases, Shri Sayajirao General Hospital and Medical College, Vadodara, Gujarat, India, from July 2005 to November 2007. Five hundred consecutive HIV-positive cases were screened for TB during the study period.
Case Definition: Active tuberculosis was defined as definitive disease, if the patient had a positive smear or culture, and as probable disease, if the patient had signs and symptoms suggestive of tuberculosis in addition to response to anti-tuberculosis treatment. Both definitive and probable cases were counted together “as diagnosed with tuberculosis” in this study.
Inclusion criteria
All HIV-positive cases diagnosed with active TB necessitating Anti-Tuberculosis Treatment (ATT) during the length of investigation were enrolled. These included:
HIV-positive cases already known to have TB.
HIV-positive cases screened for TB and recently detected as having TB.
HIV-positive cases not initially presenting with demonstrable TB who, subsequently, during the monitoring period, developed the disease.
Data collection
A detailed history of every case was obtained including past medical history, family history and treatment history of TB. Also considered in all cases were: date of HIV diagnosis, interval duration between HIV diagnosis and subsequent development of TB and initial indication for HIV testing. In addition, patients underwent rigorous physical examination to detect any opportunistic infection, the presence of lymphadenopathy and sexually transmitted diseases.
Baseline assessment
Hemoglobin, total lymphocyte count, differential lymphocyte count, erythrocyte sedimentation rate (ESR), urine (albumin, sugar and microscopic examination), Venereal Disease Research Laboratory test (VDRL), hepatitis B surface antigen (HBsAg) and sputum acid fast bacilli (AFB) were carried out in each case.
Fine needle aspiration (FNA) of enlarged and clinically palpable lymph nodes was executed and specimens were processed with hematoxylin and eosin (H and E), Giemsa and Ziehl-Neelsen (ZN) staining.
Chest radiography (PA view) and abdominal ultrasonography (USG) were performed in all cases in order to detect a focus of tuberculosis. The ultrasounds were specifically scrutinized for the presence of either retroperitoneal lymph nodes or splenic microabscesses , since they are clinically useful sentinel markers of tuberculosis.
In all cases with a normal chest x-ray and USG abdomen at baseline, the studies were repeated at six month intervals, or at more patient-specific intervals, whenever other signs and symptoms warranted it, in order to detect an early stage presentation of TB.
Lymphocyte enumeration was performed in patients able to afford the test.
Monitoring process
All patients receiving ATT were subjected to clinical and laboratory monitoring on a periodic basis. Liver function testing was performed every three months or whenever indicated by individual symptoms. Chest x-ray, USG abdomen and FNA of enlarged lymph nodes were repeated at the conclusion of ATT. In patients on Highly Active Antiretroviral Therapy (HAART), CD4 count was assessed every six months, or more frequently, if clinically indicated. Patients completing a course of ATT whose financial duress precluded efavirenz-based HAART were relegated to nevirapine-based HAART.
Data analysis
The data accrued on all HIV-TB coinfected patients was tabulated in Microsoft Office Excel software and analyzed using CDC Epi Info software version 3.4.1 (July 2007).
RESULTS
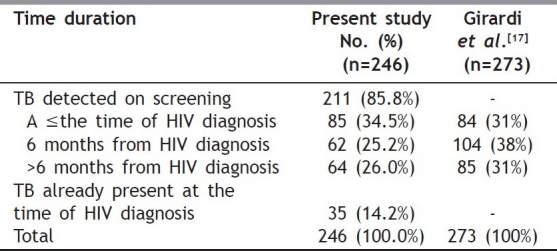
During the study period, of 500 HIV-positive patients who presented to the clinical site of investigation, , 246 (49.2%) were determined to be infected with TB per case definition outlined in the methods section. Out of 246 co infected cases, 35(14.2%) had TB at the time of presentation whereas in 211(85.8%), TB was extemporaneously detected by actively screening the patients [Table 1].
Table 1.
Time duration between HIV diagnosis and development of TB (n=246)
Epidemiological profile
Data accrued from coinfected patients during the study period revealed that participants had the following characteristics: 68.7% were males and 31.3% cases were females, 10.5% cases were of younger than 15 years of age, and of these 82.5% were between the ages of 15 to 50 years of age, representing the most socioeconomically productive segment of the population [Table 2]. Civil status within this group was predominantly married (77.6%). The most common mode of transmission was heterosexual in nature (65%), while, vertical HIV transmission was present in 24(9.8%) cases [Table 3].
Table 2.
Age-wise sex distribution of HIV-TB co infected cases (n=246)
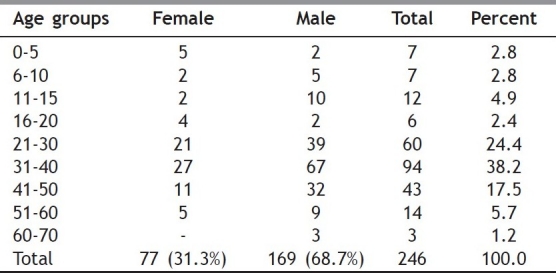
Table 3.
Mode of transmission of HIV in HIV-TB co infected cases (n=246)

Clinical profile
A past history of TB had already been documented in 14.2% of the patients. It can be safely assumed that these cases were more likely to have developed reactivation of TB as HIV-induced immunosupression eventually took its toll. In the majority of cases (59.7%), TB was diagnosed within six months after HIV diagnosis, suggestive of delayed HIV testing and advanced tuberculous disease. In 85.8% of cases, TB was detected by rigorously screening the patients at the onset of the study, or subsequently, during follow up visits as well as implementing radiographic and ultrasound studies, as described [Table 1]. Such a high diagnostic yield reflects the importance of actively screening HIV-positive patients for (early) diagnosis and treatment of TB. In 34.5% of these patients, TB was detected concomitantly at the time of HIV diagnosis. The most common indication for HIV testing was chronic illness in 42.7% cases, while 14.2% of the cases were tested for HIV only because the diagnosis of TB was already established.
Manifestation types
The majority of our study subjects manifested (68.3%) extrapulmonary TB. In contrast, pulmonary TB, a common presentation in HIV-negative cases, was present in only 54.8% of HIV-TB coinfected cases. Fifty seven patients (23.1%) presented with disseminated TB involving both pulmonary and extrapulmonary sites. In 94.8% of the patients, ESR was elevated and 57.4% of these had ESR values of over 100. Therefore, an HIV-positive patient with an abnormal ESR should always raise the index of suspicion for TB; in such cases, a thorough investigation for a possible focus of TB is warranted. Purified protein derivative (PPD) testing was reactive in only 12 out of 146 (8.2%) cases which may be attributed to HIV-induced immunosupression.
Utility of screening as a TB diagnostic tool
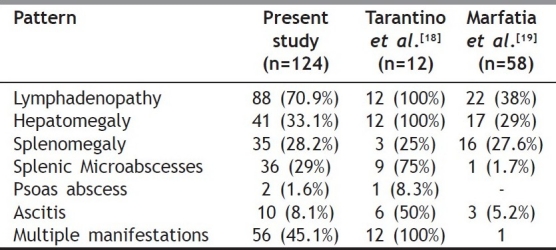
Chest x-ray and USG abdomen were demonstrated as possibly being the most useful diagnostic tools to detect TB. Chest x-ray detected pleural effusion and lesions suggestive of pulmonary TB in 151 (61.4%) of the cases. USG abdomen revealed lesions suggestive of abdominal TB in 124(42.2%) of the cases [Table 4] and diagnosis of probable TB was made if there was resolution of these lesions on repeat USG after a course of ATT. All the cases detected with abdominal tuberculosis on the basis of USG abdomen were either clinically asymptomatic (11.3%), or had occasional complaints like diarrhea, abdominal pain (12%) or other nonspecific complaints such as fever or weight loss (73.4%). Thus, all HIV cases should be aggressively screened for TB with the additional implementation of USG, irrespective of initial clinical symptoms.
Table 4.
Findings observed on USG abdomen suggestive of tuberculosis (n=124)
Out of 135 cases of pulmonary tuberculosis, sputum AFB was positive in only 2.4% of patients. Out of 73 cases with clinically palpable lymph nodes, FNA of lymph nodes, (along with H and E and ZN staining), detected TB lymphadenitis in 36(49.3%) cases. Cerebral spinal fluid (CSF) sampling detected TB meningitis in 11 (five per cent) cases. Moreover, non-healing perianal ulcer in three cases and epididymoorchitis in two cases were presumed to be coursing with TB on clinical grounds alone and diagnosis of probable TB was made after positive response to a therapeutic trial of ATT.
Patterns of pulmonary TB
Pulmonary consolidation was the most common finding observed in 72.6% of patients with pulmonary TB (98 out of 135). In contrast, to the fact that upper lobe lung consolidation is more common in HIV-negative patients, middle and lower lobe lung consolidation was the more common manifestation in coinfected patients, accounting for 42.5% of all cases. Hilar lymphadenopathy was a marker for tuberculosis in 14.9% cases. Additionally, fibrocavitary tuberculosis, which is a more common finding in HIV-negative patients, was present in only 6.6% of HIV positive patients.
Patterns of extrapulmonary TB
Abdominal TB (73.8%) was the most frequent site of involvement among extrapulmonary TB cases, followed by clinically palpable lymph nodes (21.5%) and pleural effusion (17.3%). Pericardial effusion was a marker for TB in two cases. Abdominal lymphadenopathy retroperitoneal, pre and periaortic, (retrocecal and splenic) was the most common finding observed in 70.9% cases of abdominal TB; therefore it should serve as a focus of attention for the interpreting radiologist in such studies. Retroperitoneal lymph nodes were the most common site of lymphadenopathy, found to be enlarged in 72.2% of all cases, whereas splenic microabscesses were present in only 29% of cases. Lymph node enlargement was present in 140 cases and the most common site was found to be within the abdomen in 49.7% of all cases followed by extraabdominal clinically palpable sites in 25.7%
Follow up of HIV-TB coinfected cases
Of 246 cases, only 174 were available for final evaluation; 92% of these had a successful curative treatment. Such a finding suggests that Directly Observed Therapy (DOT), in the manner administered to HIV-negative TB cases, is sufficient to successfully cure HIV-positive TB cases as well. Fourteen (8%) of 174 patients experienced treatment failure; 10 (71.4%) of them were cured only after extending the courses of ATT or changing the ATT regimen. Twelve of the fourteen treatment failures had a different focus of TB (on follow up) after the completion of their course of ATT. ATT was well tolerated in all except one patient who experienced exfoliative dermatitis due to Rifampin (RFM). Seventy two (29%) were lost to follow up and were not available for final evaluation.
DISCUSSION
Coinfection with TB and HIV has already been reported as one of the most significant global public health concerns.[9–11] However, the same concern applies in developing countries with poor resources. As demonstrated in this study, 246 (49.2%) HIV-positive subjects were coinfected with TB. Out of these 246 cases, 35 (14.2%) cases presented with previously diagnosed TB; in 211(85.8%) cases, TB was detected by active screening as demonstrated in our study patients. The prevalence of TB cases in HIV-positive subjects observed in this study was substantially higher than that reported in other studies; for example Noeske et al.reported that 256 out of 799 (only 32%) HIV-positive subjects were culture-positive for TB, of which 195 (76%) were AFB smear-positive.[12] It is plausible to suggest that the inclusion of abdominal ultrasound in the diagnostic maneuvers for TB may indeed increase the sensitivity (and decrease the rate of false negative results) of the currently available diagnostic methods. This notion was substantiated by the high level of previously unknown coinfection in developing countries with limited resources such as India, where exclusively implementing skin tests alone for screening may not have revealed such a high incidence of coinfection in HIV-positive patients. Therefore, the need for more aggressive screening, as delineated in our study, is made self-evident. However, one must be cautious in concluding that the higher prevalence found in this study represents a true increase, since it could be related to diagnosis bias. In this context, it is worth noting that cases detected by abdominal USG were presumed to be from probable TB based only on treatment response since no biopsy, culture or other definitive confirmatory tests were performed.
The diagnosis of abdominal tuberculosis is made more difficult in limited-resource countries where laparoscopy and colonoscopy are rarely available.[13,14] Therefore abdominal ultrasound can very well be deemed an important tool for such a diagnosis in TB patients coinfected with HIV. In this study, we make a compelling argument for the use of abdominal ultrasound as constituting an important tool to detect foci of tuberculosis in the abdominal cavity. It is worth noting that common presenting symptoms and signs of probable abdominal TB consist of abdominal pain (76.6%); ascites (59.6%); weight loss (53.2%) and fever (29.8%).[15] Since TB cases in this study were detected rather early due to the benefit of having a more sophisticated screening protocol, these patients were either entirely clinically asymptomatic or had occasional dysfunctional digestive complaints (such as diarrhea or abdominal pain) or had other nonspecific complaints such as fever and weight loss. In other studies the most common presenting abdominal features were ascites, periaortic lymphadenopathy (over one cm in size), and hepatomegaly.[13] In this study abdominal lymphadenopathy (i.e. retroperitoneal, pre and periaortic, retrocecal and splenic) was the most common finding observed in 70.9% cases of abdominal TB. Retroperitoneal lymph nodes were the most frequent site of lymph node enlargement (in 72.2% of cases), and splenic microabscesses were present in 29% cases. Another important finding of this and other studies relates to the probability of developing abdominal TB in HIV coinfected patients which seems to be independent of their CD4 cell count.
Therefore, we would confidently recommend routine screening for TB with the implementation of USG in addition to other routine tuberculosis tests like PPD, AFB sputum smears and cultures, etc., regardless of the absence of clinical symptoms in HIV-positive patients. TB is one of the most common opportunistic infections (OI) occurring in HIV-positive patients and can be easily discovered by simple investigations such as chest x-ray, USG abdomen and FNA, all of which are available in low-resource countries. Moreover, every HIV-positive patient should be screened for TB with the greater reach of the aforementioned studies, both during the initial visit and subsequent testing at specified intervals or extemporaneously if symptoms so warrant.
As indicated, our coinfected cases most likely developed active TB from the reactivation of TB, mainly due to immunosuppression expected in HIV-positive patients. In the majority of cases (59%), TB was diagnosed within six months of HIV diagnosis, suggestive of a delayed HIV diagnosis, most likely due to advanced tuberculous disease. Some of the patients could have developed Immune Reconstitution Inflammatory Syndrome (IRIS) which is quite common in HIV/TB coinfected patients started on HAART. In our population, the most common indication for HIV testing consisted of chronic illness in 42.7% of cases, while 14.2% of the patients were tested for HIV due to the presence of TB. This is consistent with the fact that a delayed diagnosis of HIV tends to occur in developing countries, which necessitates a more active surveillance strategy to detect cases in their early stages of evolution.
Most of the subjects (68.3%) in our study coursed with extrapulmonary TB. While pulmonary TB is a common presentation in HIV negative cases, it was present in only 54.8% of HIV-TB coinfected cases in our study. Disseminated TB involving both pulmonary and extrapulmonary sites was also frequent (23.1%) in our study population. ESR was elevated in most of the study cases, and therefore may buttress the assertion that a high ESR increases the index of suspicion for TB and therefore, a more intense search for a focus of TB should be pursued. Tuberculin skin testing (PPD) was reactive in only 8.2% of the cases which may be a correlate to HIV-induced immunosuppression. Therefore, such a test, albeit inexpensive may be of scant relevance in the diagnosis of TB in the late stages of HIV.
In the course of treating HIV/TB, coinfected patients in a setting of limited resources, interactions between ATT (rifampin) and HAART (nevirapine, protease inhibitors) should always be considered. Rifampin lowers nevirapine levels, thus impacting response to treatment. Hence, efavirenz or high dose of nevirapine (300mg twice daily) may be given as a substitute for efavirenz in any HAART regimen. Efavirenz can be supplanted with nevirapine after completion of ATT in order to make the regimen more economical. ATT given as DOT within the guidelines of the Revised National Tuberculosis Control Program (RNTCP) proved to be quite satisfactory in managing most of the HIV/TB coinfected cases.[9]
The impact and extent of the TB/HIV epidemic in the Indian region will depend on the future evolution of the HIV epidemic itself, as well as whether the efforts to control TB are effective and well orchestrated. Such an endeavor may very well require full implementation of DOT, as well as prevention of HIV infection, preventing the progression of latent TB infection to active disease and the facilitated provision of HIV/AIDS care and antiretroviral treatment on a long-term basis.
Finally, other specific strategies to consider would entail exploring the additional benefits of using Interferon gamma (IFN-γ) assays (based on M. tuberculosis specific peptides, ESAT6 and CFP10) to diagnose latent tuberculosis infection (LTBI) in HIV-infected patients. Additionally, it may be worth considering the role of lifelong prophylactic treatment with isoniazid to prevent TB reinfection.[16] Many quandaries are yet to be explored and new trajectories to be defined, all “grist for the mill” for further scientific queries and investigations.
ACKNOWLEDGMENTS
The authors would like to thank Fogarty International Center, AIDS International Training and Research program faculty for their valuable technical support (AITRP Grant # D43 TW006793). We would also like to thank Drs. Patricia Emmanuel and Sadaf Aslam for their valuable comments and suggestions.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Marfatia YS SA, Modi M. Overview of HIV/AIDS in India. Indian J Sex Transm Dis. 2007;28:1–5. [Google Scholar]
- 2.Sharma SK. Co-infection of human immunodeficiency virus (HIV) and tuberculosis: Indian perspective. Indian J Tuberc. 2004;51:5–16. [Google Scholar]
- 3.Tillekeratne LG, Kiwera RA, Chu HY, Kaale L, Morpeth SC, Ostermann J, Mtweve SP, Shao JF, Bartlett JA, Crump JA. Morbidity and mortality among a cohort of HIV-infected adults in a programme for community home-based care, in the Kilimanjaro Region of Tanzania (2003-2005) Ann Trop Med Parasitol. 2009;103:263–73. doi: 10.1179/136485909X398203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harries A MD, Graham S., MD . 2 ed. Geneva: World Health Organization; 2004. TB/HIV: TB/HIV: A clinical manual. WHO/HTM/TB/2004. [Google Scholar]
- 5.Sharma SK, Kadhiravan T, Banga A, Goyal T, Bhatia I, Saha PK. Spectrum of clinical disease in a series of 135 hospitalised HIV-infected patients from north India. BMC Infect Dis. 2004;4:52. doi: 10.1186/1471-2334-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD CG. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–33. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low SY EP. Human immunodeficiency virus testing in patients with newly-diagnosed tuberculosis in Singapore. Singapore Med J. 2009;50:479–81. [PubMed] [Google Scholar]
- 8.O’Donnell MR, Padayatchi N, Master I, Osburn G, Horsburgh CR. Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. Int J Tuberc Lung Dis. 2009;13:855–61. [PMC free article] [PubMed] [Google Scholar]
- 9.Arora VK. Trends of extra-pulmonary tuberculosis under revised national tuberculosis control programme: A study from South Delhi. Indian J Tuberc. 2006;53:77–83. [Google Scholar]
- 10.Yamada NN. The current and future situations of TB/HIV (co-infection of tuberculosis and HIV) in Japan. Kekkaku. 2009;84:203–11. [PubMed] [Google Scholar]
- 11.Sinkala E, Gray S, Zulu I, Mudenda V, Zimba L, Vermund SH, et al. Clinical and ultrasonographic features of abdominal tuberculosis in HIV positive adults in Zambia. BMC Infect Dis. 2009;9:44. doi: 10.1186/1471-2334-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noeske J, Dopico E, Torrea G, Wang H, Van Deun A. Two vs. three sputum samples for microscopic detection of tuberculosis in a high HIV prevalence population. Int J Tuberc Lung Dis. 2009;13:842–7. [PubMed] [Google Scholar]
- 13.9th Conference on Retroviruses and Opportunistic Infections. Seattle, Washington: 2002. N. B. Molecular Epidemiology of Tuberculosis (TB) in a High-Incidence Setting: New Insights into TB Dynamics and Transmission; p. 2002. [Google Scholar]
- 14.Akinkuolie AA, Agbakwuru EA, Egharevba PA, Adesunkanmi AR. Abdominal tuberculosis in a Nigerian teaching hospital. Afr J Med Med Sci. 2008;37:225–9. [PubMed] [Google Scholar]
- 15.Prasad R, Saini JK, Gupta R, Kannaujia RK, Sarin S, Suryakant , et al. A comparative study of clinic-radiological spectrum of tuberculosis among HIV seropositive and HIV seronegative patients. Indian J Chest Dis Allied Sci. 2004;46:99–103. [PubMed] [Google Scholar]
- 16.Paton NI, Ng YM, Chee CB, Persaud C, Jackson AA. Effects of tuberculosis and HIV infection on whole-body protein metabolism during feeding, measured by the [15N] glycine method1-3. Am J Clin Nutr. 2003;78:319–25. doi: 10.1093/ajcn/78.2.319. [DOI] [PubMed] [Google Scholar]
- 17.Girardi E AG, Vanacore P, et al. 9th Conference on Retroviruses and Opportunistic Infections, Seattle, Washington; Seattle, Washington: 2002. Who Gets HIV-Associated Tuberculosis in the Era of HAART. [Google Scholar]
- 18.Tarantino L, Giorgio A, de Stefano G, Farella N, Perrotta A, Esposito F. Disseminated mycobacterial infection in AIDS patients: abdominal US features and value of fine-needle aspiration biopsy of lymph nodes and spleen. Abdom Imaging. 2003;28:602–8. doi: 10.1007/s00261-003-0035-9. [DOI] [PubMed] [Google Scholar]
- 19.Marfatia YS, Makrandi S, Mistry D. Role of abdominal sonography (USG) in the management of HIV positive cases. Indian J Sex Transm Dis. 2006;27:92–4. [Google Scholar]


