Abstract
The clinical effect of imiquimod stems from cytokine-induced activation of the immune system. A randomized study was conducted to study the efficacy and safety of daily applications of 5% imiquimod cream in female patients with external genital warts and molluscum contagiosum (MC). The clearance rate of lesions was 75% in genital MC patients and 50% in patients with genital warts. Erythema was the commonest adverse reaction seen in 24% patients with the use of 5% imiquimod. Other side effects were excoriation seen in 16% patients, erosions in 10% patients, excoriation in 6% patients and pain was seen in 4% patients.
Keywords: Application, genital, imiquimod, immune, molluscum contagiosum, topical, treatment, warts
INTRODUCTION
The exact mechanism of action none in which imiquimod and its analogs activate the immune system is not yet known. Nevertheless, it is known that imiquimod activates immune cells through the toll-like receptor 7none (TLR7), commonly involved in pathogen recognition, on the cell surface.[1] Cells activated by imiquimod via TLR-7 secrete cytokinesnone [primarily interferon-α (IFN-α)none , interleukin-6none (IL-6) and tumor necrosis factor-αnone (TNF-α)].[2] There is evidence that imiquimod, when applied to skin, can lead to the activation of Langerhans cellsnone , which subsequently migrates to local lymph nodes to activate the adaptive immune system.[3] Other cell types activated by imiquimod include natural killer cells, macrophages and B-lymphocytes.[4] New research has shown that imiquimod's antiproliferative effect is totally independent of immune system activation or function. Imiquimod exerts its effect by increasing levels of the opioid growth factor receptornone (OGFr). Blocking OGFr function with siRNA technology resulted in loss of any antiproliferative effect of imiquimod.[5] Topical application of imiquimod elevates the production of cytokines, including the principal cytokine for antiviral activity, interferon-α. This is the initial event in an immunological cascade resulting in the stimulation of the innate immune response as well as the cell-mediated pathway of acquired immunity. This immune modification mediates the indirect antiviral, antiproliferative and antitumor activity of imiquimod in vivo. These properties highlight the potential of imiquimod as an effective treatment for genital warts and MC. The present study was carried out to study the efficacy and safety of 5% imiquimod for the treatment of genital molluscum contagiosum and genital warts in female patients.
MATERIALS AND METHODS
A randomized study was conducted to study and assess the efficacy and safety of daily applications of 5% imiquimod cream in female patients with external genital warts and MC. All patients gave written informed consent before enrolment. The protocol and consent forms were reviewed and approved by appropriate institutional review boards at each institution. Patients were randomized by study center to receive daily applications of 5% imiquimod cream for a maximum of 16 weeks. Before bedtime, patients rubbed the study cream into clean, dry, lesional skin until it disappeared and washed the area with soap and water 8 ± 2 h after application.
Inclusion criteria
All female patients were seronegative for the human immunodeficiency virus.
Female patients who agreed to use effective birth-control measures.
Exclusion criteria
Female patients with pre-study pap smear showing a high-grade squamous intraepithelial lesion were excluded from our study.
Genital wart and MC therapy in the 4 weeks prior to treatment initiation.
Pregnant or lactating females.
To investigate wart and MC recurrence, patients who had complete clearance of their baseline lesions at any time during the treatment period stopped treatment and entered a 12-week treatment-free follow-up period. Patients were evaluated weekly for the first 4 weeks and every 2 weeks thereafter for the remainder of the 16-week treatment period as well as during the 12-week follow-up period. A detailed wart and MC assessment, including photographs, measurements, counts and location, were completed before the study treatment was initiated and at each evaluation visit. New lesions that developed during the treatment period were treated with study medication and were followed separately from the baseline target warts and MC. Both the patient and study personnel assessed local skin reactions at the treatment sites using a four-point scale from 0 (no reaction) to 3 (severe). Clinical laboratory tests (e.g., hematology, blood chemistry and urinalysis) were performed before treatment, at week 8 and after treatment. The skin biopsy of the patients was done wherever the diagnosis was in doubt. All patients were asked about adverse experiences at each evaluation visit and these were recorded on a Performa. The primary efficacy was assessed by the proportion of patients in each treatment group who had complete clearance of their baseline warts and MC during the treatment period.
RESULTS
The results were tabulated and the data was analyzed statistically.
DISCUSSION
The present prospective randomized, trial evaluated the efficacy and safety of daily patient-applied imiquimod for up to 16 weeks for the treatment of external genital warts and MC. The commonest (48%) age group of patients was between 30 and 40 years, followed by 24% between 20 and 30 years, followed by 20% between 40 and 50 years, followed by 6% between 50 and 60 years and 2% between 10 and 20 years [Table 1]. The genital MC was seen in 72% patients and genital warts were seen in 28% patients [Table 2]. In our study, 34% patients had 2–5 lesions, 50% patients had 5–10 lesions, 14% patients had 10-15 lesions and 2% patients had more than 15 lesions [Table 3]. Baseline warts cleared from 7 out of 14 (50%) [Figure 1a–c] and genital MC lesions cleared from 27 of 36 (75%) [Figure 2a–c][Table 4]. In a study conducted by Metkar et al.,[6] complete clearance of molluscum contagiosum was seen in 44% patients with imiquimod. The commonest side effect observed in their study was erythema and crusting. In our study, there were no systemic reactions, although local skin reactions (generally of mild or moderate severity) were common. Local reactions caused two patients to discontinue treatment. The most frequently reported local skin reactions were erythema seen in 24% patients, excoriation in 16% patients, itching was seen in 12% patients, erosions were seen in 10% patients, excoriation was seen in 6% patients and pain was seen in 4% patients [Table 5]. In a study by Diamantis et al.,[7] imiquimod 5% cream showed an acceptable safety profile; local inflammatory reactions were the most frequent adverse effects, with local erythema being the most common. The local reactions noted with imiquimod are most likely due to cytokine-induced inflammation and/or an immune response. Local reactions, which were predominantly of mild and moderate severity, required the discontinuation of treatment in only two patients. In preclinical trials, the cumulative rate of irritation produced by 5% imiquimod cream was less than that produced by Vaseline Intensive Care Lotion, indicating that imiquimod is not inherently irritating to normal skin. In this clinical study, there was a significant correlation between the clearance of warts and MC and erythema. An inflammatory response was not required to achieve clearance of the warts; however, patients with such a response were more likely to have wart clearance.
Table 1.
Age distribution of patients
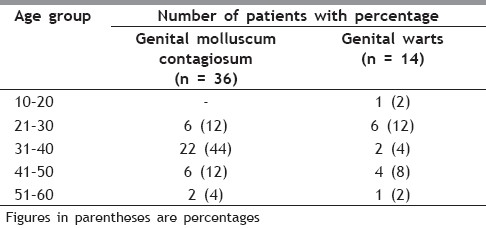
Table 2.
Types of lesions

Table 3.
Number of lesions
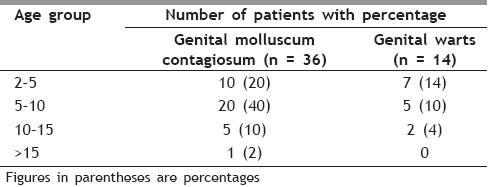
Figure 1a.
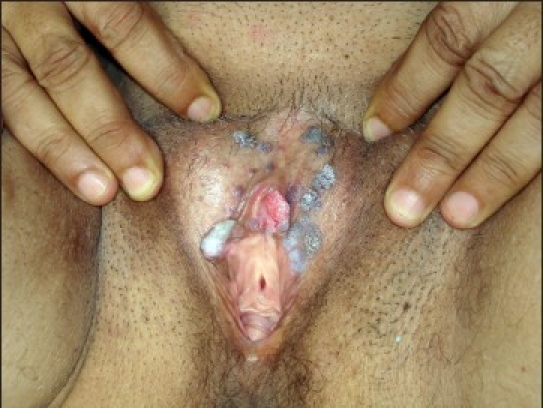
Genital warts in a 15 year old female
Figure 1c.
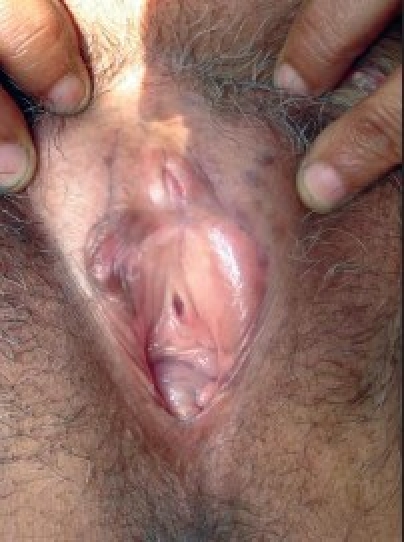
Genital warts after 12 weeks of treatment
Figure 2a.
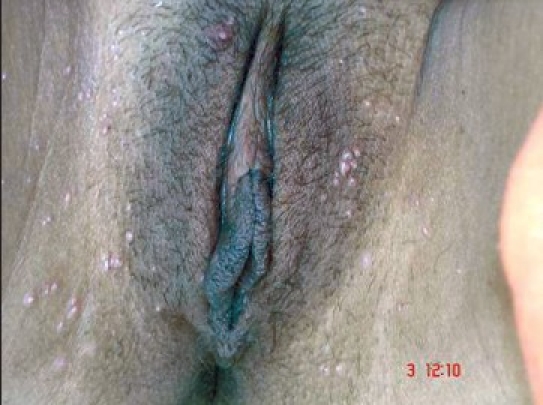
Genital MC in a 35 year old female
Figure 2c.
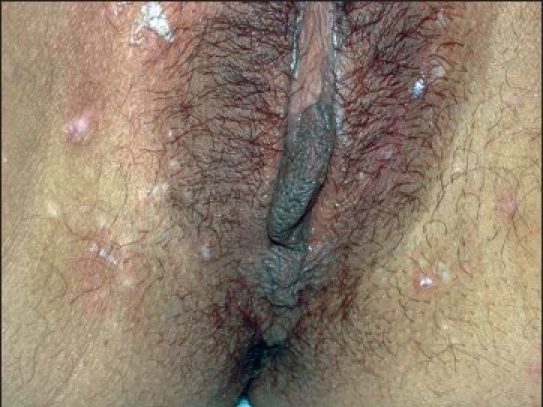
Genital MC after 10 weeks of treatment
Table 4.
Clearance of lesions

Table 5.
Adverse reactions to 5% Imiquimod
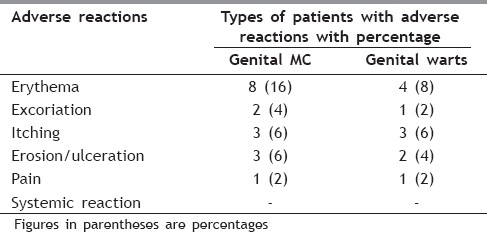
Figure 1b.
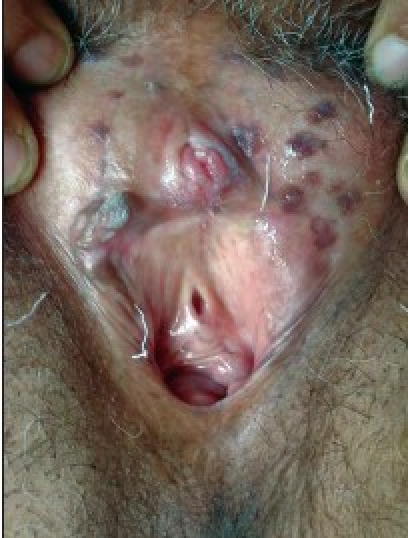
Genital warts after 6 weeks of treatment
Figure 2b.
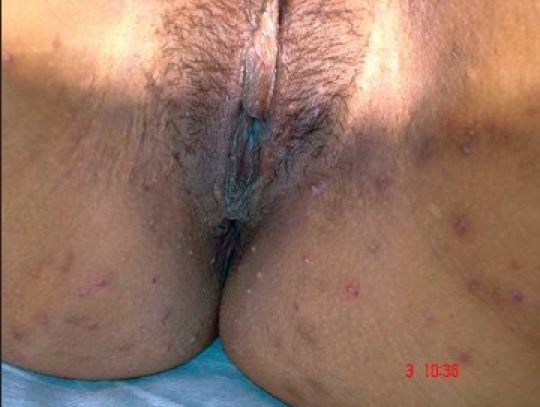
Genital MC after 4 weeks of treatment
Patient applied 5% imiquimod cream is effective for the treatment of external genital warts and genital MC and has a favorable safety profile.
Genital human papillomavirus (HPV) infection is a common sexually transmitted disease.[8] The most frequent clinical manifestation of infection is genital warts. An estimated 30 to 50% of sexually active adults in the United States are infected with HPV, although only 1% may have visible genital warts.[9] The natural history of genital warts in humans appears to be that they may persist, progress or regress. Regression of genital warts is thought to be due to an immune response.[10] Most treatments currently available for genital warts (e.g., cryotherapy, excision, electrosurgery, coagulation, laser surgery, trichloroacetic acid treatment and podophyllin resin treatment) are directed at the lesions and not at the etiologic agent, HPV. Interferons (IFNs) and patient-applied podofilox (podophyllotoxin) are currently available therapies that have been evaluated in well-controlled trials.[11]
Imiquimod is a new agent, an immune-response modifier, that has been demonstrated to have potent in vivo antiviral and antitumor effects in animal models.[12] Imiquimod, 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine (also known as R-837 and S-26308), is a nonnucleoside heterocyclic amine that belongs to a class of products known as immune-response modifiers. In vivo studies have demonstrated that imiquimod is a potent inducer of alpha IFN (IFN-α), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) and that it has adjuvant properties greater than those of Freund's complete adjuvant. In animal models, imiquimod has been demonstrated to have potent antiviral and antitumor effects.[13] The current study was designed to evaluate the efficacy and safety of imiquimod for the treatment of external genital warts and MC.
Non-specific inflammation and dermatitis can occur during use of Imiquimod for genital warts and molluscum.[14,15] This often occurs where the skin is traumatized from scratching, or between skin folds. Massive blisters, bloody dry eschar, pain and discomfort often follows the use of Imiquimod for skin cancers and precancerous growths.[16,17] Many individuals with extensive actinic keratosis cannot tolerate the resulting reaction either. Fortunately, after completion of the therapy, the skin often heals with barely any scarring. Imiquimod can also cause subclinical lesions to become visible. This unmasking effect is felt to be of clinical benefit as lesions that may have otherwise have been missed are being treated. Other side effects include headache, back pain, muscle aches, tiredness, flu-like symptoms, swollen lymph nodes, diarrhea and fungal infections.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Zagon IS, Donahue RN, Rogosnitzky M, McLaughlin PJ. Imiquimod upregulates the opioid growth factor receptor to inhibit cell proliferation independent of immune function. Exp Biol Med. 2008;233:968–79. doi: 10.3181/0802-RM-58. [DOI] [PubMed] [Google Scholar]
- 2.Miller RL, Gerster JF, Owens ML, Slade HB, Tomai MA. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol. 1999;21:1–14. doi: 10.1016/s0192-0561(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 3.Bilu D, Sauder DN. Imiquimod: modes of action. Br J Dermatol. 2003;149:5–8. doi: 10.1046/j.0366-077x.2003.05628.x. [DOI] [PubMed] [Google Scholar]
- 4.Kono T, Kondo S, Pastore S, Shivji GM, Tomai MA, McKenzie RC, et al. Effects of a novel topical immunomodulator, imiquimod, on keratinocyte cytokine gene expression. Lymphokine Cytokine Res. 1994;13:71–6. [PubMed] [Google Scholar]
- 5.Reiter MJ, Testerman TL, Miller RL, Weeks CE, Tomai MA. Cytokine induction in mice by the immunomodulator imiquimod. J Leukoc Biol. 1994;55:234–40. doi: 10.1002/jlb.55.2.234. [DOI] [PubMed] [Google Scholar]
- 6.Metkar A, Pande S, Khopkar U. An open, nonrandomized, comparative study of imiquimod 5% cream versus 10% potassium hydroxide solution in the treatment of molluscum contagiosum. Indian J Dermatol Venereol Leprol. 2008;74:614–8. doi: 10.4103/0378-6323.45104. [DOI] [PubMed] [Google Scholar]
- 7.Diamantis ML, Bartlett BL, Tyring SK. Safety, efficacy & recurrence rates of imiquimod cream 5% for treatment of anogenital warts. Skin Therapy Lett. 2009;14:1–5. [PubMed] [Google Scholar]
- 8.Stone KM. Human papillomavirus infection and genital warts: update on epidemiology and treatment. Clin Infect Dis. 1995;20:91–7. doi: 10.1093/clinids/20.supplement_1.s91. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 10.Heaton CL. Clinical manifestations and modern management of condylomata acuminata: a dermatologic perspective. Am J Obstet Gynecol. 1995;172:1344–50. doi: 10.1016/0002-9378(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 11.Jablonska S, Majewski S. Immunology of genital papillomavirus infections. In: Gross G, Jablonska S, Pfister H, Stegner HE, editors. Genital papillomavirus infection. Berlin Germany: Springer-Verlag; 1990. pp. 263–81. [Google Scholar]
- 12.Garland SM. Imiquimod. Curr Opin Infect Dis. 2003;16:85–9. doi: 10.1097/00001432-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Garland SM, Sellors JW, Wikstrom A, Petersen CS, Aranda C, Aractingi S, et al. Imiquimod 5% cream is a safe and effective self-applied treatment for anogenital warts--results of an open-label, multicentre Phase IIIB trial. Int J STD AIDS. 2001;12:722–9. doi: 10.1258/0956462011924218. [DOI] [PubMed] [Google Scholar]
- 14.Benson E. Imiquimod: potential risk of an immunostimulant. Australas J Dermatol. 2004;45:123–4. doi: 10.1111/j.1440-0960.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- 15.Beutner KR, Tyring SK, Trofatter KF, Jr, Douglas JM, Jr, Spruance S, Owens ML, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42:789–94. doi: 10.1128/aac.42.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards L, Ferenczy A, Eron L, Baker D, Owens ML, Fox TL, et al. Self-administered topical 5% imiquimod cream for external anogenital warts.HPV Study Group. Human PapillomaVirus. Arch Dermatol. 1998;134:25–30. doi: 10.1001/archderm.134.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Gilson RJ, Shupack JL, Friedman-Kien AE, Conant MA, Weber JN, Nayagam AT, et al. A randomized, controlled, safety study using imiquimod for the topical treatment of anogenital warts in HIV-infected patients.Imiquimod Study Group. AIDS. 1999;13:2397–404. doi: 10.1097/00002030-199912030-00011. [DOI] [PubMed] [Google Scholar]
- 18.Perry CM, Lamb HM. Topical imiquimod: a review of its use in genital warts. Drugs. 1999;58:375–90. doi: 10.2165/00003495-199958020-00017. [DOI] [PubMed] [Google Scholar]
- 19.Dahl MV. Imiquimod: An immune response modifier. J Am Acad Derm. 2000;43:1–5. doi: 10.1067/mjd.2000.107809. [DOI] [PubMed] [Google Scholar]
- 20.Edwards L. Imiquimod in clinical practice. J Am Acad Dermatol. 2000;43:12–7. doi: 10.1067/mjd.2000.107806. [DOI] [PubMed] [Google Scholar]
- 21.Edwards L, Ferenczy A, Eron L, Baker D, Owens ML, Fox TL, et al. Self-administered topical 5% imiquimod cream for external anogenital warts.HPV Study Group. Human Papilloma Virus. Arch Dermatol. 1998;134:25–30. doi: 10.1001/archderm.134.1.25. [DOI] [PubMed] [Google Scholar]
- 22.Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis. 2003;30:124–8. doi: 10.1097/00007435-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Genital warts. Cutaneous warts and molluscum contagiosum. Br J Dermatol. 2003;149:15–9. doi: 10.1046/j.0366-077x.2003.05623.x. [DOI] [PubMed] [Google Scholar]
- 24.Bilu D, Sauder DN. Imiquimod: modes of action. Br J Dermatol. 2003;149:5–8. doi: 10.1046/j.0366-077x.2003.05628.x. [DOI] [PubMed] [Google Scholar]
- 25.Barton JC. Angioedema associated with imiquimod. J Am Acad Dermatol. 2004;51:477–8. doi: 10.1016/j.jaad.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Bharti AC, Shukla S, Mahata S, Hedau S, Das BC. Anti-human papillomavirus therapeutics: facts & future. Indian J Med Res. 2009;130:296–310. [PubMed] [Google Scholar]


