Abstract
The in vitro immune responses to mycobacterial antigens have been linked to the H-2 loci in mice. We evaluated in vitro and in vivo immune responses during early Mycobacterium tuberculosis (M.tb) pulmonary infection of C57BL/6 (H-2b), C57BL/6 (H-2k), CBA/J (H-2k), and C3H/HeJ (H-2k) mice to determine H-2k-dependent and -independent effects. H-2k-dependent effects included delayed and diminished Ag85-specific Th1 cell priming, a reduced frequency of Ag85-specific IFN-γ producing cells, reduced IFN-γ protein in vivo, and increased M.tb lung burden as demonstrated by C57BL/6 H-2k mice vs C57BL/6 mice. H-2k-independent factors controlled the amount of Ag85-specific IFN-γ produced by each cell, T cell numbers, granuloma size, and lymphocytic infiltrates in the lungs. Overall, these results suggest that an H-2k-dependent suboptimal generation of Ag85-specific cells impairs control of early M.tb growth in the lungs. H-2k-independent factors influence the potency of IFN-γ producing cells and on immune cell trafficking during pulmonary M.tb infection.
Keywords: tuberculosis, H-2 haplotype, MHC haplotype, antigen 85, interferon-gamma, mouse
1. Introduction
Tuberculosis (TB) caused by infection with Mycobacterium tuberculosis (M.tb) remains an important global health threat, infecting approximately 2 billion people worldwide [1] and causing TB disease in approximately 8–9 million individuals annually [2]. Many immunogenetic factors are associated with host immune responses and susceptibility to M.tb in mice [3–7], including the major histocompatibility (MHC) haplotype (known as the H-2 haplotype in mice) [8–14]. A direct relationship between H-2k and increased susceptibility to M.tb has been established, whereby the H-2k alleles reduced survival of BALB/c H-2k congenic mice following aerosol infection [9] and impaired the control of M.tb growth in the lungs following intraperitoneal infection [15]. The H-2k haplotype also reduced survival of B10.BR (H-2k) compared to C57BL/10J (H-2b) mice following M.tb aerosol infection [16]. The route or dose of exposure may be important, as H-2k mice that received a high dose, intravenous M.tb infection were not more susceptible to M.tb [10]. It is also apparent, however, that the H-2k genes do not always contribute to M.tb susceptibility, as C3H/HeSnJ H-2b congenic mice (normally H-2k haplotype) survived an aerosol infection equally well as controls [16].
A genetic predisposition [17] with H-2b-linked control [18] of abundant in vitro IFN-γ production has been demonstrated using spleen cells from M. bovis BCG sensitized mice. Using spleen cells from M.tb infected mice, it has been further elucidated that abundant in vitro IFN-γ production by H-2b haplotype cells is T cell dependent, demonstrable in response to complex mixtures of M.tb antigens and to Ag85 [16]. Questions that we address, some of which have been posed by others [17], are whether the H-2 dependent variability of in vitro IFN-γ production reflects differences in the frequency of responding cells, differences in the amount of IFN-γ per cell, or other host factors. We also address whether this in vitro phenomenon corresponds to in vivo IFN-γ protein levels, and whether there is an association between IFN-γ levels and control of M.tb growth during early infection. The immune responses of H-2 mismatched (C57BL/6 and C57BL/6 H-2K mice) and H-2k matched (C57BL/6 H-2k, CBA/J and C3H/HeJ) mouse strains were used to identify H-2k-dependent and -independent contributions. We focused on lung cells, because the in vitro spleen cell responses may not always reflect lung IFN-γ immune responses in M.tb infected mice [19, 20]. Finally, Ag85 was chosen as a model antigen because this antigen is expressed by M.tb bacilli during early pulmonary infection [21] and host recognition of Ag85 may be important in establishing protective immunity. Furthermore, the lung responses to this antigen have not been investigated in detail in H-2k mice.
Lung cell cultures from H-2k mice produced significantly less Ag85-specific IFN-γ, as has been shown from splenocyte responses to Ag85 [16]. This reflected a reduced frequency of Ag85-responsive cells in H-2k mice. In CBA/J mice each responding cell produced low levels of IFN-γ, showing an H-2k-independence. Low IFN-γ responses in vitro indeed corresponded to in vivo levels, and furthermore, were associated with impaired control of M.tb growth. Both H-2k-dependent and -independent factors contributed to T cells accumulations, granuloma numbers, and lymphocytic cuffs in the lungs of M.tb infected mice during early infection.
Finally, in all mouse strains the pattern of antigen-specific IFN-γ produced from blood cultures mirrored that from lung cells, indicating that immune responses from blood samples can reflect immunity in the lungs, a concept which we have previously advocated [22]. Therefore, blood samples may accurately reflect immunologic responses to M.tb infection of humans.
2. Materials and Methods
2.1 Mice
Specific-pathogen-free eight-week-old female C57BL/6, B6.AK-H2k/FlaEgJ (C57BL/6 H-2k congenic), CBA/J, and C3H/HeJ mice (The Jackson Laboratory, Bar Harbor, ME) were maintained in ventilated cages within Biosafety Level-3 facilities and provided with sterile food and water ad libitum. All protocols were approved by The Ohio State University’s Institutional Laboratory Animal Care and Use Committee.
2.2 M.tb stocks
M.tb Erdman (ATCC #35801) was obtained from American Type Culture Collection (Manassas, VA.). Stocks were grown in Proskauer-Beck liquid medium containing 0.05% Tween 80 to mid-log phase and frozen in 1ml aliquots at −80°C.
2.3 M.tb infection and M.tb burden
Each mouse was infected with 37 ± 32 (SD) CFU of M.tb Erdman using an Inhalation Exposure System (Glas-col, Terre Haute, IN) [23]. Initial M.tb dose (day 0 of infection) and M.tb burden were determined by plating serial dilutions of lung homogenates onto OADC-supplemented 7H11 agar. M.tb CFUs were counted after 3 weeks at 37°C.
2.4 Lung cell isolation
Mice were euthanized by CO2 on day 21 of M.tb infection. Following perfusion through heart with 10ml of PBS containing 50U/ml of heparin (Sigma; St. Louis, MO), the lungs from individual mice were placed in Dulbecco’s modification of Eagle’s medium (DMEM; 500ml) (Mediatech; Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (Atlas Biologicals; Fort Collins, CO), 1% HEPES buffer (Sigma), 10ml of 100X non-essential amino acid solution (Sigma), 5ml of penicillin/streptomycin solution (50,000U penicillin, 50mg streptomycin; Sigma) and 0.14% mercaptoethanol (complete DMEM). The lung lobes were diced with sterile razor blades and digested for 30 minutes at 37°C with 4ml of complete DMEM containing collagenase XI (0.7mg/ml; Sigma) and bovine pancreatic DNase (30 μg/ml; Sigma). 6ml of complete DMEM was then added to dilute enzymatic activity and the lung pieces were pressed through sterile 70 μm nylon mesh screens (BD Biosciences; San Jose, CA) to obtain single cell suspensions. Residual red blood cells were lysed using 2ml of lysis buffer (0.15 M NH4Cl, 1mM KHCO3) for 3 minutes at room temperature followed by washing with complete DMEM. Viable cells were counted using Trypan blue exclusion and re-suspended at working concentrations in complete DMEM.
2.5 Blood collection
Mice were euthanized by CO2 on day 21 of M.tb infection. Immediately following euthanasia, 200–300 μl of blood per mouse were collected by cardiac puncture and placed into 20U of heparin (Sigma) diluted in 12 μl of sterile PBS. Heparinized whole blood was then diluted 1:10 in complete DMEM [22].
2.6 Mediastinal lymph node isolation
Mice were euthanized by CO2 on day 14, 21, and 28 of M.tb infection. The lung-draining mediastinal lymph modes from individual mice were isolated, passed through 70 μm cell strainers to create a single cell suspension, red blood cells lysed as above, and single cell suspensions washed in complete DMEM. Viable cells were counted using Trypan blue exclusion and re-suspended at working concentrations in complete DMEM.
2.7 Lung, blood, and lymph node ELISAs
2×105 lung cells (100 μl), 1:10 diluted blood (100 μl), or 2×105 lymph node cells (100 μl) from individual mice were cultured in duplicate or triplicate in 96-well sterile tissue culture plates (BD Falcon Microtest™) with 100 μl of antigens to achieve final concentrations of ovalbumin (10 μg/ml; Sigma), M.tb CFP (10 μg/ml), M.tb ESAT-6 (5 μg/ml), M.tb Ag85 (5 μg/ml), concanavalin-A (10 μg/ml; Sigma), or purified anti-CD3 and anti-CD28 (1 μg/ml and 0.1 μg/ml (BD)). Purified M.tb antigens were titrated in 1:1 serial dilutions from 20 μg/ml to 0.02 μg/ml, and the maximal responses were achieved with 5–10 μg/ml (not shown). Anti-CD3/anti-CD28 concentrations were also titrated in 1:1 serial dilutions from 1 μg/ml and 0.1 μg/ml to 0.001 μg/ml and 0.0001 μg/ml, and the maximal responses were achieved at or above 0.5 μg/ml (anti-CD3) and 0.05 μg/ml (anti-CD28) (not shown). Lymph node cultures were supplemented with congenic adherent spleen cells as haplotype-matched antigen presenting cells, following 1-hour adherence to plastic at 37°C and multiple washes to remove non-adherent cells. Cultures were incubated for 72 hours at 37°C and frozen at −80°C prior to analysis.
IFN-γ was quantified using antibody pairs (BD Biosciences), except from CBA/J and C3H/HeJ Ag85-stimulated blood cultures where antibody pairs from eBioscience (Ready-Set-Go IFN-γ ELISPOT kit) were necessary to increase assay sensitivity. Briefly, high-binding 96-well flat bottom plates (BD Falcon, Fisher Scientific) were coated overnight with anti-IFN-γ and blocked with complete DMEM for 2 hours at room temperature. Neat and diluted (1:20 and 1:50 in PBS) samples and recombinant murine IFN-γ standards (BD Biosciences) were incubated overnight at 4°C, followed by detection with a secondary biotinylated anti-IFN-γ overnight at 4°C. After a 30 minute room temperature conjugation with streptavidin horseradish peroxidase (Invitrogen, Carlsbad, CA), ELISAs were developed with 3,3′, 5,5′-tetramethylbenzidine substrate (DAKO, Carpinteria, CA) and stopped with 0.18M H2SO4. Positive samples were quantified by optical density at 450nm wavelength absorbance with 570nm correction, compared to the linear standard curve (ng/ml) and corrected for dilution factors. The amount of antigen-specific IFN-γ was calculated by subtracting the IFN-γ produced in the presence of ovalbumin. Lung cells cultured with ovalbumin produced averages of 7.64 ng/ml (C57BL/6), 2.32 ng/ml (C57BL/6 H-2k), 1.03 ng/ml (CBA/J), and 0.25 ng/ml (C3H/HeJ). Strain-dependent differences were not detected in blood cultures from ovalbumin control wells (average 0.42 ng/ml).
In some experiments, TNF in cell culture supernatants was measured by ELISA (eBioscience TNF ELISA kit) as per the kit instructions. In some experiments, IL-2 was measured in cell culture supernatants by ELISA using antibody pairs from BD Biosciences and a recombinant murine IL-2 standard (Peprotech), following the protocol outlined above for IFN-γ.
2.8 Lung IFN-γ ELISPOTs
Lung cells from individual mice were serially diluted in complete DMEM starting at 1×105 cells per well and cultured with ovalbumin, M.tb CFP, ESAT-6 and Ag85 (concentrations as described above), in pre-coated and blocked ELISPOT plates (Millipore, Billerica, MA). As a positive control, lung cells were cultured with purified anti-CD3 (BD Biosciences) plus anti-CD28 (BD Biosciences) at final concentrations of 1 μg/ml and 0.1 μg/ml, respectively. Samples were incubated for 24–36 hours at 37°C. IFN-γ spot forming units (SFU) were detected and developed as per the manufacturer’s instructions (eBioscience, Ready-Set-Go Mouse IFN-γ ELISpot kit). Spots were counted and analyzed in the linear response range using an Immunospot Analyzer (C.T.L., Cleveland, OH). The numbers of antigen-specific IFN-γ SFU were determined by subtracting the number of SFU in the presence of ovalbumin, which were on average 16 (C57BL/6), 27 (C57BL/6 H-2k), 9 (CBA/J) and 28 (C3H/HeJ) SFU per 2 × 105 lung cells.
2.9 Flow Cytometry
The following antibodies and isotype controls were purchased from BD Biosciences: Fc Block™ (clone 2.462), PerCP-Cy5.5 anti-CD3ε (145-2C11), APC-Cy7 anti-CD4 (GK1.5), PE-Cy7 anti-CD8 (53–6.7), APC anti-CD11b (MI/70), PE anti-CD11c (HL3), FITC anti-I-A/I-E (2G9) and FITC rat IgG2aκ; PE-Cy7 anti-IFN-γ (XMG1.2) and PE-Cy7 rat IgG1κ; PE anti-IL-2 (JEH6-5H4) and PE rat IgG2b. PerCP-Cy5.5 anti-TNF (MP6-XT22) and rat IgG1κ were purchased from BioLegend. 1–5×106 lung cells or lymph node cells per mouse were fixed in FACS buffer for 30 minutes at 4°C and incubated with 0.31 μg of Fc Block™ for 10 minutes at room temperature to minimize non-specific antibody binding. Samples were then incubated with 0.31 μg of fluorescent antibodies in the dark for 20 minutes at 4°C followed by 3–5 washes in FACS buffer. Lymphocytes were identified according to characteristic forward and side scatter profiles and at least 10,000 events were counted within the lymphocyte gate using a BD LSRII flow cytometer. Results were analyzed with FACSDiva software (BD Biosciences).
2.11 Lymph node T cell intracellular flow cytometry
In some experiments, 1×106 lymph node cells were cultured for 3 hours in the presence of 1 μg/ml purified anti-CD3 (BD Biosciences) and 0.1 μg/ml anti-CD28 (BD Biosciences) plus monensin. Intracellular cytokine production was detected in CD4 T cells as per instructions (Cytofix/Cytoperm kit, BD Biosciences).
2.12 Histology and histomorphometry
Lung lobes from individual mice were inflated with 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin or for acid-fast bacilli by a modified Ziehl-Neelson stain. Slides were digitally scanned using an Aperio ScanScope (Vista, CA) and multiple sections per group were analyzed by a board-certified veterinary anatomic pathologist (GB). Morphometric analysis was performed using Aperio ImageScope v8.2.5. Immune cell aggregates within the lung were first identified at 4X magnification. Granulomas were defined as focal aggregates of mixed inflammatory cells (neutrophils, macrophages, lymphocytes). Lymphocytic cuffs were defined as partial or complete concentric layers of one or more lymphocytes within perivascular or peribronchial/peribronchiolar adventitia. The number of granulomas, average granuloma size, number of lymphocytic cuffs, average lymphocytic cuff size, and total (area of granulomas plus area of lymphocytic cuffs) immune cell infiltrates were calculated.
2.13 Statistics
Statistical analyses were performed using Prism 4 software (GraphPad Software, San Diego, CA). Multigroup comparisons were analyzed by one-way ANOVA with Tukey’s post-test. Pairwise comparisons used the Student’s t-test. Statistical significance in all analyses was defined as *p<0.05, **p<0.01, ***p<0.001.
3. Results
3.1 Low production of M.tb Ag85-specific IFN-γ production depends on H-2k alleles
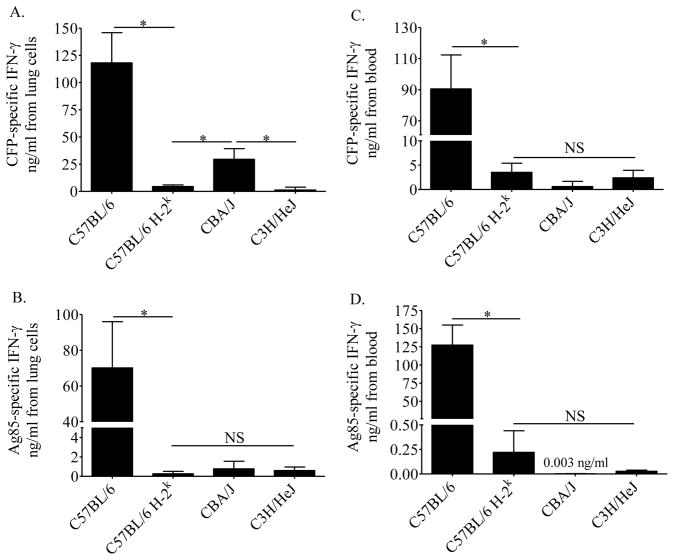
To determine H-2k-dependent and independent effects on antigen-specific responses, cells from M.tb-infected C57BL/6, C57BL/6 H-2k, CBA/J (H-2k), and C3H/HeJ (H-2k) mice were stimulated with a complex mixture of antigens (CFP), and purified antigens (Ag85 and ESAT-6). Lung cells from C57BL/6 H-2k produced significantly less IFN-γ in vitro as compared to cells from C57BL/6 mice when stimulated with M.tb CFP (Figure 1A), M.tb Ag85 (Figure 1B), and M.tb ESAT-6 (not shown). These findings support previous work showing H-2 control of antigen-specific IFN-γ using bronchoalveolar and spleen cells from other mouse strains [16]. Of the three H-2k mouse strains in our studies, an H-2k independent effect was identified by the CBA/J IFN-γ response to CFP (Figure 1A), which may have been due to enhanced responses to ESAT-6 (not shown), a protein found in abundance in M.tb CFP [24].
Figure 1. Antigen-specific IFN-γ produced in vitro from lung and blood cells of M.tb infected mice.
Mice were infected with 37 ± 32 CFU of M.tb Erdman by aerosol and euthanized at day 21 of infection. Lung cells (A, B) and 1:10 diluted whole blood (C, D) were cultured for 72 hours with M.tb CFP (A, C) or M.tb Ag85 (B, D). Antigen-specific IFN-γ was calculated by subtracting the amount of IFN-γ produced in the presence of ovalbumin (lung averages ng/ml: C57BL/6 = 7.6; C57BL/6 H-2k = 2.3; CBA/J = 1.0; C3H/HeJ = 0.25; blood average ng/ml = 0.42, no strain dependent variation). Data shown are the average plus SEM from one experiment with 5–10 mice per group and are representative of three independent experiments. Statistical significance was calculated by the Student’s t-test (C57BL/6 vs C57BL/6 H-2k) and one-way ANOVA with Tukey’s post test (C57BL/6 H-2k, CBA/J, C3H/HeJ), *p<0.05, NS not significant.
In these in vitro assays, the culture wells from each mouse strain contained similar numbers of CD4 T cells, CD8 T cells, and antigen presenting cells (discriminated by CD11c and CD11b [25]) as determined by flow cytometry on separate aliquots (not shown). Low amounts of IFN-γ in vitro, therefore, cannot be due to insufficient cell numbers. All mouse strains produced IFN-γ to non-specific stimuli (concanavalin-A, not shown), indicating that low IFN-γ was not due to an inherent defect in pathways leading to IFN-γ production.
Diluted blood samples from the same mice were stimulated with M.tb antigens. Similar to the lung cell cultures, blood cells from C57BL/6 H-2k produced significantly less antigen-specific IFN-γ as compared to C57BL/6 mice in response to M.tb CFP (Figure 1C), Ag85 (Figure 1D) and ESAT-6 (not shown). No significant differences were detected amongst the blood cells’ antigen-specific IFN- γ responses from three H-2k mouse strains.
Overall, the amount of M.tb antigen-specific IFN-γ produced in vitro by lung cells during primary M.tb infection was reduced by the H-2k haplotype, and was common amongst the three H-2k mouse strains tested. With few exceptions, Ag85-specific IFN-γ responses from blood cells reflected IFN-γ responses from lung cells, a pattern we have previously shown using additional M.tb antigens [22].
3.2 H-2 alleles control the numbers of M.tb Ag85-specific IFN-γ producing lung cells, but not the amount of Ag85-specific IFN-γ produced per cell
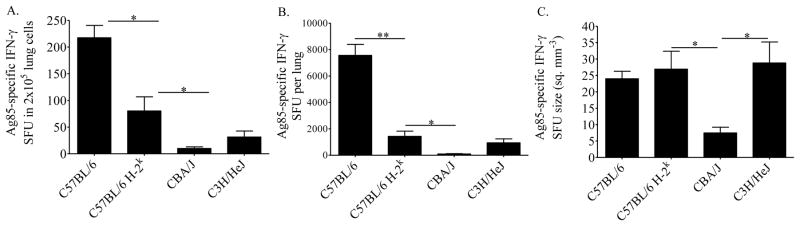
To determine whether a reduced frequency of Ag85-specific cells or poor IFN-γ production per cell contributed to low IFN-γ from the H-2k lung cell cultures (Figure 1), lung cells from M.tb-infected mice were stimulated with Ag85 and IFN-γ-producing cells were quantified by ELISPOT. The H-2k haplotype (C57BL/6 vs C57BL/6 H-2k) reduced the frequency (Figure 2A) and absolute numbers (Figure 2B) of Ag85-specific IFN-γ-producing cells in the lungs, but had no effect on the amount of Ag85-specific IFN-γ produced by each responding cell, as determined by the SFU size (Figure 2C). In contrast, the frequency, absolute numbers, and SFU size of ESAT-6-specific IFN-γ-producing cells was not affected by the H-2k alleles on the C57BL/6 background (not shown).
Figure 2. Frequency and potency of antigen-specific IFN-γ producing cells.
Mice were infected with 37 ± 32 CFU of M.tb Erdman by aerosol and euthanized at day 21 of infection. Lung cells were cultured for 24 hours in the presence of M.tb Ag85. The frequency of IFN-γ SFU (A), absolute numbers of SFU (B), and SFU size (C) were quantified by ELISPOT. Antigen-specific IFN-γ was calculated by subtracting the numbers of IFN-γ SFU produced in the presence of ovalbumin (average 30.1 per 2 × 105 cells, no strain dependent variation). Results are the average and SEM from two independent experiments, each with 5 mice per strain: total per strain, n=10. Statistical significance was calculated by the Student’s t-test (C57BL/6 vs C57BL/6 H-2k) and one-way ANOVA with Tukey’s post test (C57BL/6 H-2k, CBA/J, C3H/HeJ) *p<0.05, **p<0.01.
H-2k-independent effects were also identified in the frequency, absolute number, and SFU size of Ag85-specific IFN-γ responsive cells. Comparison of the three H-2k strains (C57BL/6 H-2k, CBA/J, and C3H/HeJ) demonstrates lower frequencies (Figure 2A) and absolute numbers (Figure 2B) of Ag85 responsive cells in CBA/J and C3H/HeJ lungs than C57BL/6 H-2k, lungs which was statistically significant CBA/J mice. Similar results were demonstrated with ESAT-6 (not shown). The amount of Ag85-specific IFN-γ produced on a per cell basis was significantly less from CBA/J cells as compared to cells from C57BL/6 H-2k or C3H/HeJ mice (Figure 2C), showing that the H-2k independent factors affect the production of IFN-γ at the single-cell level. The SFU size of ESAT-6-specific IFN-γ-producing cells was similar from all H-2k strains (not shown).
To confirm that low IFN-γ was not a result of defective TCR signaling, lung cells were stimulated with anti-CD3 and anti-CD28. TCR-induced IFN-γ production on a per-cell basis was similar across all mouse strains (data not shown) indicating that TCR mediated pathways that contribute to IFN-γ production were intact in the responding cells.
3.3 Numbers of lung cells and kinetics of the acquired immune response
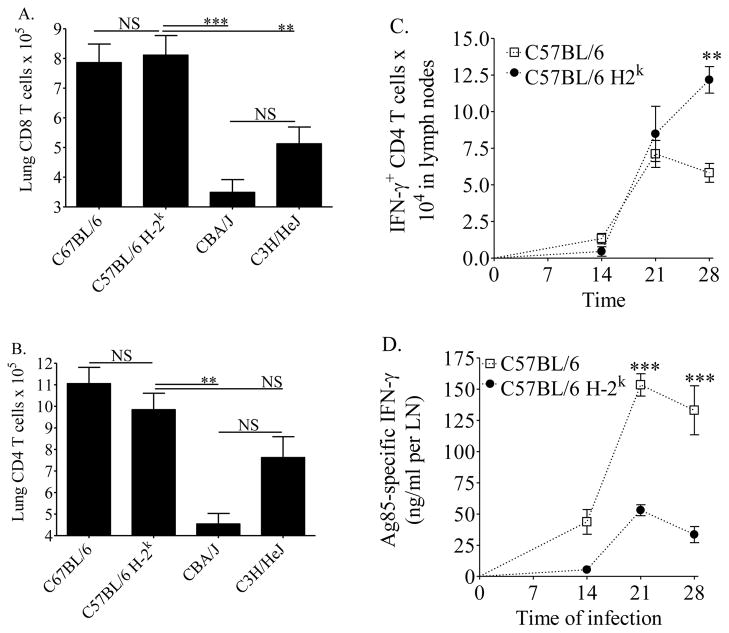
To determine whether the H-2k alleles specifically affected the kinetics of the acquired immune response, cells from the lungs and mediastinal (lung-draining) lymph nodes were evaluated. Total cell numbers (not shown), CD8 T cells (Figure 3A) and antigen presenting cells (not shown) from the lungs of C57BL/6 H-2k mice were similar to C57BL/6 mice, indicating that H-2k alleles did not significantly affect these cell populations in the lungs at 3 weeks of infection. A slight reduction in the numbers of lung CD4 T cells was apparent due to the H-2k haplotype, but the difference was not statistically significant (Figure 3B).
Figure 3. Lung cell numbers and kinetics of the acquired immune response in mice.
Mice were infected with 37 ± 32 CFU of M.tb Erdman by aerosol. At day 21 of infection, mice were euthanized and single cell suspensions from the lungs were fixed and stained with anti-CD3, anti-CD8 (A), and anti-CD4 (B) for flow cytometry. At days 14, 21, and 28 of infection, lymph node cells were isolated and cultured with anti-CD3 and anti-CD28 (1 μg/ml and 0.1 μg/ml, respectively) and monensin for 3 hours. Intracellular IFN-γ was then quantified in CD4 T cells by flow cytometry (C). Separate aliquots of lymph node cells were cultured for 72 hours in the presence of congenic adherent spleen cells and 5 μg/ml of Ag85, and IFN-γ was quantified in supernatants by ELISA and calculated to reflect IFN-γ from the total cells isolated per LN (D). Results are the average and SEM of at least two independent experiments, each with 5 mice per group, total per strain: n = 10–15 (A, B). Results are the average and SEM from one experiment with 4–5 mice per strain (C, D). For A and B, statistical significance was calculated by the Student’s t-test (C57BL/6 vs C57BL/6 H-2k) and one-way ANOVA with Tukey’s post test (C57BL/6 H-2k, CBA/J, C3H/HeJ). For C and D, statistical significance was calculated by one-way ANOVA with Tukey’s post test (C57BL/6 vs C57BL/6 H-2k over time). *p<0.05, **p<0.01, ***p<0.001, NS not significant.
H-2k independent effects were identified by comparing C57BL/6 H-2k, CBA/J, and C3H/HeJ mice. The numbers of CD4 T cells and CD8 T cells present in the lungs at day 21 of M.tb infection were reduced in CBA/J and C3H/HeJ mice, as compared to C57BL/6 H-2k (Figure 3A and 3B). These results indicate that loci outside of the H-2k alleles also influence CD4 T cell and CD8 T cell accumulation in the lungs at 3 weeks of M.tb infection.
We sought to determine whether the H-2k alleles affected the kinetics of Th1 cell priming in the mediastinal lymph nodes. The lymph nodes of C57BL/6 H-2k contained equivalent or more CD4 T cells capable of producing TCR-induced IFN-γ than did C57BL/6 mice, and the responses were not delayed (Figure 3C). In contrast, there was an H-2k-dependent delay and reduction in Ag85-specific IFN-γ produced in lymph node cell cultures (Figure 3D). The differences were statistically significant at days 21 and 28 of M.tb infection in C57BL/6 H-2k lymph node cells as compared to C57BL/6 cells (Figure 3D). These results indicate that the low Ag85-specific IFN-γ responses from the lungs at 3 weeks of M.tb infection in C57BL/6 H-2k mice compared to C57BL/6 mice (Figures 1 and 2) could be a consequence of delayed and diminished T cell priming, specifically to M.tb antigens. Similar results, indicative of an H-2k dependent alteration (delay and decrease) in the TH1 response were obtained from TNF+ CD4 T cells by intracellular flow cytometry (not shown) and TNF secretion into cell culture supernatants ELISAs (not shown).
The acquired immune response in C3H/HeJ mice has also been reported to be diminished and delayed [20], and unpublished data from our laboratory indicates the same is true for the acquired immune response against M.tb in CBA/J mice as compared to C57BL/6 mice. Together, these results indicate that H-2k-dependent and -independent genes contribute to anti-M.tb responses.
3.4 H-2k-dependent and -independent effects on pulmonary immune cell infiltrates during early M.tb infection
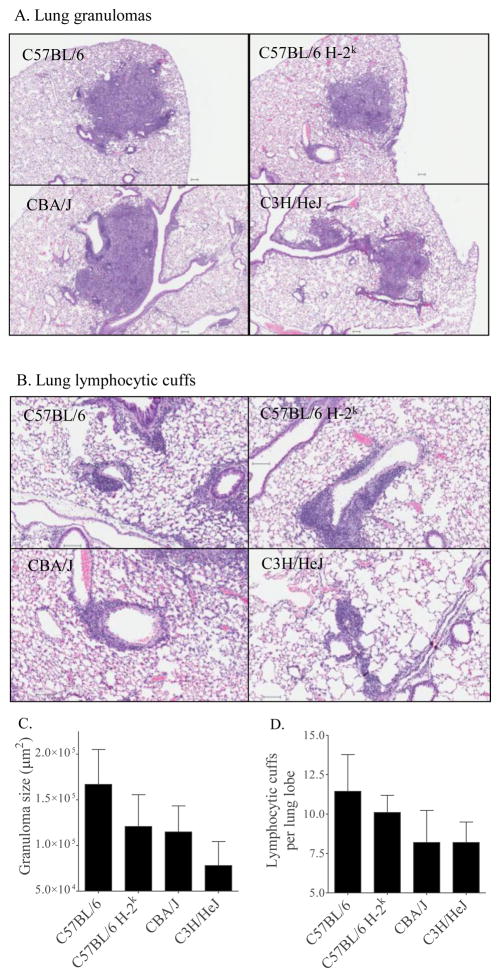
To address whether the H-2k haplotype affected granuloma formation and evidence of lymphocyte trafficking, histology and histomorphometry were performed. All mouse strains had formed pulmonary granulomas (Figure 4A) and had accumulated perivascular and peribronchiolar lymphocytic cuffs (Figure 4B) by three weeks of M.tb infection. On high magnification, granulomas were similarly composed of macrophages, lymphocytes, and neutrophils (not shown). There was a trend for smaller granulomas (Figure 4C) and fewer lymphocytic cuffs (Figure 4D) in C57BL/6 H-2k mice as compared to C57BL/6 mice, although statistically significant differences were not detected. These results suggest a modest H-2k dependent effect on immune cell trafficking to M.tb-infected lungs.
Figure 4. Immune cell aggregates in lungs of M.tb infected mice.
Mice were infected with 37 ± 32 CFU of M.tb Erdman by aerosol and euthanized at day 21 of infection. Lung lobes from individual mice were fixed in neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Slides were digitally scanned. Examples of representative granulomas (A) and lymphocytic cuffs (B) are shown for each mouse strain. Granuloma size (C) and the number of lymphocytic cuffs (D) were quantified. Results are the average and SEM of two independent experiments, each with five mice per group: total group size per strain: n=10. Statistical calculations by the Student’s t-test (C57BL/5 vs C57BL/6 H-2k) and one-way ANOVA with Tukey’s post test (C57BL/6 H-2k, CBA/J, C3H/HeJ) did not identify significant (p<0.05) differences.
To determine H-2k independent effects on granuloma size and lymphocytic cuffs, the lungs of C57BL/6 H-2k, CBA/J, and C3H/HeJ were compared at day 21 of M.tb infection. Similarly sized granulomas were present in C57BL/6 H-2k and CBA/J lungs, while the granulomas of C3H/HeJ mice were smaller (Figure 4C); however, the differences were not statistically significant.
There was a trend for fewer lymphocytic cuffs in the lungs of CBA/J and C3H/HeJ mice as compared to C57BL/6 H-2k mice (Figure 4D), although again, the results were not statistically significant. There were no detectable differences in the numbers or localization of acid-fast bacilli in the lungs of C57BL/6, C57BL/6 H-2k, CBA/J, or C3H/HeJ mice (not shown). Overall, granuloma formation and lymphocyte trafficking were moderately affected by H-2k-dependent and -independent effects. The failure to reach statistical significance may reflect limited tissue for analysis (two 5 μm sections per mouse).
3.5 Reduced IFN-γ is associated with elevated M.tb burden
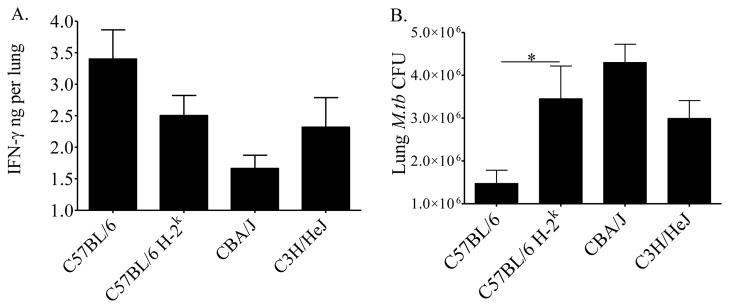
The in vitro assays quantify IFN-γ responses to abundant exogenous antigen. It has not been shown previously whether these in vitro results truly reflect IFN-γ protein levels in vivo. To compare in vitro and in vivo levels, IFN-γ was quantified in homogenized lung tissue from M.tb-infected mice (Figure 5A). Comparison of C57BL/6 H-2k and C57BL/6 lung samples show that IFN-γ levels were moderately reduced by the H-2k alleles. Although the difference was not statistically significant, this trend supports an H-2k dependent control of IFN-γ production, as seen in the antigen-stimulated cell cultures in vitro in Figures 1, 2, and 3. Furthermore, the H-2k-dependent reduction in IFN-γ in vivo was associated with significantly more M.tb bacilli isolated from the lungs of C57BL/6 H-2k mice than from C57BL/6 mice (Figure 5B).
Figure 5. In vivo IFN-γ production is associated with M.tb control.
Mice were infected with 37 ± 32 CFU of M.tb Erdman by aerosol and euthanized at day 21 of infection. IFN-γ present in vivo was quantified by ELISA in homogenized lung tissue (A). M.tb lung burdens were calculated by serially diluting the homogenized lung tissue onto supplemented 7H11 agar plates (B). Results are the average plus SEM from two (A) or two to four (B) independent experiments, each with 5 mice per group, total number per strain: n = 10–20. Statistical significance was determined by the Student’s t-test (C57BL/6 vs. C57BL/6 H-2k) and one-way ANOVA with Tukey’s post-test (C57BL/6 H-2k, CBA/J, C3H/HeJ), *p<0.05.
To assess whether H-2k-independent factors contributed to IFN-γ in vivo and the ability to control M.tb growth by day 21 of infection, C57BL/6 H-2k, CBA/J, and C3H/HeJ lung samples were compared. Similar IFN-γ levels (Figure 5A) and M.tb burdens (Figure 5B) were present in the lungs of C57BL/6 H-2k and C3H/HeJ mice. In contrast, the lungs of CBA/J mice contained less IFN-γ (Figure 5A) and more M.tb bacilli (Figure 5B) than either of the other H-2k strains. Although these levels were not statistically significant, the differences suggest that H-2k-independent factors may also affect immune control of pulmonary M.tb.
4. Discussion and Conclusions
H-2 genes control multiple aspects of the anti-M.tb immune response, including the generation of antibodies [12], survival of mycobacterial antigen-specific T cells in vitro [13, 14], and in vitro IFN-γ levels produced by T cells [16]. Here, we focused our analysis to identify H-2k-dependent (comparison of C57BL/6 and C57BL/6 H-2k data) and -independent (comparison of C57BL/6 H-2k, CBA/J, C3H/HeJ data) contributions to the anti-M.tb immune response during early M.tb infection in vitro and in vivo. In general, the amount of IFN-γ in vivo followed similar H-2k-dependent and -independent patterns than the in vitro assays, but the in vivo levels were significantly less than in vitro.
We defined the IFN-γ antigen-specific responses to Ag85 using multiple in vitro assays. Our results (Figure 1) support previous works that demonstrated H-2-linked control of Ag85-specific IFN-γ responses by ELISA in M. bovis BCG [18] and in M.tb [16] infection models. Here, we provide additional new information that indicates the H-2k alleles reduced the frequency of Ag85-specific IFN-γ-producing lung cells, as compared to H-2b alleles (Figures 2A, 2B), but did not affect IFN-γ production at the single-cell level (Figure 2C). We also show that the H-2k alleles altered Th1 cell priming to Ag85 in the local lymph nodes, resulting in a delayed and diminished generation of Ag85-responsive cells (Figures 3C, 3D). A plausible consequence of suboptimal generation of Ag85-specific IFN-γ-producing cells and reduced IFN-γ protein in vivo (Figure 5A) was impaired control of M.tb growth at day 21 of infection (Figure 5B), although further studies are needed to prove causality in this model. Ongoing studies are underway to determine whether these alterations in H-2k-dependent immune responses and impaired M.tb control affect long-term TB disease progression on the C57BL/6 background. Thus far, survival up to 200 days has been equivalent in C57BL/6 H-2k mice and C57BL/6 WT mice (not shown). In other mouse strains (BALB/c), H-2k alleles negatively impacted median survival by approximately 65 days [9] and increased M.tb burden [15], providing supportive evidence that H-2k alleles accelerate TB disease progression.
In general, it is well established that MHC genes control immune responses [26] and regulate T cell functions in self-tolerance and autoimmunity [27]. MHC genes may also influence the Th1-Th2 cytokine balance [28]. In our model, additional studies are necessary to determine the underlying molecular mechanism of the H-2k-dependent reduction of Ag85-responsiveness as compared to H-2b. These differences may be related to the affinity of Ag85 peptide(s) for the I-Ak and I-Ek class II chains as compared to the immunodominant Ag85B peptide (aa240–254 [29]) recognized by I-Ab class II chains. To our knowledge, H-2k class II restricted immunodominant Ag85 epitopes have not been identified. We can speculate, however, that H-2k genes may impact anti-M.tb immunity by influencing MHC-TCR interactions at many levels including thymic selection, priming and clonal expansion in secondary lymphoid organs, and effector functions at the sites of M.tb infection.
Additional, moderate effects attributable to the H-2k alleles were also identified in these studies. These features were primarily associated with immune cell localization to the lungs, including CD4 T cell numbers, granuloma number, and number of lymphocytic cells which were slightly reduced in C57BL/6 H-2k mice relative to C57BL/6 mice (Figure 4). A failure to reach statistical significance by histomorphometry likely reflects the small amount of lung tissue available for analysis. Overall, our results are consistent with previous work that used different H-2 matched mouse strains and followed granulomatous inflammation over time [6].
We compared the immune responses in three additional mouse strains (C57BL/6 H-2k, CBA/J, and C3H/HeJ) to identify H-2k independent or epistatic effects during early M.tb infection. As has been shown in other combinations of mouse strains with some different analyses [6, 16, 30], features associated with immune cell migration to the lungs were influenced by genes outside of the H-2 region (Figure 4). The mechanism(s) by which non-H-2k genes reduced granuloma numbers and lymphocytic cuffs in CBA/J and C3H/HeJ mice during early M.tb infection was not identified but could be related to a delay in the acquired immune response as has been shown for C3H/HeJ mice [20] or to impaired chemotaxis, as we have suggested [31] for CBA/J mice. We also identified a second H-2k-independent effect apparent from the study of CBA/J Ag85-specific IFN-γ responsive cells. The amount of IFN-γ per cell from CBA/J mice was much less than that produced by individual cells from other mouse strains (Figure 2C). This effect was dependent on Ag85, as individual T cells from this mouse strain were fully capable of producing TCR-induced IFN-γ [22, and not shown].
Overall, our results show that H-2 alleles contribute to the frequency of Ag85-specific IFN-γ producing cells during M.tb infection. We did evaluate another M.tb antigen, ESAT-6, but the H-2-dependent and -independent results were variable and inconsistent (not shown). Our results also indicate that haplotype independent or epistatic factors control immune cell localization to the lungs. Finally by using Ag85, we have expanded our previous ESAT-6 results [22] and similarly showed that blood and lung antigen-specific responses are similar. Overall, this concept is important because blood responses from humans could be used to estimate lung responses during M.tb infection.
Our results using M.tb infected mice may be relevant to human studies because there is a genetic association between the MHC (HLA in man) and increased susceptibility to TB (reviewed in [32]). Limited information has been published regarding the in vitro antigen-specific immune responses of TB patients with DQ and DR polymorphisms that are associated with TB disease. Further studies in these patients or in humanized (HLA-expressing) mice may determine antigen-specific responses or mechanisms that can predict M.tb susceptibility in humans.
H-2k-loci affect Ag85-specific cells, which may impair control of M.tb growth.
H-2k-independent factors influence the potency of IFN-γ producing cells.
H-2k-independent factors influence immune cell trafficking during M.tb infection.
Acknowledgments
Support was provided by the NIH R01 (AI064522; JT), NIH T32 (RR07073; GB) and NIH K08 (AI071111; GB). The manuscript content is the authors’ responsibilities and does not necessarily represent the views of the NIH. M.tb CFP, M.tb Ag85, and the recombinant plasmid pMRLB7 containing Rv3875 (esat-6) were generously provided by Colorado State University’s TB Vaccine Testing and Research Material Contract Number HH5N266200400091c NIH N01AI40091. ESAT-6 was expressed in E. coli and isolated as per the Contract’s recommendations. Routine histology (embedding, sectioning, hematoxylin, and eosin staining and acid-fast staining) services were performed by The Ohio State University’s Department of Veterinary Biosciences Histotechnology Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutherland I. Recent studies in the epidemiology of tuberculosis based on the risk of being infected with the tubercle bacilli. Advances in Tuberculosis Research. 1976;19:1–63. [PubMed] [Google Scholar]
- 2.WHO. Global tuberculosis control - surveillance, planning, financing. 2009. [Google Scholar]
- 3.Mitsos LM, et al. Genetic control of susceptibility to infection with Mycobacterium tuberculosis in mice. Genes and Immunity. 2000;1:467–477. doi: 10.1038/sj.gene.6363712. [DOI] [PubMed] [Google Scholar]
- 4.Bellamy R. The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes and Infection. 1999;1:23–27. doi: 10.1016/s1286-4579(99)80010-0. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy R, et al. Genetic susceptibility to tuberculosis in Africans: A genome-wide scan. Proceedings of the National Academy of Science. 2000;97(14):8005–8009. doi: 10.1073/pnas.140201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orrell J, et al. Morphometric analysis of Mycobacterium tuberculosis infection in mice suggests a genetic influence on the generation of the granulomatous inflammatory response. Journal of Pathology. 1992;166:77–82. doi: 10.1002/path.1711660112. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez F, et al. Multigenic control of disease severity after virulent Mycobacterium tuberculosis infection in mice. Infection and Immunity. 2003;71(1):126–131. doi: 10.1128/IAI.71.1.126-131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barcenas-Morales G, et al. Recessive expression of the H2A-controlled immune response phenotype depends critically on antigen dose. Immunology. 2000;99:221–228. doi: 10.1046/j.1365-2567.2000.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medina E, North RJ. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93:270–274. doi: 10.1046/j.1365-2567.1998.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apt AS, et al. Distinct H-2 complex control of mortality, and immune responses to tuberculosis infection in virgin and BCG-infected mice. Clinical and Experimental Immunology. 1993;94:322–329. doi: 10.1111/j.1365-2249.1993.tb03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brett S, et al. Influence of H-2 genes on growth of Mycobacterium tuberculosis in the lungs of chronically infected mice. Immunology. 1992;76:129–132. [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanyi J, Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986;59:329–332. [PMC free article] [PubMed] [Google Scholar]
- 13.Pichugin AV, Petrovskaya SN, Apt AS. H-2 cpmplex controls CD4/CD8 ratio, recurrent responsiveness to repeated stimulations, and resistance to activation-induced apoptosis during T cell response to mycobacterial antigens. Journal of Leukocyte Biology. 2006;79:739–746. doi: 10.1189/jlb.0705392. [DOI] [PubMed] [Google Scholar]
- 14.Pichugin AV, et al. Capacity of murine T cells to retain long-term responsiveness to mycobacterial antigens is controlled by the H-2 complex. Clinical and Experimental Immunolology. 1998;111:316–324. doi: 10.1046/j.1365-2249.1998.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brett S, et al. Influence of H-2 genes on growth of Mycobacterium tuberculosis in the lungs of chronically infected mice. Immunology. 1992;76(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- 16.Kamath AB, et al. The major histocompatibility haplotype affects T-cell recognition of mycobacterial antigens but not resistance to Mycobacterium tuberculosis in C3H mice. Infection and Immunity. 2004;72(12):6790–6798. doi: 10.1128/IAI.72.12.6790-6798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen K, Palfliet K. In vitro production of gamma interferon is dependent on mouse genotype. Journal of Interferon Research. 1983;3(1):129–137. doi: 10.1089/jir.1983.3.129. [DOI] [PubMed] [Google Scholar]
- 18.Huygen K, et al. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovis Bacillus Calmette-Guerin. Infection and Immunity. 1988;56(12):3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner J, et al. Immunological basis for reactivation of tuberculosis in mice. Infection and Immunity. 2001;69(5):3264–3270. doi: 10.1128/IAI.69.5.3264-3270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chackerian AA, Perera TV, Behar SM. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infection and Immunity. 2001;69(4):2666–2674. doi: 10.1128/IAI.69.4.2666-2674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogerson BJ, et al. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology. 2006;118:195–201. doi: 10.1111/j.1365-2567.2006.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beamer GL, et al. Peripheral blood interferon-{gamma} release assays predict lung responses and Mycobacterium tuberculosis disease outcome in mice. Clinical and Vaccine Immunolology. 2008;15(3):474–83. doi: 10.1128/CVI.00408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vesosky B, Flaherty DK, Turner J. Th1 cytokines facilitate CD8-T-cell-mediated early resistance to infection with Mycobacterium tuberculosis in old mice. Infection and Immunity. 2006;74(6):3314–3324. doi: 10.1128/IAI.01475-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weldingh K, et al. Two-Dimensional Electrophoresis for Analysis of Mycobacterium tuberculosis Culture Filtrate and Purification and Characterization of Six Novel Proteins. Infection and Immunity. 1998;66(8):3492–3500. doi: 10.1128/iai.66.8.3492-3500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Juarrero M, et al. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. Journal of Immunology. 2003;171(6):3128–3135. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- 26.McDevitt H. Discovering the role of the major histocompatability complex in the immune response. Annual Review of Immunology. 2000;18:1–17. doi: 10.1146/annurev.immunol.18.1.1. [DOI] [PubMed] [Google Scholar]
- 27.LeGuern C. Regulation of T cell functions by MHC II self-presentation. Trends in Immunology. 2003;24(12):633–638. doi: 10.1016/j.it.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Guardiola J, et al. Functional significance of polymorphism among MHC class II gene promoters. Tissue Antigens. 1996;48(6):615–625. doi: 10.1111/j.1399-0039.1996.tb02684.x. [DOI] [PubMed] [Google Scholar]
- 29.Yanagisawa S, et al. Mapping of the V beta 11+ helper T cell epitopes on mycobacterial antigen in mouse primed with Mycobacterium tuberculosis. International Immunology. 1997;9(2):227–237. doi: 10.1093/intimm/9.2.227. [DOI] [PubMed] [Google Scholar]
- 30.Lyadova IV, et al. Comparative Analysis of T Lymphocytes Recovered from the Lungs of Mice Genetically Susceptible, Resistant, and Hyperresistant to Mycobacterium tuberculosis-Triggered Disease. Journal of Immunology. 2000;165(10):5921–5931. doi: 10.4049/jimmunol.165.10.5921. [DOI] [PubMed] [Google Scholar]
- 31.Beamer GL, Turner J. Murine models of susceptibility to tuberculosis. Arch Immunol Ther Exp. 2005;53:469–483. [PubMed] [Google Scholar]
- 32.Yim JJ, Selvaraj P. Genetic susceptibility in tuberculosis. Respirology. 2010;15(2):241–256. doi: 10.1111/j.1440-1843.2009.01690.x. [DOI] [PubMed] [Google Scholar]